Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries
Abstract
:1. Introduction
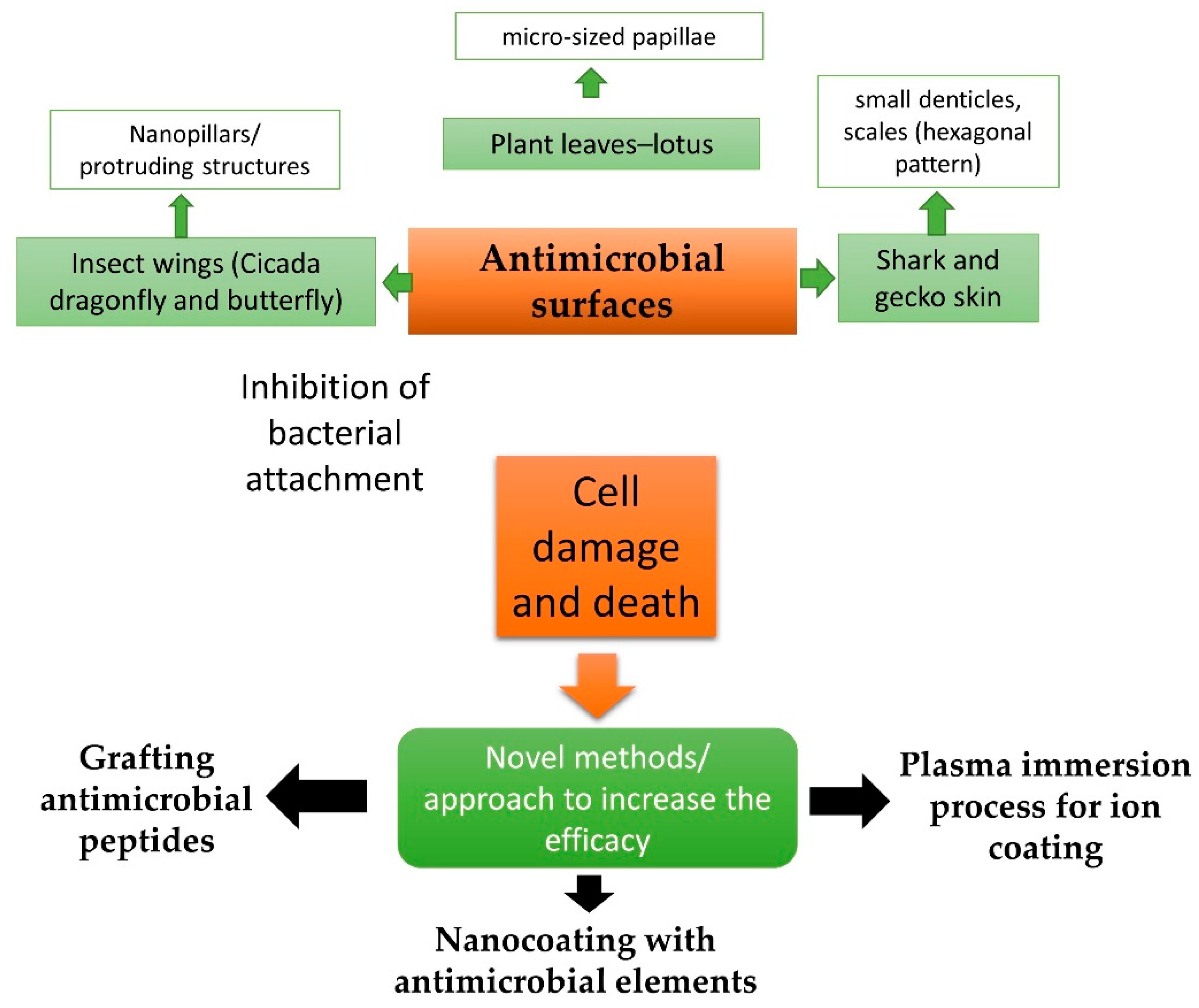
2. Antimicrobial Surfaces in Nature
3. Mimicking Antimicrobial Structures/Materials
Advantages and Shortcomings of Nature-Inspired Antimicrobial Surfaces
4. Effect of Antimicrobial Structures on Biofilms
4.1. Novel Techniques Used for the Development of Nanostructures
4.2. Bioinspired Antimicrobial Peptides and Their Applications on Antimicrobial Surfaces
5. Challenges and Research Gap
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singap. Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef] [Green Version]
- da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Brauge, T.; Faille, C.; Leleu, G.; Denis, C.; Hanin, A.; Midelet, G. Treatment with disinfectants may induce an increase in viable but non culturable populations of Listeria monocytogenes in biofilms formed in smoked salmon processing environments. Food Microbiol. 2020, 92, 103548. [Google Scholar] [CrossRef]
- Baquero, F.; Negri, M.-C.; Morosini, M.-I.; Blázquez, J. Antibiotic-selective environments. Clin. Infect. Dis. 1998, 27, S5–S11. [Google Scholar] [CrossRef] [Green Version]
- Russell, A. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 2000, 52, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, Y.; Gleichauf, K.; Lou, J.; Li, Q. Nanostructure on Taro Leaves Resists Fouling by Colloids and Bacteria under Submerged Conditions. Langmuir 2011, 27, 10035–10040. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, A.; Chen, Y.; Yang, Y.; Zhang, Q.; Luo, S.; Ye, M.; Zhou, Y.; An, Y.; Huang, W.; et al. Lotus leaf inspired antiadhesive and antibacterial gauze for enhanced infected dermal wound regeneration. Chem. Eng. J. 2020, 402, 126202. [Google Scholar] [CrossRef]
- Dundar Arisoy, F.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2016, 18, 1311–1316. [Google Scholar] [CrossRef]
- Jiang, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef]
- LakshmiBalasubramaniam, S.; Patel, A.S.; Nayak, B.; Howell, C.; Skonberg, D. Antioxidant and antimicrobial modified cellulose nanofibers for food applications. Food BioSci. 2021, 44, 101421. [Google Scholar] [CrossRef]
- Kesel, A.; Liedert, R. Learning from nature: Non-toxic biofouling control by shark skin effect. Comp. Biochem. Physiol. A 2007, 4, S130. [Google Scholar] [CrossRef]
- Choi, W.; Lee, C.; Lee, D.; Won, Y.J.; Lee, G.W.; Shin, M.G.; Chun, B.; Kim, T.-S.; Park, H.-D.; Jung, H.W. Sharkskin-mimetic desalination membranes with ultralow biofouling. J. Mater. Chem. A 2018, 6, 23034–23045. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene materials in antimicrobial nanomedicine: Current status and future perspectives. Adv. Healthc. Mater. 2018, 7, 1701406. [Google Scholar] [CrossRef] [Green Version]
- Morka, K.D.; Wernecki, M.; Kędziora, A.; Książczyk, M.; Dudek, B.; Gerasymchuk, Y.; Lukowiak, A.; Bystroń, J.; Bugla-Płoskońska, G. The Impact of Graphite Oxide Nanocomposites on the Antibacterial Activity of Serum. Int. J. Mol. Sci. 2021, 22, 7386. [Google Scholar] [CrossRef]
- Alhadrami, H.; Al-Hazmi, F. Antibacterial activities of titanium oxide nanoparticles. J. Bioelectron. Nanotechnol. 2017, 2, 5. [Google Scholar]
- Zhou, J.; Wang, X. The osteogenic, anti-oncogenic and antibacterial activities of selenium-doped titanium dioxide coatings on titanium. Surf. Coat. Technol. 2020, 403, 126408. [Google Scholar] [CrossRef]
