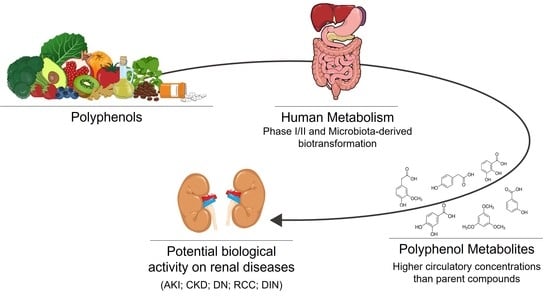
Polyphenols and Their Metabolites in Renal Diseases: An Overview
Abstract
:1. Introduction
2. Implication of Polyphenols in Renal Pathophysiology
2.1. Acute Kidney Injury
2.2. Chronic Kidney Disease
2.3. Diabetic Nephropathy
2.4. Renal Cancer
2.5. Drug-Induced Nephrotoxicity
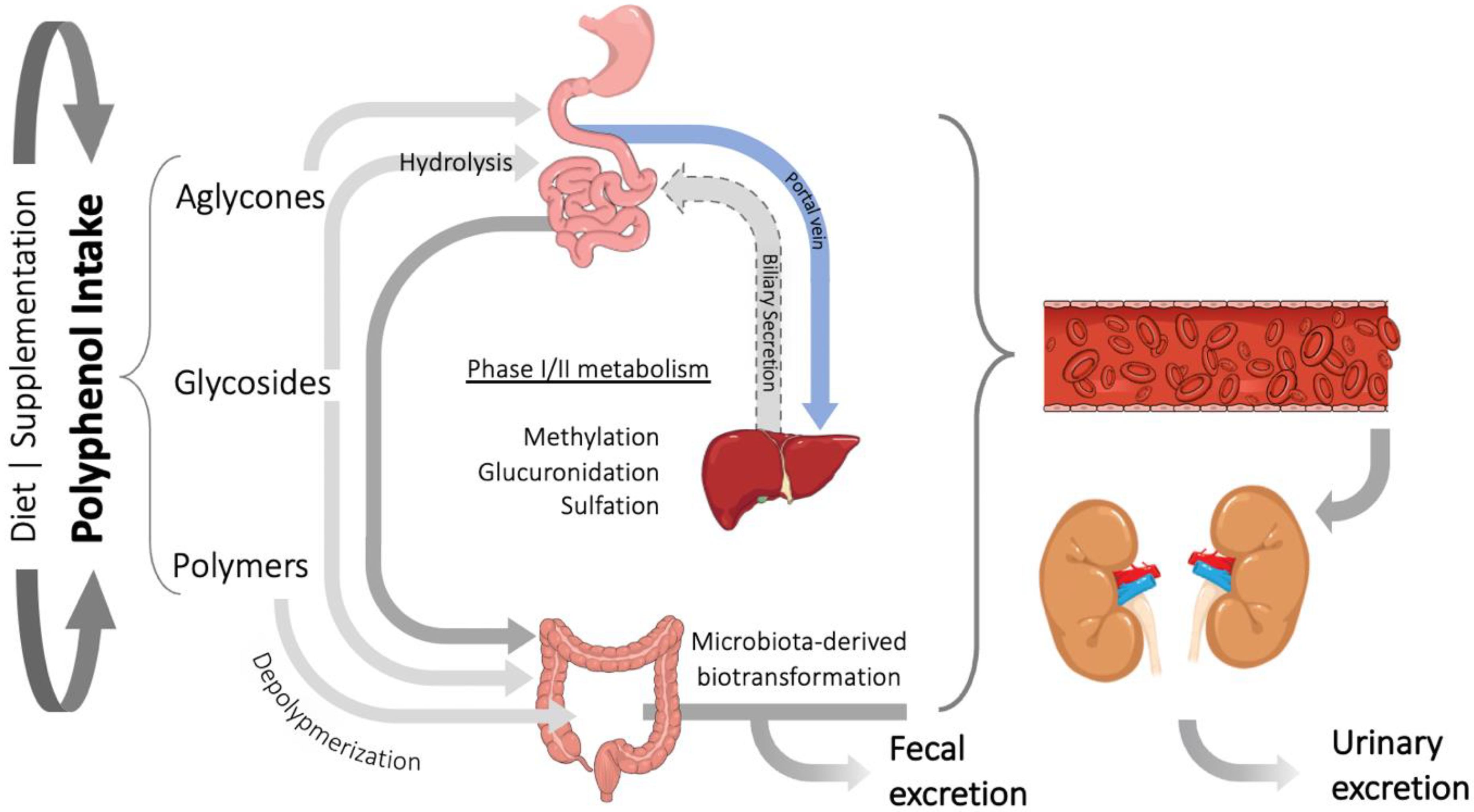
3. Polyphenols Bioavailability and Their Relevant Metabolites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75, A6–A7. [Google Scholar] [CrossRef]
- Levey, A.S.; Titan, S.M.; Powe, N.R.; Coresh, J.; Inker, L.A. Kidney disease, race, and gfr estimation. Clin. J. Am. Soc. Nephrol. 2020, 15, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Maggo, J.K.; Campbell, K.L.; Craig, J.C.; Johnson, D.W.; Sutanto, B.; Ruospo, M.; Tong, A.; Strippoli, G.F.M. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2017, 2017, CD011998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Jia, Z.; Yan, Z.; Yang, J. Consumption of fruits and vegetables and risk of renal cell carcinoma: A meta-analysis of observational studies. Oncotarget 2017, 8, 27892–27903. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A pilot study of a natural food supplement as new possible therapeutic approach in chronic kidney disease patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef]
- Turki, K.; Charradi, K.; Boukhalfa, H.; Belhaj, M.; Limam, F.; Aouani, E.; Unit, H.; Unit, H.; Hospital, H.B. Grape seed powder improves renal failure of chronic kidney disease patients. EXCL1 J. 2016, 15, 424–433. [Google Scholar]
- Lin, Y.F.; Lee, Y.H.; Hsu, Y.H.; Chen, Y.J.; Lin, Y.F.; Cheng, F.Y.; Chiu, H.W. Resveratrol-loaded nanoparticles conjugated with kidney injury molecule-1 as a drug delivery system for potential use in chronic kidney disease. Nanomedicine 2017, 12, 2741–2756. [Google Scholar] [CrossRef]
- Hu, Y.; Mou, L.; Yang, F.; Tu, H.; Lin, W. Curcumin attenuates cyclosporine A-induced renal fibrosis by inhibiting hypermethylation of the klotho promoter. Mol. Med. Rep. 2016, 14, 3229–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms—Biomarkers—Interventions, and Future Perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Gómez-Sierra, T.; Jiménez-Uribe, A.P.; Bellido, B.; Pedraza-Chaverri, J. Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction. BioFactors 2020, 46, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Li, Y.R.; Trush, M. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef]
- Levey, A.S.; James, M.T. Annals graphic medicine—The problem list. Ann. Intern. Med. 2017, 167, ITC65–ITC79. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, Y.Y.; Lai, Y.H.; Cheng, C.C.; Lin, T.C.; Su, Y.S.; Liu, C.H.; Lai, P.C. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Int. J. Mol. Med. 2016, 37, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, F.; Liu, M.; Xue, J.; Huang, H. Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Exp. Ther. Med. 2018, 16, 3233–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Mao, L.; Yang, L.; Zou, J.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 2017, 8, 36449–36461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.J.; Luo, F.; Bu, Q.D.; Jiang, W.; Zhang, W.; Liu, X.M.; Che, L.; Luan, H.; Zhang, H.; Ma, R.X.; et al. Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomed. Pap. 2020, 164, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holthoff, J.H.; Wang, Z.; Seely, K.A.; Gokden, N.; Mayeux, P.R. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012, 81, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K.S. SIRT1/3 activation by Resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell. Longev. 2016, 2016, 7296092. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, Z.; Li, T.; Xu, S.; Wang, X.; Chen, Z.; Lin, C. Polydatin inhibits mitochondrial dysfunction in the renal tubular epithelial cells of a rat model of sepsis-induced acute kidney injury. Anesth. Analg. 2015, 121, 1251–1260. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, A.P.; Bhatti, R. Explicit role of peroxisome proliferator-activated receptor gamma in gallic acid-mediated protection against ischemia-reperfusion-induced acute kidney injury in rats. J. Surg. Res. 2014, 187, 631–639. [Google Scholar] [CrossRef]
- Bao, G.H.; Xu, J.; Hu, F.L.; Wan, X.C.; Deng, S.X.; Barasch, J. EGCG inhibit chemical reactivity of iron through forming an Ngal-EGCG-iron complex. BioMetals 2013, 26, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Twal, M.; Kiefer, P.; Salameh, A.; Schnabel, J.; Ossmann, S.; Von Salisch, S.; Krämer, K.; Sobiraj, A.; Kostelka, M.; Mohr, F.W.; et al. Reno-protective effects of epigallocatechingallate in a small piglet model of extracorporeal circulation. Pharmacol. Res. 2013, 67, 68–78. [Google Scholar] [CrossRef]
