The Use of Electronic Nose in the Quality Evaluation and Adulteration Identification of Beijing-You Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Intramuscular Fat (IMF) and Crude Protein (CP)
2.3. Determination of Nucleotide Compound Contents
2.4. Determination of Amino Acid Contents
2.5. Determination of Fatty Acids
2.6. Determination of VOCs
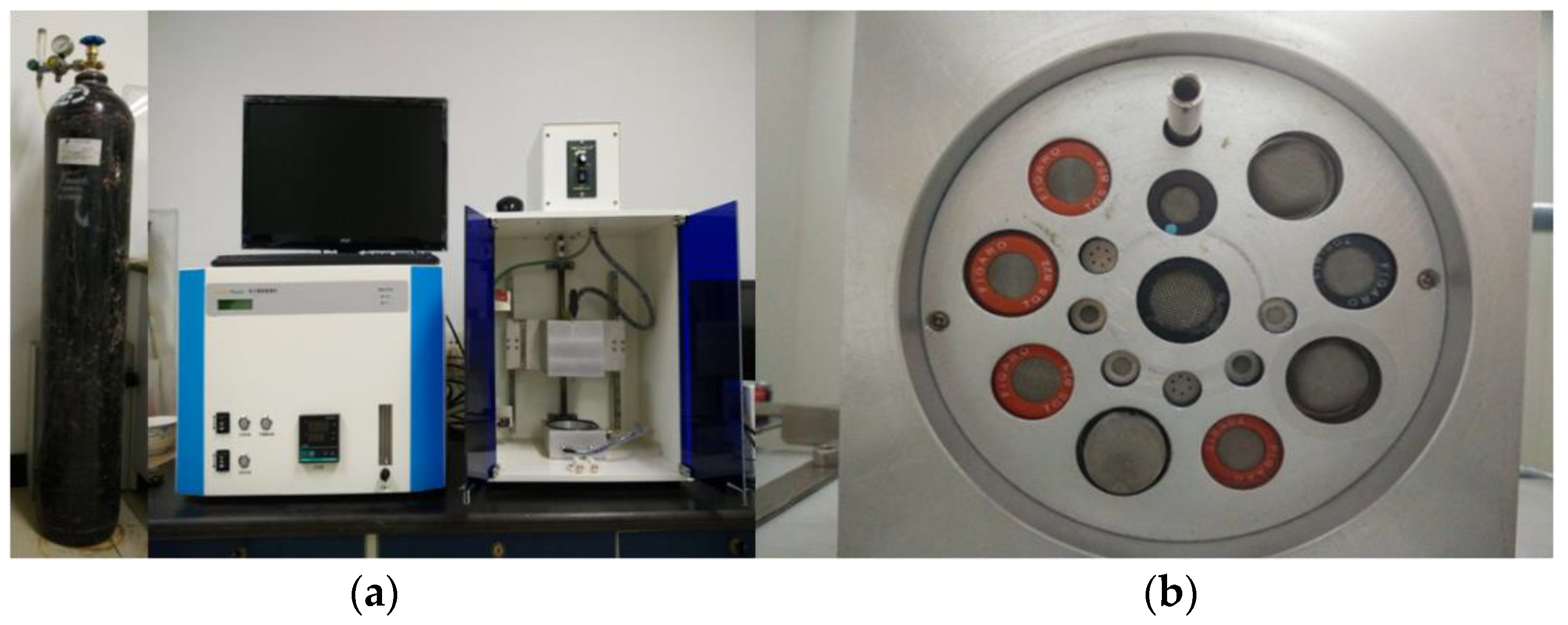
2.7. Electronic Nose Evaluation
2.8. Reagent Section
2.9. Statistical Analysis
3. Results and Discussion
3.1. Contents of Muscle IMF and CP
3.2. Contents of Nucleotide Compound
3.3. Contents of Amino Acids
3.4. Contents of Fatty Acids
3.5. VOCs Analysis
3.6. Electronic Nose Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, D.; Zhang, D.; Xu, G.; Li, K.; Wang, Q.; Zhang, Z.; Li, J.; Chen, Y.; Jia, Y.; Qu, L.J. Effects of different rearing systems on meat production traits and meat fiber microstructure of Beijing-you chicken. Anim. Sci. J. 2015, 86, 729–735. [Google Scholar] [CrossRef]
- Zheng, P. Breeds of Domesticated Animal and Poultry in China; Shanghai Scientific and Technical Publishers: Shanghai, China, 1988. [Google Scholar]
- Zhao, G.P.; Cui, H.X.; Liu, R.R.; Zheng, M.Q.; Chen, J.L.; Wen, J. Comparison of breast muscle meat quality in 2 broiler breeds. Poult. Sci. 2011, 90, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Zdanowska-Sąsiadek, Ż.; Marchewka, J.; Horbańczuk, J.O.; Wierzbicka, A.; Lipińska, P.; Jóźwik, A.; Atanasov, A.G.; Huminiecki, Ł.; Sieroń, A.; Sieroń, K.; et al. Nutrients composition in fit snacks made from ostrich, beef and chicken dried meat. Molecules 2018, 23, 1267. [Google Scholar] [CrossRef] [PubMed]
- Yulian, C.; Yan, Q.; Yu, X.; Haochun, C.; Liang, Z.; Ming, H.; Guanghong, Z. Differences in Physicochemical and Nutritional Properties of Breast and Thigh Meat from Crossbred Chickens, Commercial Broilers, and Spent Hens. Asian Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar]
- Abrodo, P.A.; Llorente, D.D.; Corujedo, S.J.; de la Fuente, E.D.; Álvarez, M.D.G.; Gomis, D.B. Characterisation of Asturian cider apples on the basis of their aromatic profile by high-speed gas chromatography and solid-phase microextraction. Food Chem. 2010, 121, 1312–1318. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Shi, L.K.; Zhang, D.D.; Liu, Y.L. Survey of polycyclic aromatic hydrocarbons of vegetable oils and oilseeds by GC-MS in China. Food Addit. Contam. A 2016, 33, 603–611. [Google Scholar] [CrossRef]
- Bartlett, G. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 211–220. [Google Scholar]
- Górska-Horczyczak, E.; Guzek, D.; Molęda, Z.; Wojtasik-Kalinowska, I.; Brodowska, M.; Wierzbicka, A. Applications of electronic noses in meat analysis. Food Sci. Technol. 2016, 7, 389–395. [Google Scholar] [CrossRef]
