Differentiating between Enterococcusfaecium and Enterococcuslactis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Enterococcus Isolates and Growth Conditions
2.2. 16S rRNA Sequencing
2.3. Identifying Specific Mass Peaks
2.3.1. Sample Preparation for MALDI-TOF MS
2.3.2. MALDI-TOF MS Analysis
2.4. Creating an In-House Database
2.5. Identifying Isolates Using Specific Peaks
3. Results and Discussion
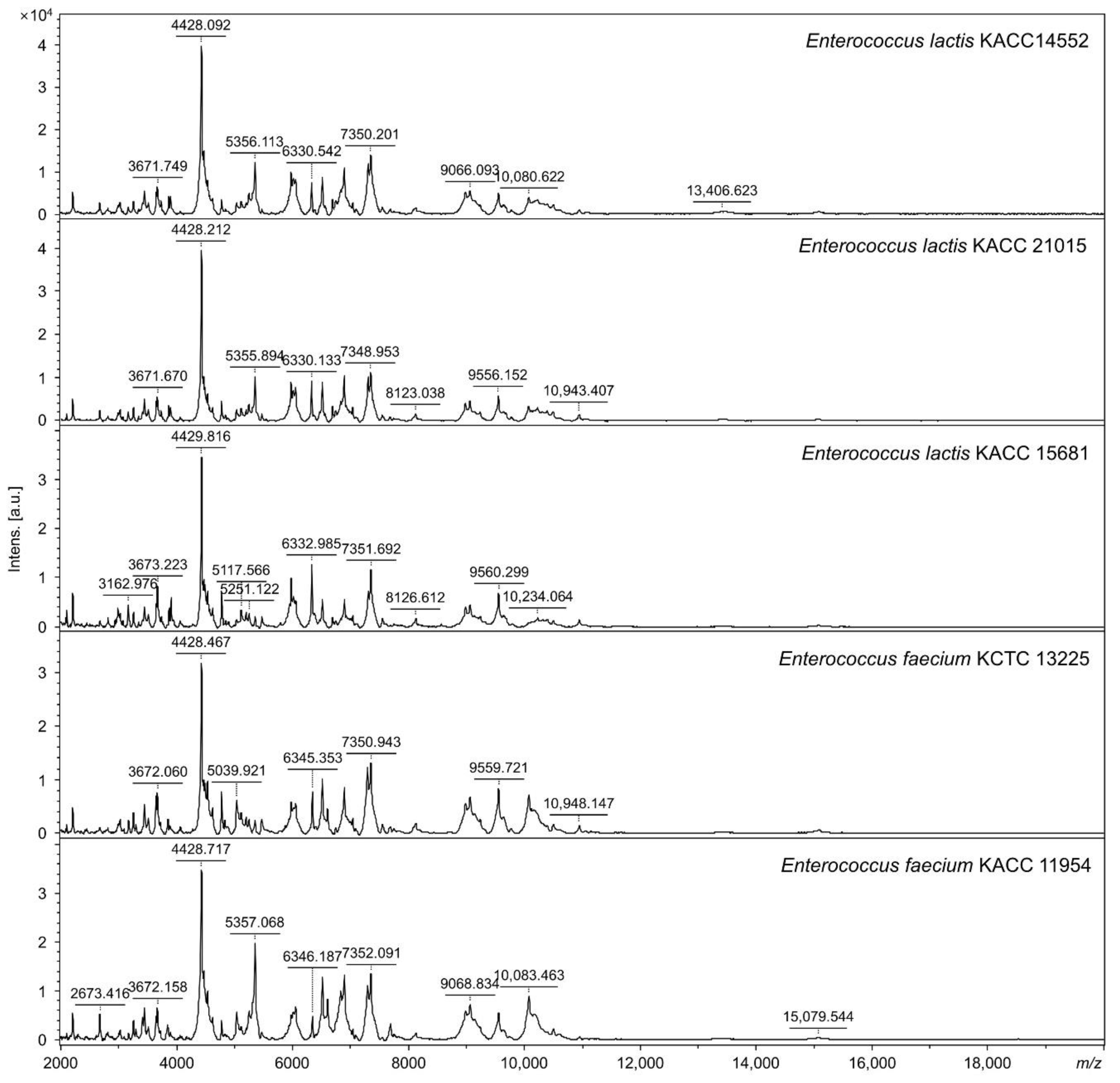
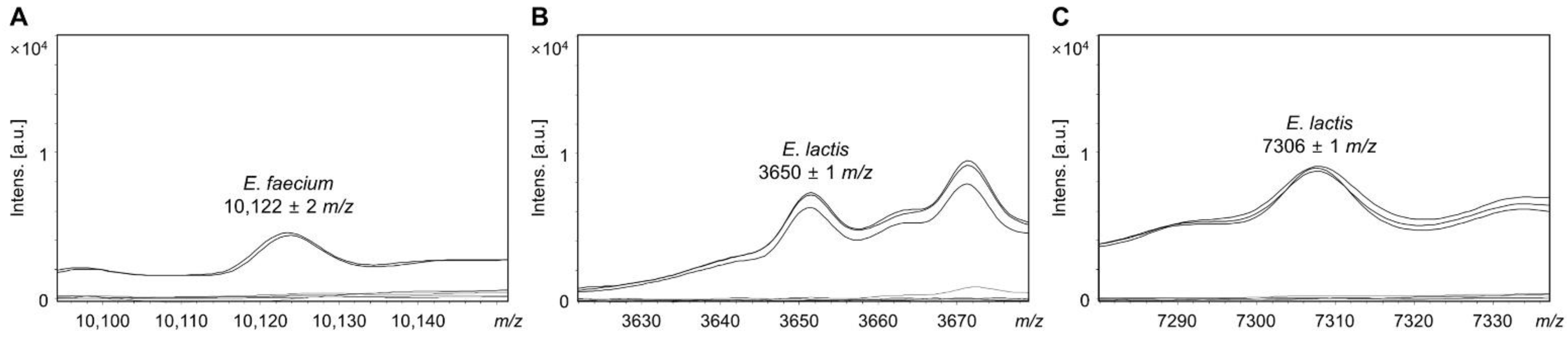
3.1. Identifying Specific Mass Peaks
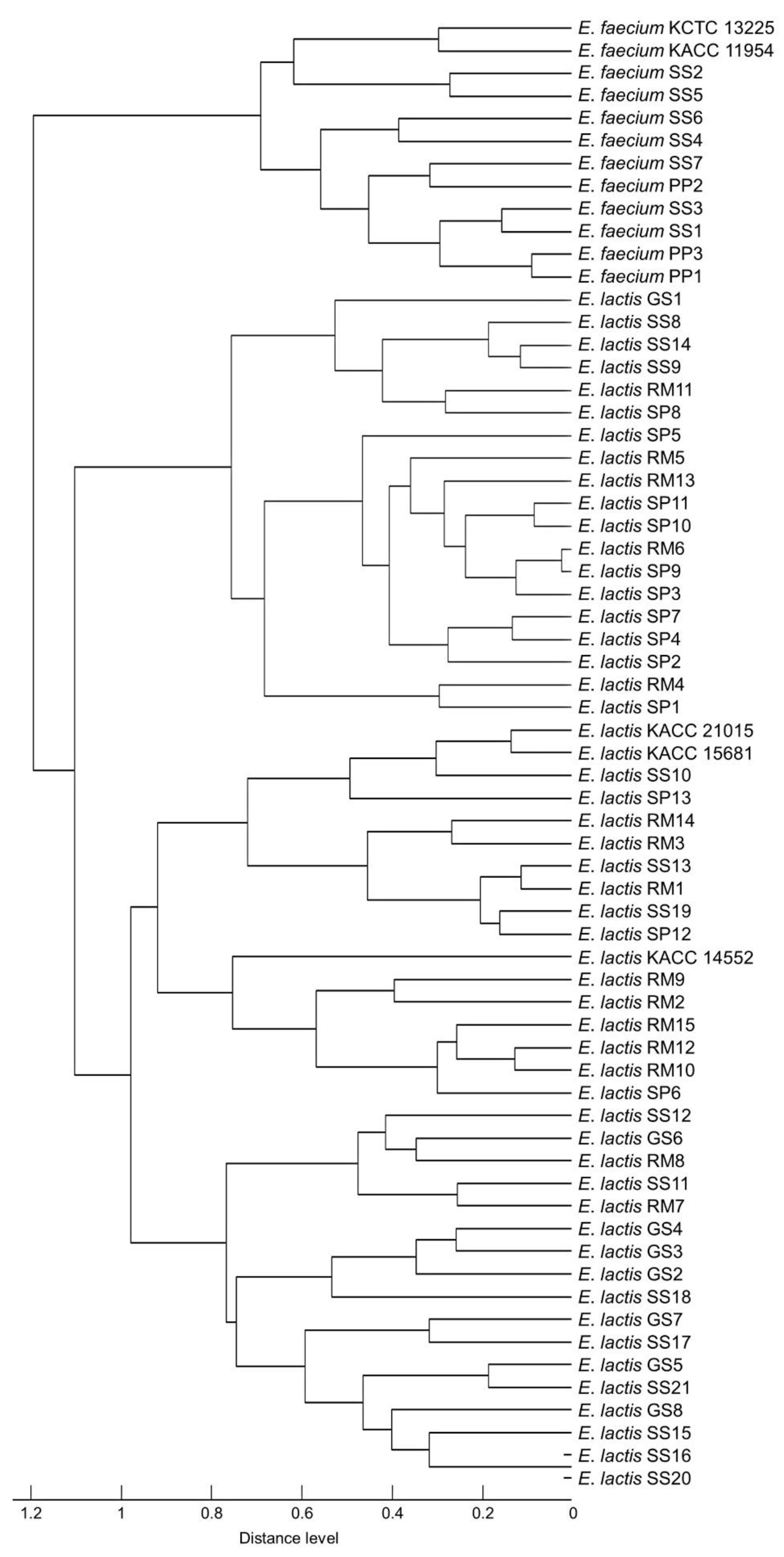
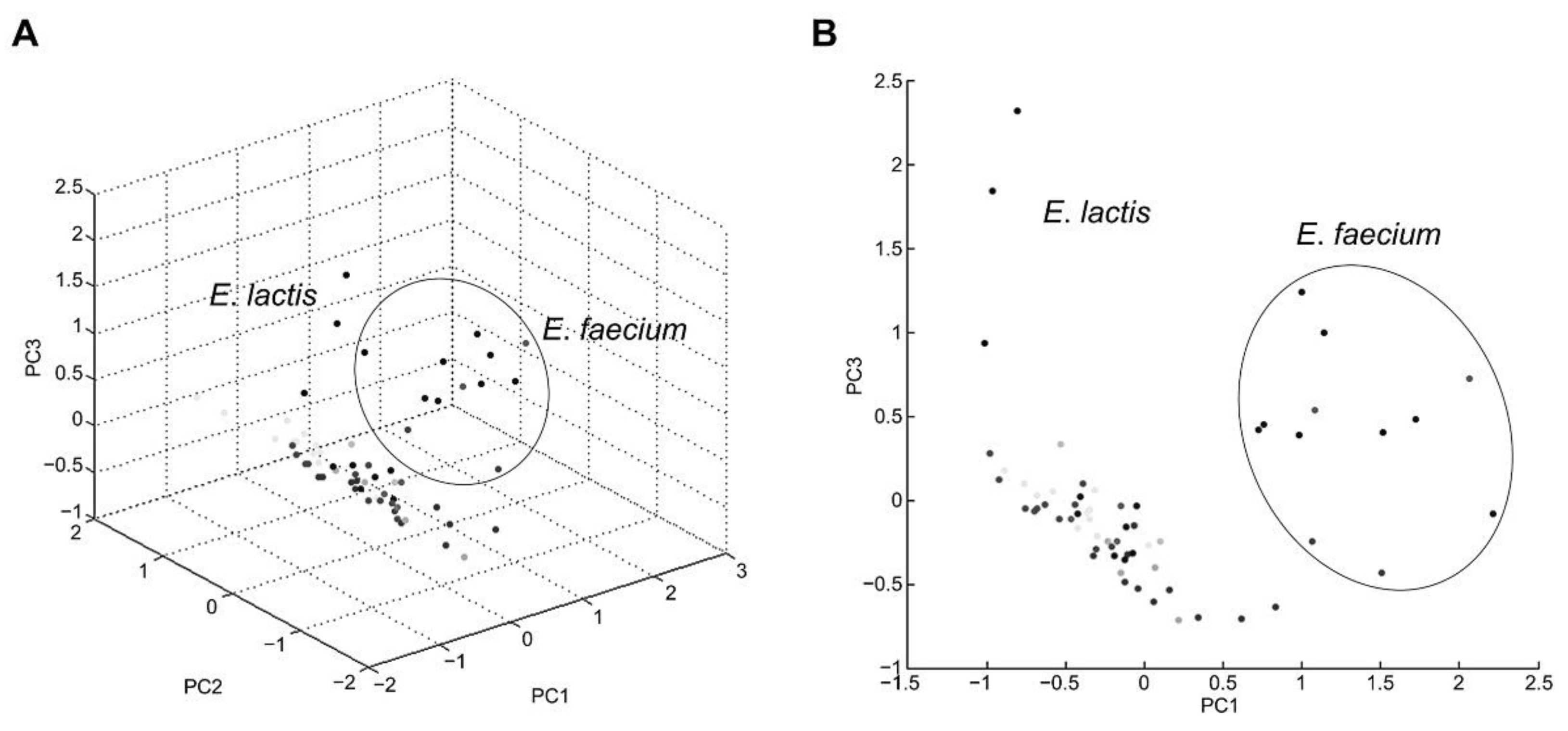
3.2. Evaluating Commercial and In-House Databases
3.3. Identifying Isolates Using Specific Mass Peaks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morandi, S.; Cremonesi, P.; Povolo, M.; Brasca, M. Enterococcus lactis sp. nov., from Italian raw milk cheeses. Int. J. Syst. Evol. Microbiol. 2012, 62, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Berreta, A.; Baumgardner, R.M.; Kopper, J.J. Evaluation of commercial veterinary probiotics containing enterococci for transferrable vancomycin resistance genes. BMC Res. Notes 2020, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Daza, M.V.B.; Cortimiglia, C.; Bassi, D.; Cocconcelli, P.S. Genome-based studies indicate that the Enterococcus faecium clade b strains belong to Enterococcus lactis species and lack of the hospital infection associated markers. Int. J. Syst. Evol. Microbiol. 2021, 71, 004948. [Google Scholar] [CrossRef]
- Fu, X.; Lyu, L.; Wang, Y.; Zhang, Y.; Guo, X.; Chen, Q.; Liu, C. Safety assessment and probiotic characteristics of Enterococcus lactis JDM1. Microb. Pathog. 2022, 163, 105380. [Google Scholar] [CrossRef]
- Kostinek, M.; Specht, I.; Edward, V.A.; Pinto, C.; Egounlety, M.; Sossa, C.; Mbugua, S.; Dortu, C.; Thonart, P.; Taljaard, L.; et al. Characterisation and biochemical properties of predominant lactic acid bacteria from fermenting cassava for selection as starter cultures. Int. J. Food Microbiol. 2007, 114, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, L. Rapid species- and subspecies-specific level classification and identification of Lactobacillus casei group members using MALDI Biotyper combined with ClinProTools. J. Dairy Sci. 2018, 101, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Jonas, E. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 33, 152–155. [Google Scholar]