- Funamoto, M.; Masumoto, H.; Takaori, K.; Taki, T.; Setozaki, S.; Yamazaki, K.; Minakata, K.; Ikeda, T.; Hyon, S.H.; Sakata, R. Green Tea Polyphenol Prevents Diabetic Rats from Acute Kidney Injury after Cardiopulmonary Bypass Presented at the American Heart Association Scientific Session, Chicago, IL, Nov 15–19, 2014. Ann. Thorac. Surg. 2016, 101, 1507–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakuta, Y.; Okumi, M.; Isaka, Y.; Tsutahara, K.; Abe, T.; Yazawa, K.; Ichimaru, N.; Matsumura, K.; Hyon, S.H.; Takahara, S.; et al. Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl. Int. 2011, 24, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, H.; Peng, H.; Huang, F.; Zhong, J.; Zhou, J. Molecular mechanisms of curcumin renoprotection in experimental acute renal injury. Front. Pharmacol. 2017, 8, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Liang, X.; Liang, M.; Qin, R.; Qin, F.; Wang, X. Ellagic Acid Ameliorates Renal Ischemic-Reperfusion Injury Through NOX4/JAK/STAT Signaling Pathway. Inflammation 2020, 43, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lin, H.; Liu, H.; Lu, Z.; Wang, H.; Fan, S.; Li, N. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation 2019, 42, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Prim. 2017, 3, 17088. [Google Scholar] [CrossRef]
- Li, P.; Song, X.; Zhang, D.; Guo, N.; Wu, C.; Chen, K.; Liu, Y.; Yuan, L.; Chen, X.; Huang, X. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. BioFactors 2020, 46, 168–179. [Google Scholar] [CrossRef]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.; Lu, M.; Han, Y.; Zhou, H.; Liu, W.; Li, L.; Jin, R. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 2017, 119, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef] [Green Version]
- Hongtao, C.; Youling, F.; Fang, H.; Huihua, P.; Jiying, Z.; Jun, Z. Curcumin alleviates ischemia reperfusion-induced late kidney fibrosis through the APPL1/Akt signaling pathway. J. Cell. Physiol. 2018, 233, 8588–8596. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, H.F.; Wang, J.H.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Zhang, Z. Salvianolic Acid A Protects the Kidney against Oxidative Stress by Activating the Akt/GSK-3 β/Nrf2 Signaling Pathway and Inhibiting the NF- B Signaling Pathway in 5/6 Nephrectomized Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2853534. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Pak, W.L.W.; Tanaka, T.; Tang, S.C.W.; Nangaku, M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology 2021, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Pani, A.; Baratta, F.; Pastori, D.; Coronati, M.; Scaglione, F.; del Ben, M. Prevention and management of type II diabetes chronic complications: The role of polyphenols (Mini-Review). Curr. Med. Chem. 2021, 29, 1099–1109. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Health benefits of resveratrol in kidney disease: Evidence from in vitro and in vivo studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowd, V.; Kang, Q.; Wang, Q.; Wang, Q.; Chen, F.; Cheng, K.W. Resveratrol: Evidence for Its Nephroprotective Effect in Diabetic Nephropathy. Adv. Nutr. 2020, 11, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Li, K.X.; Ji, M.J.; Sun, H.J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Tsatsakis, A.; Skaperda, Z.; Kouretas, D.; Shahraki, J. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, X.J.; Ding, M.R.; You, C.Y.; Lin, X.; Wang, Y.; Wu, M.J.Y.; Xu, G.F.; Wang, G.D. Resveratrol decreases high glucose-induced apoptosis in renal tubular cells via suppressing endoplasmic reticulum stress. Mol. Med. Rep. 2020, 22, 4367–4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, R.; Zhao, L.; Ma, S.; Qin, G. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Eur. J. Pharmacol. 2020, 885, 173387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Qi, X.; Zhang, X.; Ren, K. Resveratrol Protects Against Post-Contrast Acute Kidney Injury in Rabbits with Diabetic Nephropathy. Front. Pharmacol. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, W.; Wang, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, J.; Chen, W.; Feng, F.; Deng, Y. Resveratrol inhibits lipopolysaccharide-induced extracellular matrix accumulation and inflammation in rat glomerular mesangial cells by sphk1/s1p2/nf-κb pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Su, H.; Liang, D.; Li, J.; Ting, W.J.; Liao, S.C.; Huang, C.Y. Ramipril and resveratrol co-treatment attenuates RhoA/ROCK pathway-regulated early-stage diabetic nephropathy-associated glomerulosclerosis in streptozotocin-induced diabetic rats. Environ. Toxicol. 2019, 34, 861–868. [Google Scholar] [CrossRef]
- Xie, X.; Peng, J.; Huang, K.; Huang, J.; Shen, X.; Liu, P.; Huang, H. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2012, 362, 183–193. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, J.; Chen, Z.; Huang, J.; Chen, Q.; Cai, W.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic. Biol. Med. 2017, 106, 393–405. [Google Scholar] [CrossRef] [PubMed]