- Chen, J.; Gu, J.; Zhang, R.; Mao, Y.; Tian, S. Freshness evaluation of three kinds of meats based on the Electronic Nose. Sensors 2019, 19, 605. [Google Scholar] [CrossRef]
- Wijaya, D.R.; Sarno, R.; Zulaika, E.; Sabila, S.I. Development of mobile electronic nose for beef quality monitoring. Procedia Comput. Sci. 2017, 124, 728–735. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a tool for monitoring the authenticity of food. A Review. Food Anal. Methods 2016, 10, 1800–1816. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Cui, S. Analysis of pork adulteration in minced mutton using electronic nose of metal oxide sensors. J. Food Eng. 2013, 119, 744–749. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Hayat, K.; Karangwa, E.; Zhang, X.; Xiao, Z. Effect of enzymatic hydrolysis with subsequent mild thermal oxidation of tallow on precursor formation and sensory profiles of beef flavours assessed by partial least squares regression. Meat Sci. 2014, 96, 1191–1200. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Rajkumar, U.; Muthukumar, M.; Haunshi, S.; Niranjan, M.; Raju MV, L.N.; Rama Rao, S.V.; Chatterjee, R.N. Comparative evaluation of carcass traits and meat quality in native Aseel chickens and commercial broilers. Br. Poult. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef]
- Phatthawin, L.; Rawiwan, W.; Thanaporn, B.; Arpamas, C.; Niraporn, C.; Sanchai, J. Taste-Active and Nutritional Components of Thai Native Chicken Meat: A Perspective of Consumer Satisfaction. Food Sci. Anim. Resour. 2021, 41, 237–246. [Google Scholar]
- Farkaš, P.; Sádecká, J.; Kováč, M.; Siegmund, B.; Leitner, E.; Pfannhauser, W. Key odourants of pressure-cooked hen meat. Food Chem. 1997, 60, 617–621. [Google Scholar] [CrossRef]
- Xiaojun, J.; Jingting, S.; Ming, Z.; Yifan, L.; Yunjie, T.; Gaige, J.; Yanju, S.; Jianmin, Z. Comparative analysis of quality and flavor of broiler chickens in different breeds and feeding cycles. Chin. J. Anim. Nutr. 2018, 30, 2421–2430. [Google Scholar]
- Jung, S.; Lee, K.H.; Nam, K.C.; Jeon, H.J.; Jo, C. Quality Assessment of the Breast Meat from WoorimatdagTM and Broilers. Korean J. Food Sci. Anim. Resour. 2014, 34, 709–716. [Google Scholar] [CrossRef]
- Li, X.; Rezaei, R.; Peng, L.; Wu, G. Composition of amino acids in feed ingredients for animal diets. Amino Acids 2011, 40, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Gecgel, U. Changes in some physicochemical properties and fatty acid composition of irradiated meatballs during storage. J Food Sci. Technol. 2013, 50, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.T.; Luo, Y.K. Changes in microbial communities and quality attributes of white muscle and dark muscle from common carp (Cyprinus carpio) during chilled and freeze-chilled storage. Food Microbiol. 2018, 73, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J. Influence of intramuscular fat content on the quality of pig meat—1. Composition of the lipid fraction and sensory characteristics of m. longissimus lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef]