- Kim, E.; Cho, E.J.; Yang, S.M.; Kim, M.J.; Kim, H.Y. Novel approaches for the identification of microbial communities in kimchi: MALDI-TOF MS analysis and high-throughput sequencing. Food Microbiol. 2021, 94, 103641. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yang, S.M.; Kim, H.Y. Analysis of cultivable microbial community during kimchi fermentation using MALDI-TOF MS. Foods 2021, 10, 1068. [Google Scholar] [CrossRef]
- Jang, K.S.; Kim, Y.H. Rapid and robust MALDI-TOF MS techniques for microbial identification: A brief overview of their diverse applications. J. Microbiol. 2018, 56, 209–216. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.J.; Yang, S.M.; Kim, C.G.; Choo, D.W.; Kim, H.Y. Rapid identification of Staphylococcus species isolated from food samples by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Microbiol. Biotechnol. 2019, 29, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Umemura, H.; Nakayama, T. Current status of matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) in clinical diagnostic microbiology. Molecules 2020, 25, 4775. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cho, Y.; Lee, Y.; Han, S.K.; Kim, C.G.; Choo, D.W.; Kim, Y.R.; Kim, H.Y. A proteomic approach for rapid identification of Weissella species isolated from Korean fermented foods on MALDI-TOF MS supplemented with an in-house database. Int. J. Food Microbiol. 2017, 243, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, M.; Zautner, A.E.; Rabsch, W.; Zimmermann, O.; Weig, M.; Bader, O.; Groß, U. Rapid discrimination of Salmonella enterica serovar Typhi from other serovars by MALDI-TOF mass spectrometry. PLoS ONE 2012, 7, e40004. [Google Scholar] [CrossRef]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a French cheese: The Maroilles. Int. J. Food Microbiol. 2017, 247, 24–32. [Google Scholar] [CrossRef]
- Troncoso, C.; Pavez, M.; Cerda, A.; Oporto, M.; Villarroel, D.; Hofmann, E.; Rios, E.; Sierralta, A.; Copelli, L.; Barrientos, L. MALDI-TOF MS and 16S RNA identification of culturable gastric microbiota: Variability associated with the presence of Helicobacter pylori. Microorganisms 2020, 8, 1763. [Google Scholar] [CrossRef]
- Ha, M.; Jo, H.J.; Choi, E.K.; Kim, Y.; Kim, J.; Cho, H.J. Reliable identification of Bacillus cereus group species using low mass biomarkers by MALDI-TOF MS. J. Microbiol. Biotechnol. 2019, 29, 887–896. [Google Scholar] [CrossRef]