- El-Hameed, A.; Abeer, M. Polydatin-loaded chitosan nanoparticles ameliorates early diabetic nephropathy by attenuating oxidative stress and inflammatory responses in streptozotocin-induced diabetic rat. J. Diabetes Metab. Disord. 2020, 19, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Tao, L.; Xiaohui, X.; Zelin, Z.; Jiangang, L.; Zhao, S.; Weikang, H.; Hongchao, X.; Qiujing, W.; Xin, L. Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. J. Cell. Physiol. 2017, 232, 2776–2787. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Q.; Sun, X.H.; Li, X.J.; Xu, Z.C.; Yang, Y.; Lin, Z.Y.; Xiao, H.M.; Zhang, M.; Quan, S.J.; Huang, H.Q. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharmacol. Sin. 2020, 41, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients 2020, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from Black Rice Ameliorates Diabetic Nephropathy via Reducing Blood Glucose, Suppressing Oxidative Stress and Inflammation, and Regulating Transforming Growth Factor β1/Smad Expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhai, Q.; Li, Y.; Cao, M.; Xu, Y.; Zhao, K.; Wang, T. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed. Pharmacother. 2018, 103, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, Y.; Luo, G.; Yang, X.; Huang, W. Cyanidin-3-o-glucoside attenuates high glucose–induced podocyte dysfunction by inhibiting apoptosis and promoting autophagy via activation of sirt1/ampk pathway. Can. J. Physiol. Pharmacol. 2021, 99, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stepkowski, T.M.; Brzóska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Liu, S.; Li, N.; Ye, L.F.; Zha, M.; Li, C.Y.; Zhao, Y.; Pu, Q.; Bao, J.J.; Chen, X.J.; et al. Quercetin Antagonizes Glucose Fluctuation Induced Renal Injury by Inhibiting Aerobic Glycolysis via HIF-1α/miR-210/ISCU/FeS Pathway. Front. Med. 2021, 8, 219. [Google Scholar] [CrossRef]
- Du, L.; Li, C.; Qian, X.; Chen, Y.; Wang, L.; Yang, H.; Li, X.; Li, Y.; Yin, X.; Lu, Q. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol. Res. 2019, 146, 104320. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, J.; Wang, X.; Ge, J.; Li, N. Quercetin improves lipid metabolism via SCAP-SREBP2-LDLr signaling pathway in early stage diabetic nephropathy. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, F.; Liu, S.; Yan, B.; Li, X.; Ruan, S.; Yang, S. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int. J. Nanomed. 2017, 12, 7799–7813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, S.; Sun, X.; Lou, Y.; Bao, J.; Yu, J. Hyperoside ameliorates diabetic nephropathy induced by STZ via targeting the miR-499–5p/APC axis. J. Pharmacol. Sci. 2021, 146, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Wang, S.; Zhang, X.; Zai, W.; Fan, J.; Chen, W.; Bian, Q.; Luan, J.; Shen, Y.; Zhang, Y.; et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine 2018, 41, 45–53. [Google Scholar] [CrossRef]
- Dua, T.K.; Joardar, S.; Chakraborty, P.; Bhowmick, S.; Saha, A.; De Feo, V.; Dewanjee, S. Myricitrin, a glycosyloxyflavone in myrica esculenta bark ameliorates diabetic nephropathy via improving glycemic status, reducing oxidative stress, and suppressing inflammation. Molecules 2021, 26, 258. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Antioxidant, anti-apoptotic, and protective effects of myricitrin and its solid lipid nanoparticles on streptozotocin-nicotinamideinduced diabetic nephropathy in type 2 diabetic male mice. Iran. J. Basic Med. Sci. 2019, 22, 1424–1431. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Protective Effects of Epigallocatechin-3-Gallate from Green Tea in Various Kidney Diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Velusamy, P.; Chakrapani, L.N.; Srinivasan, A.K.; Singh, A.; Johnson, T.; Periandavan, K. Impact of EGCG Supplementation on the Progression of Diabetic Nephropathy in Rats: An Insight into Fibrosis and Apoptosis. J. Agric. Food Chem. 2017, 65, 8028–8036. [Google Scholar] [CrossRef]
- Yoon, S.P.; Maeng, Y.H.; Hong, R.; Lee, B.R.; Kim, C.G.; Kim, H.L.; Chung, J.H.; Shin, B.C. Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014, 116, 1210–1215. [Google Scholar] [CrossRef]
- Mohan, T.; Narasimhan, K.K.S.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Srinivasan, A.; Karthikeyan, P.; Kannan, P.; Tamilarasan, B.; et al. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Wang, L.; Ueda, S.; Yamanoue, M.; Ashida, H.; Shirai, Y. The mechanisms of ameliorating effect of a green tea polyphenol on diabetic nephropathy based on diacylglycerol kinase α. Sci. Rep. 2020, 10, 11790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Q.; Yang, X.Y.; Zhang, X.J.; Yu, C.J.; Pang, Q.Q.; Huang, Y.W.; Wang, X.J.; Sheng, J. EGCG targeting Notch to attenuate renal fibrosis: Via inhibition of TGFβ/Smad3 signaling pathway activation in streptozotocin-induced diabetic mice. Food Funct. 2020, 11, 9686–9695. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Xiao, X.; Jiang, B.; Zhou, M.; Zhang, Y.; Li, H.; Hu, Z. Epigallocatechin-3-gallate protects from high glucose induced Podocyte apoptosis via suppressing endoplasmic reticulum stress. Mol. Med. Rep. 2017, 16, 6142–6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez Cilleros, D.; López-Oliva, M.E.; Martín, M.Á.; Ramos, S. (−)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling. Food Funct. 2020, 11, 8811–8824. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y.; Zhang, Z.; Li, Y. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014, 5, 1872–1880. [Google Scholar] [CrossRef]