- Talpur, M.Z.; Wang, K.; Ahmed, I.; Li, Z.; Liu, L.; Li, Q.; Dou, T.; Gu, D.; Xu, Z.; Rong, H.; et al. Analysis of differentially expressed genes related to intramuscular fat and chemical composition in different breeds of chicken. Pak. J. Agric. Sci. 2018, 55, 615–623. [Google Scholar]
- Intarapichet, K.O.; Suksombat, W.; Maikhunthod, B. Chemical Compositions, Fatty Acid, Collagen and Cholesterol Contents of Thai Hybrid Native and Broiler Chicken Meats. J. Poult. Sci. 2008, 45, 7–14. [Google Scholar] [CrossRef][Green Version]
- Dikeman, M.E.; Pollak, E.J.; Zhang, Z.; Moser, D.W.; Gill, C.A.; Dressler, E.A. Phenotypic ranges and relationships among carcass and meat palatability traits for fourteen cattle breeds, and heritabilities and expected progeny differences for Warner-Bratzler shear force in three beef cattle breeds. J. Anim. Sci. 2005, 83, 2461–2467. [Google Scholar] [CrossRef]
- Ranran, L.; Hongyang, W.; Jie, L.; Jie, W.; Maiqing, Z.; Xiaodong, T.; Siyuan, X.; Huanxian, C.; Qinghe, L.; Guiping, Z. Uncovering the embryonic development-related proteome and metabolome signatures in breast muscle and intramuscular fat of fast-and slow-growing chickens. BMC Genom. 2017, 18, 816. [Google Scholar]
- Tang, H.; Gong, Y.Z.; Wu, C.X.; Jiang, J.; Wang, Y.; Li, K. Variation of meat quality traits among five genotypes of chicken. Poult. Sci. 2009, 88, 2212–2218. [Google Scholar] [CrossRef]
- Samooel, J.; Sik, B.Y.; Joo, K.H.; Jayasena, D.D.; Heon, L.J.; Bok, P.H.; Nyung, H.K.; Cheorun, J. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar]
- Misako, K.; Atsushi, O.; Yoichi, U. Taste enhancements between various amino acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar]
- Fukuuchi, T.; Iyama, N.; Yamaoka, N.; Kaneko, K. Simultaneous quantification by HPLC of purines in umami soup stock and evaluation of their effects on extracellular and intracellular purine metabolism. Nucleos. Nucleot. Nucl. 2018, 37, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Hu, X. Effects of fermentive Chinese herbal medicine, antibiotics and age on slaughter performance and meat quality of broilers. China Poult. 2016, 38, 33–36. [Google Scholar]
- Shi, J.; Wang, S.; Gong, J.; Gu, S.Q.; Zhuang, J.; Wang, X. Effect of different fatting models on non-volatile taste active compounds of Chinese mitten crab (Eriocheir sinensis) meat. Sci. Technol. Food Ind. 2015, 36, 347–351. [Google Scholar]