- Sagadevan, S.; Anita Lett, J.; Vennila, S.; Varun Prasath, P.; Saravanan Kaliaraj, G.; Fatimah, I.; Léonard, E.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; et al. Photocatalytic activity and antibacterial efficacy of titanium dioxide nanoparticles mediated by Myristica fragrans seed extract. Chem. Phys. Lett. 2021, 771, 138527. [Google Scholar] [CrossRef]
- Subhadarshini, S.; Singh, R.; Mandal, A.; Roy, S.; Mandal, S.; Mallik, S.; Goswami, D.K.; Das, A.K.; Das, N.C. Silver Nanodot Decorated Dendritic Copper Foam As a Hydrophobic and Mechano-Chemo Bactericidal Surface. Langmuir 2021, 37, 9356–9370. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Mohammed Ghilan, A.-K.; Arasu, M.V. Characterization of silver nanomaterials derived from marine Streptomyces sp. al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Mohamad Zain, N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Werner, M.; Baulin, V.A.; Truong, V.K.; Al Kobaisi, M.; Nguyen, S.H.; Balcytis, A.; Juodkazis, S.; Wang, J.Y.; Mainwaring, D.E. Subtle variations in surface properties of black silicon surfaces influence the degree of bactericidal efficiency. Nano-Micro Lett. 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Nowlin, K.; Boseman, A.; Covell, A.; LaJeunesse, D. Adhesion-dependent rupturing of Saccharomyces cerevisiae on biological antimicrobial nanostructured surfaces. J. R. Soc. Interface 2015, 12, 20140999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; You, W.; Shen, Q.; Wang, X.; Sheng, J.; Cheng, D.; Cao, X.; Wu, C. Preparation of lotus-leaf-like antibacterial film based on mesoporous silica microcapsule-supported Ag nanoparticles. RSC Adv. 2014, 4, 2793–2796. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, K.K.-H.; Watson, J.A.; Kim, H.-Y.; Yoon, K.-S.; Kim, E.-J.; Lee, J.-M.; Watson, G.S.; Jung, H.-S. High quality bioreplication of intricate nanostructures from a fragile gecko skin surface with bactericidal properties. Sci. Rep. 2017, 7, 41023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Zhou, Y.; Xu, X.; Shi, W.; Hu, J.; Li, G.; Qu, X.; Guo, Y.; Tian, X.; Zaman, A. Progress in construction of bio-inspired physico-antimicrobial surfaces. Nanotechnol. Rev. 2020, 9, 1562–1575. [Google Scholar] [CrossRef]
- Vaze, N.; Pyrgiotakis, G.; Mena, L.; Baumann, R.; Demokritou, A.; Ericsson, M.; Zhang, Y.; Bello, D.; Eleftheriadou, M.; Demokritou, P. A nano-carrier platform for the targeted delivery of nature-inspired antimicrobials using Engineered Water Nanostructures for food safety applications. Food Control 2019, 96, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, J.; Raghupathi, P.K.; Houf, K.; Burmølle, M.; Sørensen, S.J.; Gunde-Cimerman, N. Synergistic interactions in microbial biofilms facilitate the establishment of opportunistic pathogenic fungi in household dishwashers. Front. Microbiol. 2018, 9, 21. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Lencova, S.; Svarcova, V.; Stiborova, H.; Demnerova, K.; Jencova, V.; Hozdova, K.; Zdenkova, K. Bacterial biofilms on polyamide nanofibers: Factors influencing biofilm formation and evaluation. ACS Appl. Mater. Interfaces 2020, 13, 2277–2288. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Thermal resistance and gene expression of both desiccation-adapted and rehydrated Salmonella enterica serovar Typhimurium cells in aged broiler litter. Appl. Environ. Microbiol. 2017, 83, e00367-17. [Google Scholar] [CrossRef] [Green Version]