- Cai, X.; Bao, L.; Ren, J.; Li, Y.; Zhang, Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1α axis in vitro. Food Funct. 2016, 7, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, L.; Zhou, Q.; Yan, S.; Li, Z.; Sheng, J.; Zhang, W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol. Nutr. Food Res. 2014, 58, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Wei, C.C.; Chang, S.J. Low-molecular-weight polyphenols protect kidney damage through suppressing NF-κB and modulating mitochondrial biogenesis in diabetic: Db/db mice. Food Funct. 2016, 7, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yokozawa, T.; Noh, J.S. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates diabetes-induced renal damage through the advanced glycation end product-related pathway in db/db mice. J. Nutr. 2014, 144, 1150–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Noh, J.S.; Fujii, H.; Roh, S.-S.; Song, Y.-O.; Choi, J.S.; Chung, H.Y.; Yokozawa, T. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice. Drug Discov. Ther. 2015, 9, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/β-TrcP/Nrf2 pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, W.; Ye, S. The olive constituent oleuropein exerts nephritic protective effects on diabetic nephropathy in db/db mice. Arch. Physiol. Biochem. 2019, 128, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Qiang, G.; Zhao, Y.; Yang, X.; Chen, X.; Yan, Y.; Wang, X.; Liu, C.; Zhang, L.; Du, G. Salvianolic Acid A Protects Against Diabetic Nephropathy through Ameliorating Glomerular Endothelial Dysfunction via Inhibiting AGE-RAGE Signaling. Cell. Physiol. Biochem. 2018, 44, 2378–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, M.; Zoja, C.; Zanchi, C.; Corna, D.; Villa, S.; Bolognini, S.; Novelli, R.; Perico, L.; Remuzzi, G.; Benigni, A.; et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 2020, 10, 8418. [Google Scholar] [CrossRef]
- Wild, C.P. International Agency for Research on Cancer. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 419, pp. 1067–1069. ISBN 9780123864543. [Google Scholar]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Turajlic, S.; Rowan, A.; Nicol, D.; Farmery, J.H.R.; O’Brien, T.; Martincorena, I.; Tarpey, P.; Angelopoulos, N.; Yates, L.R.; et al. Timing the Landmark Events in the Evolution of Clear Cell Renal Cell Cancer: TRACERx Renal. Cell 2018, 173, 611–623.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic nutrients in cancer chemoprevention and metastasis: Role of the epithelial-to-mesenchymal (EMT) pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Zhang, S.; Zhang, Q.; Kang, L.; Ma, C.; Feng, L.; Li, S.; Li, J.; Yang, L.; Liu, J.; et al. Resveratrol inhibits tumor progression by down-regulation of NLRP3 in renal cell carcinoma. J. Nutr. Biochem. 2020, 85, 108489. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, H.; Zhu, L. Inhibitory effect of resveratrol on the expression of the VEGF gene and proliferation in renal cancer cells. Mol. Med. Rep. 2011, 4, 981–983. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Liao, W.; Xiong, Y. Modification of Antitumor Immunity and Tumor Microenvironment by Resveratrol in Mouse Renal Tumor Model. Cell Biochem. Biophys. 2015, 72, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Baek, S.H.; Um, J.Y.; Shim, B.S.; Ahn, K.S. Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPε and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrol. 2016, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiu, M.; Chen, L.; Liu, L.; Tan, G.; Liu, J. Resveratrol promotes regression of renal carcinoma cells via a rennin-angiotensin system suppression-dependent mechanism. Oncol. Lett. 2017, 13, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.S.; Xu, T.B.; Ruan, H.L.; Xiao, H.B.; Liu, J.; Song, Z.S.; Cao, Q.; Bao, L.; Liu, D.; Wang, C.; et al. LXRα promotes cell metastasis by regulating the NLRP3 inflammasome in renal cell carcinoma. Cell Death Dis. 2019, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Fang, Q.; Ji, S.; Han, Z.; Cheng, W.; Zhang, H. Resveratrol-mediated apoptosis in renal cell carcinoma via the p53/AMP-activated protein kinase/mammalian target of rapamycin autophagy signaling pathway. Mol. Med. Rep. 2018, 17, 502–508. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, L.; Wang, W.; Lin, P. Resveratrol inhibits ACHN cells via regulation of histone acetylation. Pharm. Biol. 2020, 58, 231–238. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; He, X. Autophagy suppresses resveratrol-induced apoptosis in renal cell carcinoma 786-O cells. Oncol. Lett. 2020, 19, 3269–3277. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Chen, L.; Zhou, M.; Guo, J.; Wang, Y.; Zheng, D.; Zhong, S. Resveratrol enhances chemosensitivity of renal cell carcinoma to paclitaxel. Front. Biosci. Landmark 2019, 24, 1452–1461. [Google Scholar] [CrossRef]