- Marín, M.; Cercenado, E.; Sánchez-Carrillo, C.; Ruiz, A.; González, Á.G.; Rodríguez-Sánchez, B.; Bouza, E. Accurate differentiation of Streptococcus pneumoniae from other species within the Streptococcus mitis group by peak analysis using MALDI-TOF MS. Front. Microbiol. 2017, 8, 698. [Google Scholar] [CrossRef]
- Veloo, A.C.M.; Elgersma, P.E.; Friedrich, A.W.; Nagy, E.; van Winkelhoff, A.J. The influence of incubation time, sample preparation and exposure to oxygen on the quality of the MALDI-TOF MS spectrum of anaerobic bacteria. Clin. Microbiol. Infect. 2014, 20, O1091–O1097. [Google Scholar] [CrossRef]
- Florio, W.; Cappellini, S.; Giordano, C.; Vecchione, A.; Ghelardi, E.; Lupetti, A. A new culture-based method for rapid identification of microorganisms in polymicrobial blood cultures by MALDI-TOF MS. BMC Microbiol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Stępień-Pyśniak, D.; Hauschild, T.; Różański, P.; Marek, A. MALDI-TOF mass spectrometry as a useful tool for identification of Enterococcus spp. from wild birds and differentiation of closely related species. J. Microbiol. Biotechnol. 2017, 27, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Demirbaş, F.; Dertli, E. Characterization of functional properties of Enterococcus spp. isolated from Turkish white cheese. LWT 2017, 75, 358–365. [Google Scholar] [CrossRef]
- Yang, S.M.; Kim, E.; Kim, D.; Baek, J.; Yoon, H.; Kim, H.Y. Rapid detection of Salmonella Enteritidis, Typhimurium, and Thompson by specific peak analysis using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Foods 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Berlamont, H.; De Witte, C.; De Bruyckere, S.; Fox, J.G.; Backert, S.; Smet, A.; Boyen, F.; Haesebrouck, F. Differentiation of gastric Helicobacter species using MALDI-TOF mass spectrometry. Pathogens 2021, 10, 366. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Kim, H.B.; Kim, H.Y. Novel specific peaks for differentiating the Lactobacillus plantarum group using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Methods 2020, 178, 106064. [Google Scholar] [CrossRef]
- Branquinho, R.; Sousa, C.; Lopes, J.; Pintado, M.E.; Peixe, L.V.; Osório, H. Differentiation of Bacillus pumilus and Bacillus safensis using MALDI-TOF-MS. PLoS ONE 2014, 9, e110127. [Google Scholar] [CrossRef]