- Almatroudi, A.; Tahir, S.; Hu, H.; Chowdhury, D.; Gosbell, I.B.; Jensen, S.O.; Whiteley, G.S.; Deva, A.K.; Glasbey, T.; Vickery, K. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms. J. Hosp. Infect. 2018, 98, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gibson, H.; Taylor, J.; Hall, K.; Holah, J. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 1999, 87, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sarkar, P.K. Bacillus cereus hazard and control in industrial dairy processing environment. Food Control 2016, 69, 20–29. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in Dairy Products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Palmer, J.; Bloemen, K.; Brooks, J.; Crawford, R. The growth of Bacillus stearothermophilus on stainless steel. J. Appl. Microbiol. 2001, 90, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meliani, A.; Bensoltane, A. Review of Pseudomonas attachment and biofilm formation in food industry. Poult. Fish. Wildl. Sci. 2015, 3, 2–7. [Google Scholar] [CrossRef]
- Allison, D.G.; Ruiz, B.; SanJose, C.; Jaspe, A.; Gilbert, P. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 1998, 167, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Elhariry, H.M. Biofilm formation by Aeromonas hydrophila on green-leafy vegetables: Cabbage and lettuce. Foodborne Pathog. Dis. 2011, 8, 125–131. [Google Scholar] [CrossRef]
- Moori Bakhtiari, N.; Tulabi, Z.; Alishahi, M. Biofilm-Producing Ability and Antibiotic Resistance Pattern of Pathogenic Strains of Aeromonas hydrophila. Jundishapur J. Microbiol. 2019, 12, e97640. [Google Scholar] [CrossRef] [Green Version]
- Daskalov, H. The importance of Aeromonas hydrophila in food safety. Food Control 2006, 17, 474–483. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Møretrø, T. Microbial diversity and ecology of biofilms in food industry environments associated with Listeria monocytogenes persistence. Curr. Opin. Food Sci. 2021, 37, 171–178. [Google Scholar] [CrossRef]
- Papaioannou, E.; Giaouris, E.D.; Berillis, P.; Boziaris, I.S. Dynamics of biofilm formation by Listeria monocytogenes on stainless steel under mono-species and mixed-culture simulated fish processing conditions and chemical disinfection challenges. Int. J. Food Microbiol. 2018, 267, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Visnovsky, S.B.; Pitman, A.R.; Cruz, C.D.; Palmer, J.; Fletcher, G.C.; Flint, S. Biofilm Formation by Listeria monocytogenes 15G01, a Persistent Isolate from a Seafood-Processing Plant, Is Influenced by Inactivation of Multiple Genes Belonging to Different Functional Groups. Appl. Environ. Microbiol. 2021, 87, e02349-20. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Visvalingam, J.; Wang, H.; Youssef, M.K.; Devos, J.; Gill, C.O.; Yang, X. Spatial and temporal distribution of Escherichia coli on beef trimmings obtained from a beef packing plant. J. Food Prot. 2016, 79, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kalchayanand, N.; King, D.A.; Luedtke, B.E.; Bosilevac, J.M.; Arthur, T.M. Biofilm formation and sanitizer resistance of Escherichia coli O157: H7 strains isolated from “high event period” meat contamination. J. Food Prot. 2014, 77, 1982–1987. [Google Scholar] [CrossRef]