- Kabel, A.M.; Atef, A.; Estfanous, R.S. Ameliorative potential of sitagliptin and/or resveratrol on experimentally-induced clear cell renal cell carcinoma. Biomed. Pharmacother. 2018, 97, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; di Li, F.; Shi, C.W.; Du, J.L.; Xue, Y.J.; Liu, X.Y.; Cao, X.; Wei, N. Mechanism and therapeutic prospect of resveratrol combined with TRAIL in the treatment of renal cell carcinoma. Cancer Gene Ther. 2020, 27, 619–623. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting miRNAs by polyphenols: Novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017, 46, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Gu, B.; Ding, Q.; Xia, G.; Fang, Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncol. Rep. 2009, 21, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.J.; Yao, X.D.; Peng, B.; Xu, Y.F.; Wang, G.C.; Huang, J.; Liu, M.; Zheng, J.H. Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp. Ther. Med. 2016, 11, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.; Zhu, G.; Jia, N.; Yang, W. Epigallocatechin-3-gallate Sensitizes Human 786-O Renal Cell Carcinoma Cells to TRAIL-Induced Apoptosis. Cell Biochem. Biophys. 2015, 72, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, W.; Li, B.; Zhang, K.; Wu, Y.; Xu, H.; Wang, J.; Zhang, J.; Fan, R.; Wei, J. Curcumin Promotes Cell Cycle Arrest and Inhibits Survival of Human Renal Cancer Cells by Negative Modulation of the PI3K/AKT Signaling Pathway. Cell Biochem. Biophys. 2015, 73, 681–686. [Google Scholar] [CrossRef]
- Seo, B.R.; Min, K.; Cho, I.J.; Kim, S.C.; Kwon, T.K. Curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability. PLoS ONE 2014, 9, e95588. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Z.; Chong, T.; Yang, J.; Li, H.; Chen, H. Curcumin enhances the radiosensitivity of renal cancer cells by suppressing NF-κB signaling pathway. Biomed. Pharmacother. 2017, 94, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, T.W.; Chen, P.N.; Wu, H.C.; Wu, S.W.; Tsai, P.Y.; Hsieh, Y.S.; Chang, H.R. Kaempferol inhibits the invasion and migration of renal cancer cells through the downregulation of AKT and FAK pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Lateef, A.; Oday-O-Hamiza; Qamar, W.; Ali, F.; Sultana, S. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: Plausible role of NF-κB. Toxicol. Lett. 2013, 216, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Moon, A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. 2012, 20, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of cisplatin-induced acute kidney injury: Pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Casanova, A.G.; Hernández-Sánchez, M.T.; Martínez-Salgado, C.; Morales, A.I.; Vicente-Vicente, L.; López-Hernández, F.J. A meta-analysis of preclinical studies using antioxidants for the prevention of cisplatin nephrotoxicity: Implications for clinical application. Crit. Rev. Toxicol. 2020, 50, 780–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Han, M.K.; Lee, S.Y.; Ramkumar, K.M.; et al. Sirt1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Ren. Physiol. 2011, 301, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentovic, M.A.; Ball, J.G.; Mike Brown, J.; Terneus, M.V.; McQuade, E.; Van Meter, S.; Hedrick, H.M.; Roy, A.A.; Williams, T. Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress. Toxicol. Vitr. 2014, 28, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Xiao, X.; Zhen, J.; Feng, J.; Song, C.; Jiang, B.; Hu, Z. Resveratrol attenuates acute kidney injury by inhibiting death receptor-mediated apoptotic pathways in a cisplatin-induced rat model. Mol. Med. Rep. 2016, 14, 3683–3689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Amaral, C.L.; Francescato, H.D.C.; Coimbra, T.M.; Costa, R.S.; D’arc Castania Darin, J.; Antunes, L.M.G.; Bianchi, M.D.L.P. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Abo-Youssef, A.M.; Khalaf, M.M.; Abo-Saif, A.A.; Saleh, I.G.; Abdelghany, T.M. Resveratrol influences platinum pharmacokinetics: A novel mechanism in protection against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2018, 290, 73–82. [Google Scholar] [CrossRef]
- Cigremis, Y.; Akgoz, M.; Ozen, H.; Karaman, M.; Kart, A.; Gecer, M.; Atalan, G. Resveratrol ameliorates cisplatin-induced oxidative injury in New Zealand rabbits. Can. J. Physiol. Pharmacol. 2015, 93, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Kamobayashi, H.; Sato, D.; Komori, M.; Yoshimura, M.; Hamada, A.; Kohda, Y.; Tomita, K.; Saito, H. Alleviation of cisplatin-induced acute kidney injury using phytochemical polyphenols is accompanied by reduced accumulation of indoxyl sulfate in rats. Clin. Exp. Nephrol. 2011, 15, 820–830. [Google Scholar] [CrossRef]
- Kuhlmann, M.K.; Horsch, E.; Burkhardt, G.; Wagner, M.; Köhler, H. Reduction of cisplatin toxicity in cultured renal tubular cells by the bioflavonoid quercetin. Arch. Toxicol. 1998, 72, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, L.; Hu, C.; Wang, Q.; Lei, Y.; Zhao, B. Myricitrin protects against cisplatin-induced kidney injury by eliminating excessive reactive oxygen species. Int. Urol. Nephrol. 2020, 52, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Song, J.; Jiang, B.; Pei, F.; Chen, B.; Yang, X.; Liu, G.; Hu, Z. Epigallocatechin-3-gallate protects against cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int. J. Clin. Exp. Pathol. 2014, 7, 4607–4616. [Google Scholar] [PubMed]