- Šedo, O.; Vávrová, A.; Vad’Urová, M.; Tvrzová, L.; Zdráhal, Z. The influence of growth conditions on strain differentiation within the Lactobacillus acidophilus group using matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry profiling. Rapid Commun. Mass Spectrom. 2013, 27, 2729–2736. [Google Scholar] [CrossRef]
- Van Prehn, J.; van Veen, S.Q.; Schelfaut, J.J.G.; Wessels, E. MALDI-TOF mass spectrometry for differentiation between Streptococcus pneumoniae and Streptococcus pseudopneumoniae. Diagn. Microbiol. Infect. Dis. 2016, 85, 9–11. [Google Scholar] [CrossRef][Green Version]
- Ruiz-Moyano, S.; Tao, N.; Underwood, M.A.; Mills, D.A. Rapid discrimination of Bifidobacterium animalis subspecies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Food Microbiol. 2012, 30, 432–437. [Google Scholar] [CrossRef]
- Gantzias, C.; Lappa, I.K.; Aerts, M.; Georgalaki, M.; Manolopoulou, E.; Papadimitriou, K.; De Brandt, E.; Tsakalidou, E.; Vandamme, P. MALDI-TOF MS profiling of non-starter lactic acid bacteria from artisanal cheeses of the Greek island of Naxos. Int. J. Food Microbiol. 2020, 323, 108586. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.; Zhu, Z.; Mao, X.; Yan, M.; Chen, Y.; Zhu, Q.; Li, H.; Gu, B. Application of MALDI-TOF MS to rapid identification of anaerobic bacteria. BMC Infect. Dis. 2019, 19, 941. [Google Scholar] [CrossRef] [PubMed]
- Abdel Samad, R.; Al Disi, Z.; Mohammad Ashfaq, M.Y.; Wahib, S.M.; Zouari, N. The use of principle component analysis and MALDI-TOF MS for the differentiation of mineral forming: Virgibacillus and Bacillus species isolated from sabkhas. RSC Adv. 2020, 10, 14606–14616. [Google Scholar] [CrossRef]
- Cherkaoui, A.; Hibbs, J.; Emonet, S.; Tangomo, M.; Girard, M.; Francois, P.; Schrenzel, J. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J. Clin. Microbiol. 2010, 48, 1169–1175. [Google Scholar] [CrossRef]
- Dieckmann, R.; Malorny, B. Rapid screening of epidemiologically important Salmonella enterica subsp. enterica serovars by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2011, 77, 4136–4146. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strains | Origins |
|---|---|
| Reference strains | |
| Enterococcus avium | KACC 10788 |
| Enterococcus avium | NCCP 10761 |
| Enterococcus casseliflavus | KCTC 3552 |
| Enterococcus devriesei | KACC 14590 |
| Enterococcus durans | KCTC 13289 |
| Enterococcus faecalis | KACC 11859 |
| Enterococcus faecalis | KCTC 3206 |