- Wang, R.; Luedtke, B.E.; Bosilevac, J.M.; Schmidt, J.W.; Kalchayanand, N.; Arthur, T.M. Escherichia coli O157: H7 strains isolated from high-event period beef contamination have strong biofilm-forming ability and low sanitizer susceptibility, which are associated with high pO157 plasmid copy number. J. Food Prot. 2016, 79, 1875–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagedar, A.; Singh, J.; Batish, V.K. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J. Basic Microbiol. 2010, 50, S98–S106. [Google Scholar] [CrossRef]
- Linklater, D.P.; Juodkazis, S.; Rubanov, S.; Ivanova, E.P. Comment on “bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli”. ACS Appl. Mater. Interfaces 2017, 9, 29387–29393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morco, S.R.; Williams, D.L.; Jensen, B.D.; Bowden, A.E. Structural biofilm resistance of carbon-infiltrated carbon nanotube coatings. J. Orthop. Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Meng, K.; Liu, P.; Cao, X.; Wang, G. Suppressive effects of gecko cathelicidin on biofilm formation and cariogenic virulence factors of Streptococcus mutans. Arch. Oral Biol. 2021, 129, 105205. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Chen, X.-Y.; Tsai, W.-P.; Lee, M. Inhibition of biofilm formation by rough shark skin-patterned surfaces. Colloids Surf. B Biointerfaces 2020, 186, 110738. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Weaver, J.C.; Thornycroft, P.J.; Lauder, G.V. Hydrodynamic function of biomimetic shark skin: Effect of denticle pattern and spacing. Bioinspir. Biomim. 2015, 10, 066010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakers, S.; Niklasson, L.; Steinhagen, D.; Kruse, C.; Schauber, J.; Sundell, K.; Paus, R. Antimicrobial Peptides (AMPs) from Fish Epidermis: Perspectives for Investigative Dermatology. J. Investig. Dermatol. 2013, 133, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Kayes, M.I.; Galante, A.J.; Stella, N.A.; Haghanifar, S.; Shanks, R.M.Q.; Leu, P.W. Stable lotus leaf-inspired hierarchical, fluorinated polypropylene surfaces for reduced bacterial adhesion. React. Funct. Polym. 2018, 128, 40–46. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Gray, J.E.; Norton, P.R.; Alnouno, R.; Marolda, C.L.; Valvano, M.A.; Griffiths, K. Biological efficacy of electroless-deposited silver on plasma activated polyurethane. Biomaterials 2003, 24, 2759–2765. [Google Scholar] [CrossRef]
- Echeverrigaray, F.; Echeverrigaray, S.; Delamare, A.; Wanke, C.; Figueroa, C.; Baumvol, I.; Aguzzoli, C. Antibacterial properties obtained by low-energy silver implantation in stainless steel surfaces. Surf. Coat. Technol. 2016, 307, 345–351. [Google Scholar] [CrossRef]
- Chen, R.; Ni, H.; Zhang, H.; Yue, G.; Zhan, W.; Xiong, P. A preliminary study on antibacterial mechanisms of silver ions implanted stainless steel. Vacuum 2013, 89, 249–253. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. Photocatalytic antibacterial capabilities of TiO2− biocidal polymer nanocomposites synthesized by a surface-initiated photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef]
- Pishbin, F.; Mouriño, V.; Gilchrist, J.B.; McComb, D.W.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013, 9, 7469–7479. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Saha, S.K.; Guha, A.; Saha, S.K. Chemical and biochemical activities of sonochemically synthesized poly(N-isopropyl acrylamide)/silica nanocomposite. Appl. Surf. Sci. 2012, 261, 598–604. [Google Scholar] [CrossRef]