- Chen, B.; Liu, G.; Zou, P.; Li, X.; Hao, Q.; Jiang, B.; Yang, X.; Hu, Z. Epigallocatechin-3-gallate protects against cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Exp. Biol. Med. 2015, 240, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- El-Mowafy, A.M.; Salem, H.A.; Al-Gayyar, M.M.; El-Mesery, M.E.; El-Azab, M.F. Evaluation of renal protective effects of the green-tea (EGCG) and red grape resveratrol: Role of oxidative stress and inflammatory cytokines. Nat. Prod. Res. 2011, 25, 850–856. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Al-Gayyar, M.M.; Salem, H.A.; El-Mesery, M.E.; Darweish, M.M. Novel chemotherapeutic and renal protective effects for the green tea (EGCG): Role of oxidative stress and inflammatory-cytokine signaling. Phytomedicine 2010, 17, 1067–1075. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Bhatia, J.; Gamad, N.; Dinda, A.K.; Gupta, Y.K.; Arya, D.S. Molecular mechanisms underlying attenuation of cisplatin-induced acute kidney injury by epicatechin gallate. Lab. Investig. 2016, 96, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Waseem, M.; Kaushik, P.; Parvez, S. Mitochondria-mediated mitigatory role of curcumin in cisplatin-induced nephrotoxicity. Cell Biochem. Funct. 2013, 31, 678–684. [Google Scholar] [CrossRef]
- Kumar, P.; Sulakhiya, K.; Barua, C.C.; Mundhe, N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol. Cell. Biochem. 2017, 431, 113–122. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Muqbil, I.; Sahin, N.; Gencoglu, H.; Guler, O.; Padhye, S.B.; Sarkar, F.H.; Mohammad, R.M. Comparative in vivo evaluations of curcumin and its analog difluorinated curcumin against cisplatin-induced nephrotoxicity. Biol. Trace Elem. Res. 2014, 157, 156–163. [Google Scholar] [CrossRef]
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodríguez-Muñoz, R.; Reyes, J.L.; Loredo, M.L.; Barrera-Oviedo, D.; Pinzón, E.; Rodríguez-Rangel, D.S.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: Relation to oxidative stress. Food Funct. 2016, 7, 279–293. [Google Scholar] [CrossRef]
- Ortega-Domínguez, B.; Aparicio-Trejo, O.E.; García-Arroyo, F.E.; León-Contreras, J.C.; Tapia, E.; Molina-Jijón, E.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107, 373–385. [Google Scholar] [CrossRef]
- Ueki, M.; Ueno, M.; Morishita, J.; Maekawa, N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J. Biosci. Bioeng. 2013, 115, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Ugur, S.; Ulu, R.; Dogukan, A.; Gurel, A.; Yigit, I.P.; Gozel, N.; Aygen, B.; Ilhan, N. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 2015, 37, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kharusi, N.; Babiker, H.A.; Al-Salam, S.; Waly, M.I.; Nemmar, A.; Al-Lawati, I.; Yasin, J.; Beegam, S.; Ali, B.H. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: A dose-dependent study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 299–310. [Google Scholar]
- Goyal, Y.; Koul, A.; Ranawat, P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell. Biochem. 2019, 453, 205–215. [Google Scholar] [CrossRef]
- Ahmad, S.T.; Sultana, S. Tannic acid mitigates cisplatin-induced nephrotoxicity in mice. Hum. Exp. Toxicol. 2012, 31, 145–156. [Google Scholar] [CrossRef]
- Chien, L.H.; Wu, C.T.; Deng, J.S.; Jiang, W.P.; Huang, W.C.; Huang, G.J. Salvianolic acid c protects against cisplatin-induced acute kidney injury through attenuation of inflammation, oxidative stress and apoptotic effects and activation of the CaMKK–AMPK–sirt1-associated signaling pathway in mouse models. Antioxidants 2021, 10, 1620. [Google Scholar] [CrossRef]
- Wang, T.E.J.; Liu, H.T.; Lai, Y.H.; Jan, T.R.; Nomura, N.; Chang, H.W.; Chou, C.C.; Lee, Y.J.; Tsai, P.S.J. Honokiol, a polyphenol natural compound, attenuates cisplatin-induced acute cytotoxicity in renal epithelial cells through cellular oxidative stress and cytoskeleton modulations. Front. Pharmacol. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.T.; Wang, T.E.; Hsu, Y.T.; Chou, C.C.; Huang, K.H.; Hsu, C.C.; Liang, H.J.; Chang, H.W.; Lee, T.H.; Tsai, P.S. Nanoparticulated honokiol mitigates cisplatin-induced chronic kidney injury by maintaining mitochondria antioxidant capacity and reducing caspase 3-associated cellular apoptosis. Antioxidants 2019, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci. 2021, 286, 120071. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Wei, Y. Hydroxytyrosol protects against cisplatin-induced nephrotoxicity via attenuating CKLF1 mediated inflammation, and inhibiting oxidative stress and apoptosis. Int. Immunopharmacol. 2021, 96, 107805. [Google Scholar] [CrossRef]