| Enterococcus faecalis | KCTC 5290 |
| Enterococcus faecium | KACC 11954 |
| Enterococcus faecium | KCTC 13225 |
| Enterococcus gallinarum | NCCP 11518 |
| Enterococcus gilvus | KACC 13847 |
| Enterococcus hirae | KACC 10779 |
| Enterococcus hirae | KACC 10782 |
| Enterococcus hirae | KACC 13884 |
| Enterococcus hirae | KACC 16328 |
| Enterococcus lactis | KACC 14552 |
| Enterococcus lactis | KACC 15681 |
| Enterococcus lactis | KACC 21015 |
| Enterococcus malodoratus | KACC 13883 |
| Enterococcus mundtii | KACC 13824 |
| Enterococcus mundtii | KCTC 3630 |
| Enterococcus pseudoavium | KACC 13781 |
| Enterococcus raffinosus | KACC 13782 |
| Enterococcus saccharolyticus | KACC 10789 |
| Isolates (no. of isolates) | |
| SP1-SP13 (13) | Soybean paste |
| RM1-RM15 (15) | Raw milk |
| PP1-PP3 (3) | Probiotic product |
| SS1-SS21 (21) | Soy sauce |
| GS1-GS8 (8) | Gajami-sikhae |
| Strains | MALDI-TOF MS Identification | Mass Peak (m/z) | |||
|---|---|---|---|---|---|
| BioTyper | In-House Database | 10,122 ± 2 | 3650 ± 1 | 7306 ± 1 | |
| KACC 11954 | E. faecium | E. faecium | + | − | − |
| KCTC 13225 | E. faecium | E. faecium | + | − | − |
| KACC 14552 | E. faecium | E. lactis | − | + | + |
| KACC 15681 | E. faecium | E. lactis | − | + | + |
| KACC 21015 | E. faecium | E. lactis | − | + | + |
| KACC 10788 | E. avium | E. avium | − | − | − |
| NCCP 10761 | E. avium | E. avium | − | − | − |
| KCTC 3552 | E. casseliflavus | E. casseliflavus | − | − | − |
| KACC 14590 | E. devriesei | E. devriesei | − | − | − |
| KCTC 13289 | E. durans | E. durans | − | − | − |
| KACC 11859 | E. faecalis | E. faecalis | − | − | − |
| KCTC 3206 | E. faecalis | E. faecalis | − | − | − |
| KCTC 5290 | E. faecalis | E. faecalis | − | − | − |
| NCCP 11518 | E. gallinarum | E. gallinarum | − | − | − |
| KACC 13847 | E. gilvus | E. gilvus | − | − | − |
| KACC 10779 | E. hirae | E. hirae | − | − | − |
| KACC 10782 | E. hirae | E. hirae | − | − | − |
| KACC 13884 | E. hirae | E. hirae | − | − | − |
| KACC 16328 | E. hirae | E. hirae | − | − | − |
| KACC 13883 | E. malodoratus | E. malodoratus | − | − | − |
| KACC 13824 | E. mundtii | E. mundtii | − | − | − |
| KCTC 3630 | E. mundtii | E. mundtii | − | − | − |
| KACC 13781 | E. pseudoavium | E. pseudoavium | − | − | − |
| KACC 13782 | E. raffinosus | E. raffinosus | − | − | − |
| KACC 10789 | E. saccharolyticus | E. saccharolyticus | − | − | − |