- Pinto, I.B.; dos Santos Machado, L.; Meneguetti, B.T.; Nogueira, M.L.; Carvalho, C.M.E.; Roel, A.R.; Franco, O.L. Utilization of antimicrobial peptides, analogues and mimics in creating antimicrobial surfaces and bio-materials. Biochem. Eng. J. 2019, 150, 107237. [Google Scholar] [CrossRef]
- Palmieri, G.; Balestrieri, M.; Proroga, Y.T.R.; Falcigno, L.; Facchiano, A.; Riccio, A.; Capuano, F.; Marrone, R.; Neglia, G.; Anastasio, A. New antimicrobial peptides against foodborne pathogens: From in silico design to experimental evidence. Food Chem. 2016, 211, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E.; Ueno, S.; Avison, M.B. Enhanced membrane permeabilization and antibacterial activity of a disulfide-dimerized magainin analogue. Biochemistry 2003, 42, 402–409. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, Y.; Ishibashi, J.; Yukuhiro, F.; Asaoka, A.; Taylor, D.; Yamakawa, M. Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. BBA Gen. Subj. 2003, 1624, 125–130. [Google Scholar] [CrossRef]
- Patterson-Delafield, J.; Martinez, R.J.; Lehrer, R.I. Microbicidal cationic proteins in rabbit alveolar macrophages: A potential host defense mechanism. Infect. Immun. 1980, 30, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, R.; Selsted, M.; Szklarek, D.; Fleischmann, J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect. Immun. 1983, 42, 10–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrick, J.W.; Hirata, M.; Shimomoura, Y.; Yoshida, M.; Zheng, H.; Zhong, J.; Wright, S.C. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob. Agents Chemother. 1993, 37, 2534–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, G.; Balestrieri, M.; Capuano, F.; Proroga, Y.T.R.; Pomilio, F.; Centorame, P.; Riccio, A.; Marrone, R.; Anastasio, A. Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry. Int. J. Food Microbiol. 2018, 279, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Barbero, R.J.; Gray, D.S.; Belcher, A.M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010, 5, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Beito, B.; Oliveira, M.; Tudela Martins, M.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Héquet, A.; Humblot, V.; Berjeaud, J.M.; Pradier, C.M. Optimized grafting of antimicrobial peptides on stainless steel surface and biofilm resistance tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef]
- Lombardi, L.; Falanga, A.; Del Genio, V.; Galdiero, S. A New Hope: Self-Assembling Peptides with Antimicrobial Activity. Pharmaceutics 2019, 11, 166. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Leong, S.S.J. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]
- Langsrud, S.; Sidhu, M.S.; Heir, E.; Holck, A.L. Bacterial disinfectant resistance—A challenge for the food industry. Int. Biodeterior. Biodegrad. 2003, 51, 283–290. [Google Scholar] [CrossRef]
- Russell, A. Bacterial resistance to disinfectants. Br. J. Infect. Control 2002, 3, 22–24. [Google Scholar] [CrossRef]
- Li, L.; Ye, L.; Kromann, S.; Meng, H. Occurrence of extended-spectrum β-lactamases, plasmid-mediated quinolone resistance, and disinfectant resistance genes in Escherichia coli isolated from ready-to-eat meat products. Foodborne Pathog. Dis. 2017, 14, 109–115. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.; Jain, S.; Prasad, K.; Seth, P.; Chaturvedi, U. Suboptimal chlorine treatment of drinking water leads to selection of multidrug-resistant Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2004, 58, 277–283. [Google Scholar] [CrossRef]