- Vicente-Vicente, L.; Casanova, A.G.; Hernández-Sánchez, M.T.; Pescador, M.; López-Hernández, F.J.; Morales, A.I. A systematic meta-analysis on the efficacy of pre-clinically tested nephroprotectants at preventing aminoglycoside nephrotoxicity. Toxicology 2017, 377, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Buitrago, J.M.; Santiago, J.M.; Fernández-Tagarro, M.; López-Novoa, J.M.; Pérez-Barriocanal, F. Protective effect of trans-Resveratrol on gentamicin-induced nephrotoxicity. Antioxid. Redox Signal. 2002, 4, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Silan, C.; Uzun, Ö.; Çomunoǧlu, N.Ü.; Gokçen, S.; Bedirhan, S.; Cengiz, M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol. Pharm. Bull. 2007, 30, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshay, O.N.; Ewees, M.G.; Abdel-Bakky, M.S.; Hafez, S.M.N.A.; Abdelrehim, A.B.; Bayoumi, A.M.A. Resveratrol reduces gentamicin-induced EMT in the kidney via inhibition of reactive oxygen species and involving TGF-β/Smad pathway. Life Sci. 2020, 258, 118178. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Rodríguez-Barbero, A.; Vicente-Sánchez, C.; Mayoral, P.; López-Novoa, J.M.; Pérez-Barriocanal, F. Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sci. 2006, 78, 2373–2377. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M.; García-Niño, W.R.; Tapia, E.; Zazueta, C.; Huerta-Yepez, S.; León-Contreras, J.C.; Hernández-Pando, R.; Aparicio-Trejo, O.E.; Madero, M.; Pedraza-Chaverri, J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid. Based Complement. Altern. Med. 2015, 2015, 917435. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Ahmad, S.F.; Mudassar, S.; Mohsin, K.; Shakeel, F.; Korashy, H.M.; Bakheet, S.A. Sinapic acid mitigates gentamicin-induced nephrotoxicity and associated oxidative/nitrosative stress, apoptosis, and inflammation in rats. Life Sci. 2016, 165, 1–8. [Google Scholar] [CrossRef]
- Dallak, M.; Dawood, A.F.; Haidara, M.A.; Abdel Kader, D.H.; Eid, R.A.; Kamar, S.S.; Shams Eldeen, A.M.; Al-Ani, B. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem. Toxicol. 2020, 45, 1–7. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Yu, X.; Song, N.; Xu, X.; Hu, J.; Zhang, T.; Ding, X. Salvianolic acid B prevents iodinated contrast media-induced acute renal injury in rats via the PI3K/Akt/Nrf2 pathway. Oxid. Med. Cell. Longev. 2016, 2016, 7079487. [Google Scholar] [CrossRef] [Green Version]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcdade, D.; Patrick, R.M.; Rizvi, S.S.H.; Knorr, D.; Labuza, T.P. Dietary Polyphenols: Metabolism and Health Effects; Tomás-Barberán, F.A., González-Sarrías, A., García-Villalba, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; ISBN 9781119563754. [Google Scholar]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Carregosa, D.; Mota, S.; Ferreira, S.; Alves-Dias, B.; Loncarevic-Vasiljkovic, N.; Crespo, C.L.; Menezes, R.; Teodoro, R.; Santos, C.N. Dos Overview of beneficial effects of (Poly)phenol metabolites in the context of neurodegenerative diseases on model organisms. Nutrients 2021, 13, 2940. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Miriam, M.-H.; Vallverdú-Quera, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Gonthier, M.-P.; Manach, C.; Morand, C.; Mennen, L.; Rémésy, C.; Scalbert, A. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br. J. Nutr. 2005, 94, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Godinho-Pereira, J.; Oudot, C.; Sequeira, C.O.; Macià, A.; Carvalho, F.; Motilva, M.J.; Pereira, S.A.; Matzapetakis, M.; Brenner, C.; et al. Berry fruits modulate kidney dysfunction and urine metabolome in Dahl salt-sensitive rats. Free Radic. Biol. Med. 2020, 154, 119–131. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Masereeuw, R.; Russel, F.G.M. Mechanisms and clinical implications of renal drug excretion. Drug Metab. Rev. 2001, 33, 299–351. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.; Giacomoni, F.; Pavot, B.; Fillatre, Y.; Rothwell, J.; Sualdea, B.B.; Veyrat, C.; Gladine, C.; Kopec, R.; Bento, A.; et al. PhytoHub V1.4: A New Release for the Online Database Dedicated to Food Phytochemicals and Their Human Metabolites. In Proceedings of the International Conference on Food Bioactives & Health, Norwich, UK, 13–15 September 2016. [Google Scholar]
- Jain, R.B. Trends in the levels of urine and serum creatinine: Data from NHANES 2001–2014. Environ. Sci. Pollut. Res. 2017, 24, 10197–10204. [Google Scholar] [CrossRef]
- Schoen, T.; Blum, J.; Paccaud, F.; Burnier, M.; Bochud, M.; Conen, D. Factors associated with 24-hour urinary volume: The Swiss salt survey. BMC Nephrol. 2013, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Llorach, R.; Khan, N.; Monagas, M.; Rotches-Ribalta, M.; Lamuela-Raventos, R.; Estruch, R.; Tinahones, F.J.; Andres-Lacueva, C. Effect of milk on the urinary excretion of microbial phenolic acids after cocoa powder consumption in humans. J. Agric. Food Chem. 2010, 58, 4706–4711. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; Van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Loke, W.M.; Jenner, A.M.; Proudfoot, J.M.; McKinley, A.J.; Hodgson, J.M.; Halliwell, B.; Croft, K.D. A metabolite profiling approach to identify biomarkers of flavonoid intake in humans. J. Nutr. 2009, 139, 2309–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, K.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Istas, G.; Heiss, C.; Rodriguez-Mateos, A. Plasma and urinary phenolic profiles after acute and repetitive intake of wild blueberry. Molecules 2016, 21, 1120. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Galli, C.; Viappiani, S.; Galli, G.; Sala, A. Olive oils rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Commun. 2000, 278, 797–799. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes Dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Lea-Henry, T.N.; Carland, J.E.; Stocker, S.L.; Sevastos, J.; Roberts, D.M. Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin. J. Am. Soc. Nephrol. 2018, 13, 1085–1095. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S.V. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