- Pan, J.; Yang, J.-J.; Zhu, S.-J.; Wu, Z.-Y. Comparison of taste compounds in pork from four different breeds. Sci. Technol. Food Ind. 2015, 36, 161–164. [Google Scholar]
- Dashmaa, D.; Yang, J.; Ba, H.V.; Ryu, K.S.; Hwang, I. Differences in the taste-active compounds between hanwoo longissimus and semitendinosus muscles and its comparision with angus longissimus beef muscle. Korean J. Food Sci. Anim. Resour. 2013, 33, 508–514. [Google Scholar] [CrossRef]
- Bo, H.; Yoon, S.S.; Han, H.R.; Wang, X. Effects of l-arginine on endothelium derived factors and cyclic nucleotides in broilers under low ambient temperature. Asian Australas. J. Anim. Sci. 2004, 17, 1570–1574. [Google Scholar]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Wen, D.; Liu, Y.; Yu, Q. Metabolomic approach to measuring quality of chilled chicken meat during storage. Poult. Sci. 2020, 99, 2543–2554. [Google Scholar] [CrossRef]
- Barton, L.; Bures, D.; Kotrba, R.; Sales, J. Comparison of meat quality between eland (Taurotragus oryx) and cattle (Bos taurus) raised under similar conditions. Meat Sci. 2014, 96, 346–352. [Google Scholar] [CrossRef]
- Geldenhuys, G.; Hoffman, L.C.; Muller, N. The fatty acid, amino acid, and mineral composition of Egyptian goose meat as affected by season, gender, and portion. Poult. Sci. 2015, 94, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Xianyong, M.; Miao, Y.; Zhichang, L.; Dun, D.; Yiyan, C.; Zhimei, T.; Gang, W. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol. 2020, 57, 404–412. [Google Scholar]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kamboh, A.A.; Zhu, W.Y. Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poult. Sci. 2013, 92, 454–461. [Google Scholar] [CrossRef]
- Kucheruk, M.; Midyk, S.; Zasekin, D.; Ushkalov, V.; Kepple, O. Comparison of fatty acid content of organic and traditionally grown broiler chickens. Food Sci. Technol. 2019, 13, 51–57. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, H.J.; Bae, Y.S.; Yong, H.I.; Lee, J.H.; Kim, J.G.; Jo, C. Comparison of Quality Traits of Meat from Korean Native Chickens and Broilers Used in Two Different Traditional Korean Cuisines. Asian Australas. J. Anim. 2013, 26, 1038–1046. [Google Scholar] [CrossRef]
- Jeon, H.J.H.; Choe, J.C.J.; Jung, Y.J.Y.; Kruk, Z.K.Z.A.; Lim, D.L.D.; Jo, C.J.C. Comparison of the Chemical Composition, Textural Characteristics, and Sensory Properties of North and South Korean Native Chickens and Commercial Broilers. Food Sci. Anim. Resour. 2010, 30, 171–178. [Google Scholar] [CrossRef]