- Brown, E.E.; Cooper, A.; Carrillo, C.; Blais, B. Selection of multidrug-resistant bacteria in medicated animal feeds. Front. Microbiol. 2019, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- M’ikanatha, N.M.; Sandt, C.H.; Localio, A.R.; Tewari, D.; Rankin, S.C.; Whichard, J.M.; Altekruse, S.F.; Lautenbach, E.; Folster, J.P.; Russo, A. Multidrug-resistant Salmonella isolates from retail chicken meat compared with human clinical isolates. Foodborne Pathog. Dis. 2010, 7, 929–934. [Google Scholar] [CrossRef]
- Zhu, S.; Naim, F.; Marcotte, M.; Ramaswamy, H.; Shao, Y. High-pressure destruction kinetics of Clostridium sporogenes spores in ground beef at elevated temperatures. Int. J. Food Microbiol. 2008, 126, 86–92. [Google Scholar] [CrossRef]
- Feeherry, F.E.; Munsey, D.T.; Rowley, D.B. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl. Environ. Microbiol. 1987, 53, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus spores in the food industry: A review on resistance and response to novel inactivation technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagripanti, J.-L.; Bonifacino, A. Bacterial Spores Survive Treatment with Commercial Sterilants and Disinfectants. Appl. Environ. Microbiol. 1999, 65, 4255–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohnen, W.; Jansen, B. Polymer materials for the prevention of catheter-related infections. Zent. Fur Bakteriol. 1995, 283, 175–186. [Google Scholar] [CrossRef]
- Atreya, C.D. Major foodborne illness causing viruses and current status of vaccines against the diseases. Foodborne Pathog. Dis. 2004, 1, 89–96. [Google Scholar] [CrossRef] [PubMed]
- White, D.G.; Zhao, S.; Simjee, S.; Wagner, D.D.; McDermott, P.F. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 2002, 4, 405–412. [Google Scholar] [CrossRef]
| Antimicrobial Surfaces | Microbial Strains Tested | References |
|---|---|---|
| Cicada wings—Nanopillars | Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae | [9] |
| Dragonfly wings | Escherichia coli | [10] |
| Chitosan from shrimp shell | Escherichia coli and Staphylococcus aureus | [11] |
| Taro leaves | Pseudomonas aeruginosa | [12] |
| Lotus leaves | E. coli and S. aureus | [13] |
| Sharkskin | E. coli or S. aureus | [14] |
| Gecko skin | Porphyromonas gingivalis | [15] |
| Butterfly wing | Escherichia coli | [16] |
| Bactericidal Compounds/Elements | Unique Properties | Efficacy Study | References |
|---|---|---|---|
| Black silicon | High aspect ratios like that of a dragonfly wing. | Antibacterial against gram-negative and gram-positive bacteria and endospores | [25] |
| Graphene, graphene oxide (GO), reduced GO (rGO), and graphene quantum dots (GQDs) | Photo activator properties of graphite oxide | Efficiency against S. aureus, E. coli, Pseudomonas aeruginosa, and Bacillus subtilis | [26,27] |
| Titanium | Optical transparency and refractive index and wide bandgap energy, photocatalytic activity | Efficient against E. coli and S. aureus | [28,29,30] |
| Silver | Hydrophobic surface to inhibit the growth of bacterial flora | Efficient against E. coli and B. subtilis | [31,32] |
| Gold | Large surface-to-volume ratio | Efficient against Salmonella typhimurium, Salmonella enteritidis, Salmonella typhi | [33] |