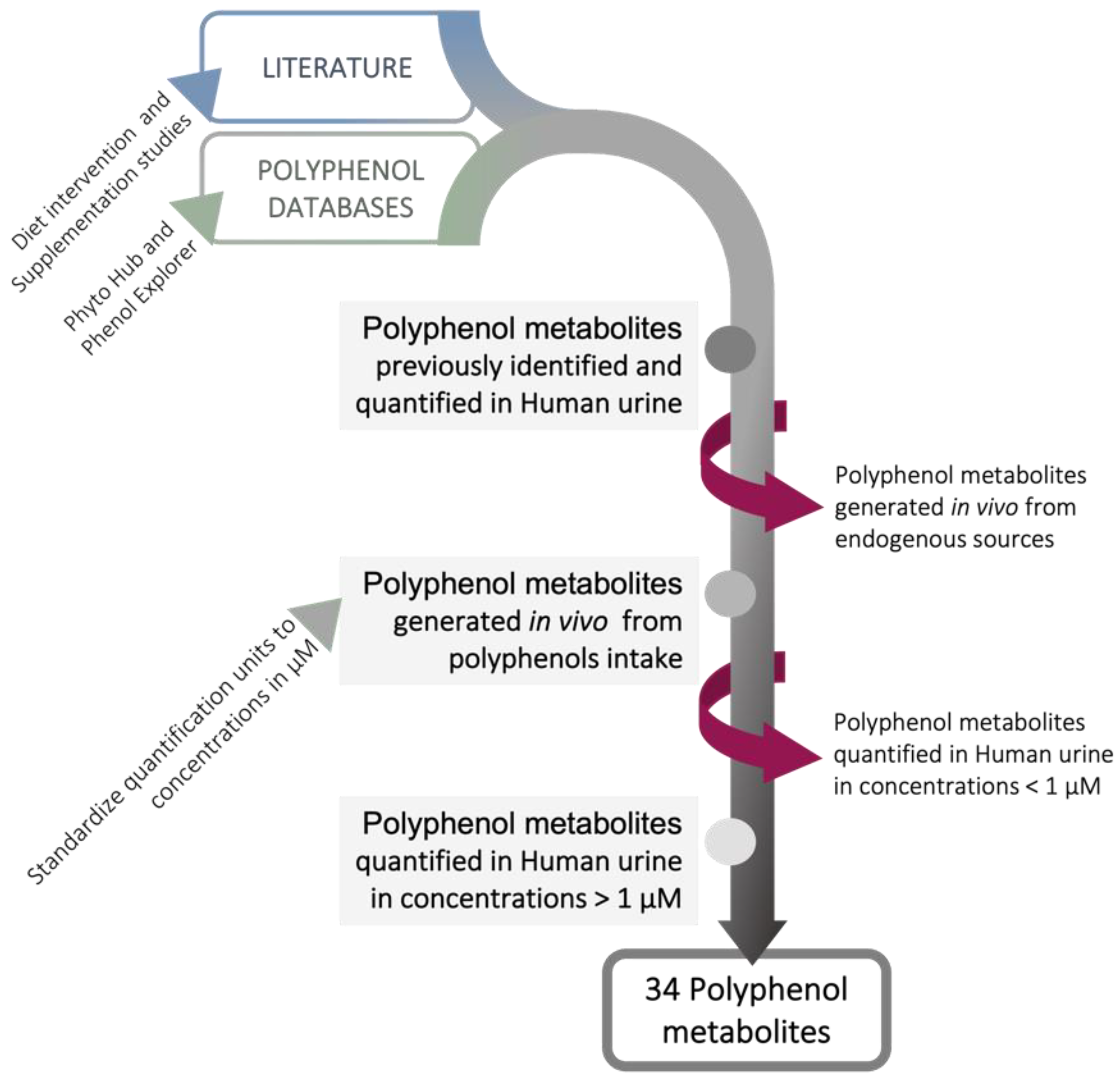
| Polyphenol Metabolites | Max. Concentration in Urine (µM) * | Experimental Conditions | Reference | |
|---|---|---|---|---|
| Polyphenol Source | Ingested Dose | |||
| 4′-Hydroxyphenylacetic acid | 406.8 | Green tea | 300 mL (single dose) | [191] |
| Phenylacetic acid | 153.3 | Cocoa powder in whole milk | 40 g (single dose) | [192] |
| 3′-Hydroxyphenylacetic acid | 136.3 | Quercetin 3-O-rutinoside | 440 mg/24 h (7 days) | [193] |
| 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | 64.8 | Quercetin | 200 mg (single dose) | [194] |
| 4′-Hydroxy-3′-methoxyphenylacetic acid (homovanillic acid) | 54.2 | Quercetin 3-O-rutinoside | 440 mg/24 h (7 days) | [193] |
| 4-Hydroxy-3-methoxybenzoic acid (Vanillic acid) | 53.6 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 3-(3′,4′-Dihydroxyphenyl)propanoic acid (Dihydrocaffeic acid) | 51.1 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 4′-Hydroxy-3′-methoxycinnamic acid (Ferulic acid) | 37.4 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 3-Hydroxybenzoic acid | 35.8 | Black tea solids | 4 g/24 h (7 days) | [193] |
| Benzoic acid | 31.5 | Quercetin | 200 mg (single dose) | [194] |
| 3′,4′-Dihydroxycinnamic acid (Caffeic acid) | 29.5 | Tablets of perilla extract | 1 tablet (single dose) | [195] |
| 3′-Hydroxycinnamic acid | 22.5 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 2,3-Dihydroxybenzoic acid | 22.5 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 4-Hydroxybenzoic acid | 19.7 | Cocoa powder in skimmed milk | 40 g/24 h (4 weeks) | [197] |
| (E)-3-(4′-Hydroxy-3′,5′-dimethoxyphenyl)prop-2-enoic acid (Sinapic acid) | 13.7 | Quercetin 3-O-rutinoside | 440 mg/24 h (7 days) | [193] |
| 4-Hydroxy-3,5-dimethoxybenzoic acid (Syringic acid) | 7.2 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 4-Ethylphenol | 7.0 | Quercetin | 200 mg (single dose) | [194] |
| 3′-Methoxycinnamic acid-4′-sulfate (Ferulic acid-sulfate) | 6.4 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 2-(3′,4′-Dihydroxyphenyl)ethanol (Hydroxytyrosol) | 6.1 | Tyrosol (140 μg/mL) | 50 mL (single dose) | [198] |
| 1,3,5-Trimethoxybenzene(Phloroglucinol) | 5.9 | (−)-epicatechin | 200 mg (single dose) | [194] |
| 3-Methoxybenzoic acid-4-glucuronide (Vanillic acid-glucuronide) | 5.7 | Red grape pomace aqueous extract | 250 mL (single dose) | [199] |
| 2′-Hydroxyhippuric acid | 5.1 | Black tea solids | 4 g/24 h (7 days) | [193] |
| 4-(2′-Hydroxyethyl)-2-methoxyphenol | 4.4 | Olive oil with phenol extract | 50 mL of 1950 mg/L total phenols extract (single dose) | [200] |
| 3,4,5-Trihydroxybenzoic acid (Gallic acid) | 3.7 | Black tea solids | 4 g/24 h (7 days) | [193] |
| Phenol-2-sulfate (Catechol-sulfate) | 3.1 | Cranberry juice | 450 mL (single dose) | [201] |
| 3′-Methoxycinnamic acid-4′-glucuronide (Ferulic acid-glucuronide) | 2.8 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 4′-Hydroxycinnamic acid (p-coumaric acid) | 2.2 | 5-caffeoylquinic acid | 2 g/24 h (7 days) | [193] |
| 4-Methylcatechol-O-sulfate | 2.1 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 3-(4′-Hydroxyphenyl)propanoic acid-3′-sulfate | 2.1 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate | 1.8 | Freeze-dried blueberry powder | 22 g (during 24 h) | [196] |
| 3-Methoxybenzoic acid-4-sulfate (Vanillic acid-4-sulfate) | 1.7 | Cyanidin-3-glucoside | 500 mg (single dose) | [202] |
| 3-Hydroxybenzoic acid-4-sulfate (Protocatechuic acid-4-sulfate | 1.2 | Cyanidin-3-glucoside | 500 mg (single dose) | [202] |
| Benzene-1,2-diol (Catechol) | 1.2 | Green tea | 300 mL (single dose) | [191] |
| 4-Hydroxybenzoic acid-3-sulfate (Protocatechuic acid-3-sulfate) | 1.1 | Cyanidin-3-glucoside | 500 mg (single dose) | [202] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods 2022, 11, 1060. https://doi.org/10.3390/foods11071060
Guerreiro Í, Ferreira-Pêgo C, Carregosa D, Santos CN, Menezes R, Fernandes AS, Costa JG. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods. 2022; 11(7):1060. https://doi.org/10.3390/foods11071060
Chicago/Turabian StyleGuerreiro, Íris, Cíntia Ferreira-Pêgo, Diogo Carregosa, Cláudia N. Santos, Regina Menezes, Ana S. Fernandes, and João G. Costa. 2022. "Polyphenols and Their Metabolites in Renal Diseases: An Overview" Foods 11, no. 7: 1060. https://doi.org/10.3390/foods11071060
APA StyleGuerreiro, Í., Ferreira-Pêgo, C., Carregosa, D., Santos, C. N., Menezes, R., Fernandes, A. S., & Costa, J. G. (2022). Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods, 11(7), 1060. https://doi.org/10.3390/foods11071060