| Strains (No. of Strains) | No. of Strains with Results | ||
|---|---|---|---|
| ≥2.300 log (Score) | 2.000–2.299 log (Score) | 1.700–1.999 log (Score) | |
| Based on the BioTyper database | |||
| Reference strains (5) | 2 (40.0%) | 3 (60.0%) | 0 (0.0%) |
| Isolates (60) | 4 (6.7%) | 50 (83.3%) | 6 (10.0%) |
| Total (65) | 6 (9.2%) | 53 (81.5%) | 6 (9.2%) |
| Based on the in-house database | |||
| Reference strains (5) | 5 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Isolates (60) | 54 (90.0%) | 6 (10.0%) | 0 (0.0%) |
| Total (65) | 59 (90.8%) | 6 (9.2%) | 0 (0.0%) |
| Strains | MALDI-TOF MS Database | Specific Peak (m/z) | |
|---|---|---|---|
| BioTyper (Score) | In-House Database (Score) | ||
| SP1 | E. faecium (2.157) | E. lactis (2.532) | E. lactis (3651.2, 7307.1) |
| SP2 | E. faecium (2.205) | E. lactis (2.476) | E. lactis (3651.2, 7307.7) |
| SP3 | E. faecium (2.2) | E. lactis (2.462) | E. lactis (3651.2, 7307.0) |
| SP4 | E. faecium (2.241) | E. lactis (2.522) | E. lactis (3651.2, 7307.0) |
| SP5 | E. faecium (2.171) | E. lactis (2.518) | E. lactis (3651.4, 7307.1) |
| SP6 | E. faecium (2.178) | E. lactis (2.586) | E. lactis (3651.1, 7307.1) |
| SP7 | E. faecium (2.171) | E. lactis (2.505) | E. lactis (3651.2, 7307.5) |
| SP8 | E. faecium (2.227) | E. lactis (2.544) | E. lactis (3650.8, 7306.7) |
| SP9 | E. faecium (1.992) | E. lactis (2.244) | E. lactis (3651.8, 7307.1) |
| SP10 | E. faecium (2.087) | E. lactis (2.409) | E. lactis (3651.4, 7307.7) |
| SP11 | E. faecium (2.128) | E. lactis (2.427) | E. lactis (3651.7, 7307.1) |
| SP12 | E. faecium (1.978) | E. lactis (2.332) | E. lactis (3651.2, 7307.8) |
| SP13 | E. faecium (2.231) | E. lactis (2.52) | E. lactis (3651.2, 7307.5) |
| RM1 | E. faecium (2.088) | E. lactis (2.469) | E. lactis (3651.3, 7307.0) |
| RM2 | E. faecium (2.193) | E. lactis (2.424) | E. lactis (3651.0, 7307.6) |
| RM3 | E. faecium (2.186) | E. lactis (2.436) | E. lactis (3651.2, 7307.4) |
| RM4 | E. faecium (1.996) | E. lactis (2.494) | E. lactis (3651.5, 7306.7) |
| RM5 | E. faecium (2.212) | E. lactis (2.495) | E. lactis (3651.2, 7305.0) |
| RM6 | E. faecium (2.012) | E. lactis (2.52) | E. lactis (3651.1, 7307.6) |
| RM7 | E. faecium (2.216) | E. lactis (2.524) | E. lactis (3651.1, 7307.4) |
| RM8 | E. faecium (2.1) | E. lactis (2.496) | E. lactis (3650.9, 7306.6) |
| RM9 | E. faecium (2.099) | E. lactis (2.47) | E. lactis (3651.6, 7305.0) |