- Jiang, L. Comparation of the Chemical Composition in Different Kinds of Broilers Meat and the Relationship with the Flavor; Huazhong Agricultural University: Wuhan, China, 2006. [Google Scholar]
- Lorenzo, J.M.; Franco, D. Fat effect on physico-chemical, microbial and textural changes through the manufactured of dry-cured foal sausage Lipolysis, proteolysis and sensory properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef]
- Garcia, C.; Berdagué, J.J.; Antequera, T.; López-Bote, C.; Córdoba, J.J.; Ventanas, J. Volatile components of dry cured Iberian ham. Food Chem. 1991, 41, 23–32. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC-MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Maarse, H.; Visscher, C. Volatile compounds in food—Qualitative and quantitative data. Food Chem. 1991, 39, 120–122. [Google Scholar]
- Farmer, L. Poultry meat flavor. Poult. Meat Sci. 1999, 12, 127–158. [Google Scholar]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography-mass spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
- Kiselev, I.; Sysoev, V.; Kaikov, I.; Koronczi, I.; Adil Akai Tegin, R.; Smanalieva, J.; Sommer, M.; Ilicali, C.; Hauptmannl, M. On the Temporal Stability of analyte recognition with an E-nose based on a metal Oxide senso array in practical application. Sensors 2018, 18, 550. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N. Nondestructive methods for quality evaluation of livestock products. J. Food Sci. Technol. 2012, 49, 342–348. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, H.; Yuan, X.; Liu, L.; Liu, X.; Wang, Y.; Ding, J.; Xiang, H.; Zhang, X.; Liu, J.; et al. Identification of the main aroma compounds in Chinese local chicken high-quality meat. Food Chem. 2021, 39, 129930. [Google Scholar] [CrossRef]
- Jiang, Q.; Zheng, L.; Tian, L.; Cheng, G.; Meng, W. Application of Electronic Nose in Aroma Prediction of Pork Balls. Food Sci. 2018, 39, 228–233. [Google Scholar]



| Reagents | Types | Manufacturer |
|---|---|---|
| ATP | Chromatographically pure | Sigma |
| ADP | Chromatographically pure | Sigma |
| AMP | Chromatographically pure | Sigma |
| IMP | Chromatographically pure | Sigma |
| GMP | Chromatographically pure | Sigma |
| HxR | Chromatographically pure | Sigma |
| Hx | Chromatographically pure | Sigma |
| Methanol | Chromatographically pure | Fisher Scientific |
| N-hexane | Chromatographically pure | Fisher Scientific |
| N-heptane | Chromatographically pure | Fisher Scientific |
| Ethanol | Analytically pure | Solarbio |
| Sulfosalicylic acid | Analytically pure | Solarbio |
| Potassium hydroxide | Analytically pure | Solarbio |
| Phthalaldehyde | Analytically pure | Solarbio |
| Perchloric acid | Analytically pure | Solarbio |
| Potassium dihydrogen phosphate | Analytically pure | Solarbio |