| Zinc | Sulfhydryl reactivity of the ionic compound | Efficient Streptococcus mutans | [34] |
| Biofilm-Forming Strains | Industrial Concern | References |
|---|---|---|
| Bacillus cereus | Negatively affects product quality and safety in dairy products. Produces emetic (cereulide) and enterotoxins (non-haemolytic enterotoxin, haemolysin BL, cytolysin K). | [53,54] |
| Geobacillus stearothermophilus | A common contaminant in powdered dairy products causing spoilage after reconstitution. | [55] |
| Pseudomonas spp. | Has been reported in dairy, meat, fresh produce, as well as ready-to-eat meal industries. Is capable of producing a high concentration of extracellular matrix (ECM) for strong attachment and is psychrophilic. | [56,57] |
| Aeromonas hydrophila | Has been reported in produce and seafood industries, leading to product contamination, food poisoning, and zoonotic diseases. | [58,59,60] |
| Listeria monocytogenes | Dairy, meat, fish, chilled vegetables, and ready-to-eat products have been known to be affected. Contamination leads to listeriosis outbreaks and therefore recalls. | [61,62,63,64] |
| Escherichia coli | Dairy, meat, seafood industries are commonly affected. Reduction of shelf life, a food poisoning outbreak, and recalls have been reported, especially with Shiga toxin-producing E. coli (STEC). | [65,66,67] |
| Staphylococcus aureus | Shelf-life reduction and food-safety concerns in meat-, poultry-, and dairy-processing industries. | [68] |
| Natural Peptides | Bioinspired Derivatives Peptides | Bactericidal Effect against Strains | References |
|---|---|---|---|
| Magainins from the skin of the African frog Xenopus laevis | Disulphide-Dimerized Magainin Analogue | Stenotrophomonas maltophilia and Escherichia coli. | [86] |
| Cathelicidins in humans | SMAP-29, a cathelicidin-derived peptide from sheep myeloid mRNA | Potent antimicrobial activity against antibiotic-resistant clinical isolates of S. aureus (MRSA), vancomycin-resistant Enterococcus faecium (VREF), and mucoid P. aeruginosa | [87,88] |
| Defensins in humans | Ornithodoros defensin A | Bactericidal activity against Bacillus cereus, Enterococcus faecalis, and methicillin-resistant Staphylococcus aureus | [89] |
| Cationic peptides (1 and 2) derived from rabbit lung macrophages | Synthetic CAP18 (106–142) | Antibacterial effect on Bacillus subtilis, Listeria monocytogenes, and Streptococcus faecalis | [90,91,92] |
| Bactenecin-Innate defence regulator peptide-1018 (IDR-1018) | 1018-derivative peptide named 1018-K6 | Bactericidal efficiency specifically against Listeria monocytogenes | [93] |
| Cecropin A, the naturally occurring peptide in moths | CM15 synthetic peptide | Bactericidal effect on Escherichia coli | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, A.; Brightwell, G. Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries. Foods 2022, 11, 844. https://doi.org/10.3390/foods11060844
Soni A, Brightwell G. Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries. Foods. 2022; 11(6):844. https://doi.org/10.3390/foods11060844
Chicago/Turabian StyleSoni, Aswathi, and Gale Brightwell. 2022. "Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries" Foods 11, no. 6: 844. https://doi.org/10.3390/foods11060844
APA StyleSoni, A., & Brightwell, G. (2022). Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries. Foods, 11(6), 844. https://doi.org/10.3390/foods11060844