| RM10 | E. faecium (2.142) | E. lactis (2.519) | E. lactis (3651.0, 7307.7) |
| RM11 | E. faecium (2.15) | E. lactis (2.522) | E. lactis (3650.7, 7306.8) |
| RM12 | E. faecium (2.061) | E. lactis (2.515) | E. lactis (3651.2, 7307.6) |
| RM13 | E. faecium (2.122) | E. lactis (2.538) | E. lactis (3651.2, 7307.6) |
| RM14 | E. faecium (2.103) | E. lactis (2.475) | E. lactis (3650.9, 7307.4) |
| RM15 | E. faecium (2.193) | E. lactis (2.59) | E. lactis (3651.1, 7307.8) |
| PP1 | E. faecium (2.226) | E. faecium (2.272) | E. faecium (10,124.6) |
| PP2 | E. faecium (2.288) | E. lactis (2.28)/E. faecium (2.275) | E. faecium (10,124.3) |
| PP3 | E. faecium (2.249) | E. lactis (2.209)/E. faecium (2.163) | E. faecium (10,123.6) |
| SS1 | E. faecium (2.232) | E. faecium (2.342) | E. faecium (10,124.2) |
| SS2 | E. faecium (2.351) | E. lactis (2.387)/E. faecium (2.37) | E. faecium (10,120.6) |
| SS3 | E. faecium (2.21) | E. lactis (2.227)/E. faecium (2.214) | E. faecium (10,123.2) |
| SS4 | E. faecium (2.279) | E. lactis (2.413)/E. faecium (2.259) | E. faecium (10,120.1) |
| SS5 | E. faecium (2.381) | E. faecium (2.348) | E. faecium (10,120.0) |
| SS6 | E. faecium (2.405) | E. lactis (2.332)/E. faecium (2.319) | E. faecium (10,121.3) |
| SS7 | E. faecium (2.381) | E. lactis (2.205)/E. faecium (2.16) | E. faecium (10,124.3) |
| SS8 | E. faecium (2.156) | E. lactis (2.435) | E. lactis (3650.7, 7306.3) |
| SS9 | E. faecium (2.166) | E. lactis (2.444) | E. lactis (3650.3, 7305.8) |
| SS10 | E. faecium (1.946) | E. lactis (2.336) | E. lactis (3651.0, 7307.5) |
| SS11 | E. faecium (2.092) | E. lactis (2.522) | E. lactis (3650.8, 7307.0) |
| SS12 | E. faecium (2.147) | E. lactis (2.378) | E. lactis (3650.9, 7306.5) |
| SS13 | E. faecium (2.037) | E. lactis (2.514) | E. lactis (3651.1, 7307.7) |
| SS14 | E. faecium (2.192) | E. lactis (2.371) | E. lactis (3650.5, 7306.1) |
| SS15 | E. faecium (2.165) | E. lactis (2.548) | E. lactis (3649.7, 7305.9) |
| SS16 | E. faecium (2.115) | E. lactis (2.501) | E. lactis (3651.2, 7305.9) |
| SS17 | E. faecium (2.158) | E. lactis (2.436) | E. lactis (3649.8, 7306.6) |
| SS18 | E. faecium (2.097) | E. lactis (2.44) | E. lactis (3650.2, 7305.2) |
| SS19 | E. faecium (1.927) | E. lactis (2.337) | E. lactis (3650.7, 7307.6) |
| SS20 | E. faecium (2.085) | E. lactis (2.39) | E. lactis (3650.2, 7306.0) |
| SS21 | E. faecium (2.225) | E. lactis (2.592) | E. lactis (3650.1, 7305.3) |
| GS1 | E. faecium (2.149) | E. lactis (2.482) | E. lactis (3650.1, 7305.1) |
| GS2 | E. faecium (2.069) | E. lactis (2.445) | E. lactis (3649.9, 7305.3) |
| GS3 | E. faecium (2.023) | E. lactis (2.53) | E. lactis (3649.8, 7305.6) |
| GS4 | E. faecium (1.975) | E. lactis (2.503) | E. lactis (3650.2, 7306.6) |
| GS5 | E. faecium (2.194) | E. lactis (2.463) | E. lactis (3650.0, 7305.2) |
| GS6 | E. faecium (2.084) | E. lactis (2.32) | E. lactis (3650.2, 7305.6) |
| GS7 | E. faecium (2.036) | E. lactis (2.537) | E. lactis (3650.1, 7306.0) |
| GS8 | E. faecium (2.092) | E. lactis (2.504) | E. lactis (3650.2, 7306.3) |
| Strains (No.) | Source | 16S rRNA Gene Sequencing | |
|---|---|---|---|
| Description | Accession No. (% Identity) | ||
| SP1-SP13 (13) | Soybean paste | E. faecium/E. lactis | MT597585.1/MG948154.1 (99.8%) |
| RM1-RM-15 (15) | Raw milk | E. faecium/E. lactis | MT597585.1/MG948154.1 (99.9%) |
| PP1-PP3 (3) | Probiotic | E. faecium | FJ378693.1 (99.0%) |
| SS1-SS3 (3) | Soy sauce | E. faecium | MN401132.1 (99.9%) |
| SS4-SS7 (4) | Soy sauce | E. faecium | MH473158.1 (99.9%) |
| SS8-SS15 (8) | Soy sauce | E. faecium/E. lactis | MT597585.1/MG948154.1 (100%) |
| SS16-SS21 (6) | Soy sauce | E. faecium/E. lactis | MT378127.1/CP082267.1 (99.9%) |
| GS1-GS8 (8) | Sikhae | E. faecium/E. lactis | MT597585.1/MG948154.1 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Yang, S.-M.; Kim, H.-J.; Kim, H.-Y. Differentiating between Enterococcusfaecium and Enterococcuslactis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Foods 2022, 11, 1046. https://doi.org/10.3390/foods11071046
Kim E, Yang S-M, Kim H-J, Kim H-Y. Differentiating between Enterococcusfaecium and Enterococcuslactis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Foods. 2022; 11(7):1046. https://doi.org/10.3390/foods11071046
Chicago/Turabian StyleKim, Eiseul, Seung-Min Yang, Hyun-Jae Kim, and Hae-Yeong Kim. 2022. "Differentiating between Enterococcusfaecium and Enterococcuslactis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry" Foods 11, no. 7: 1046. https://doi.org/10.3390/foods11071046
APA StyleKim, E., Yang, S.-M., Kim, H.-J., & Kim, H.-Y. (2022). Differentiating between Enterococcusfaecium and Enterococcuslactis by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Foods, 11(7), 1046. https://doi.org/10.3390/foods11071046