| Dipotassium phosphate | Analytically pure | Solarbio |
| Petroleum ether | Analytically pure | Solarbio |
| Anhydrous sodium sulfate | Analytically pure | Solarbio |
| Nucleotides | BJY | AA | LH |
|---|---|---|---|
| GMP | 5.87 ± 0.501 a | 4.19 ± 0.01 b | 6.15 ± 0.37 a |
| IMP | 459.77 ± 24.98 a | 247.25 ± 20.22 b | 413.49 ± 25.42 a |
| Hx | 8.63 ± 0.35 a | 63.46 ± 4.47 c | 13.81 ± 0.50 b |
| HxR | 62.27 ± 3.15 a | 147.26 ± 9.74 b | 60.73 ± 4.04 a |
| Amino Acid (mg/100 g) | BJY | LH | AA |
|---|---|---|---|
| Asp (Aspartic acid) | 7.04 ± 0.28 b | 1.13 ± 0.09 a | 18.81 ± 0.19 c |
| Thr (Threonine) | 7.71 ± 0.22 b | 6.45 ± 0.18 a | 10.34 ± 0.26 c |
| Ser (Serine) | 4.56 ± 0.04 a | 6.16 ± 0.17 b | 7.84 ± 0.40 c |
| Glu (Glutamic acid) | 82.02 ± 4.31 a | 64.66 ± 3.37 b | 57.95 ± 0.57 b |
| Gly (Glycine) | 10.97 ± 0.49 a | 8.93 ± 0.00 b | 11.38 ± 0.21 a |
| Ala (Alanine) | 15.82 ± 0.71 a | 14.56 ± 0.47 a | 21.95 ± 0.50 b |
| Cys (Cystine) | 0.76 ± 0.04 a, b | 0.92 ± 0.06 b | 0.71 ± 0.06 a |
| Val (Valine) | 19.12 ± 0.81 a | 18.98 ± 0.95 a | 14.98 ± 0.93 b |
| Met (Methionine) | 9.05 ± 0.37 b | 6.14 ± 0.26 a | 11.88 ± 0.14 c |
| Ile (Isoleucine) | 21.10 ± 0.62 a | 18.92 ± 0.55 b | 16.19 ± 0.16 c |
| Leu (Leucine) | 30.10 ± 0.60 a | 26.44 ± 0.18 b | 25.74 ± 0.55 b |
| Tyr (Tyrosine) | 19.00 ± 0.90 a, b | 18.33 ± 0.01 a | 20.21 ± 0.23 b |
| Phe (Phenylalanine) | 19.60 ± 0.05 a | 18.22 ± 0.48 b | 14.43 ± 0.14 c |
| His (Histidine) | 11.14 ± 0.25 a | 15.17 ± 0.27 b | 18.30 ± 0.14 c |
| Lys (Lysine) | 21.18 ± 0.26 a | 19.19 ± 0.89 b | 20.10 ± 0.31 ab |
| Arg (Arginine) | 8.96 ± 0.57 a | 10.00 ± 0.46 a | 19.79 ± 0.12 b |
| Pro (Proline) | 7.94 ± 1.0 a | 9.16 ± 0.29 a | 24.64 ± 0.05 b |
| EAA | 127.84 ± 0.04 a | 114.34 ± 2.76 b | 113.64 ± 0.58 b |
| Total | 296.02 ± 3.70 b | 263.33 ± 0.25 a | 315.20 ± 1.77 c |
| Fatty Acid (mg/100 g) | BJY | LH | AA |
|---|---|---|---|
| Palmitic Acid C 16:0 | 19.02 ± 1.12 a | 21.88 ± 2.41 a | 65.75 ± 2.83 b |
| Stearic Acid C 18:0 | 16.37 ± 1.87 a | 10.34 ± 2.25 a | 27.00 ± 2.12 b |
| Oleic Acid C 18:1 | 26.90 ± 3.37 a | 15.9 ± 1.12 a | 91.25 ± 5.30 b |
| Linoleic Acid C 18:2 | 34.60 ± 3.62 b | 15.37 ± 7.12 a | 76.50 ± 4.95 c |
| Arachidonic Acid C 20:4 | 14.55 ± 0.71 a | 10.87 ± 0.75 b | 6.25 ± 0.35 c |
| Nervonic Acid C 24:1 | 5.43 ± 1.31 a | 6.89 ± 1.12 a | 3.63 ± 1.94 a |
| Total | 116.86 ± 7.97 b | 79.24 ± 3.37 a | 270.38 ± 17.50 c |
| SFA | 92.75 ± 4.95 a | 32.21 ± 4.66 b | 92.75 ± 4.95 a |
| MUFA | 32.33 ± 2.06 a | 22.79 ± 2.25 a | 94.88 ± 7.25 b |
| PUFA | 49.15 ± 2.91 b | 26.24 ± 6.37 a | 82.75 ± 5.30 c |
| UFA | 81.48 ± 4.97 b | 49.03 ± 4.12 a | 177.63 ± 12.55 c |
| PUFA/MUFA | 1.52 ± 0.01 a | 1.17 ± 0.40 a | 0.87 ± 0.02 a |
| UFA/SFA | 2.31 ± 0.05 b | 1.55 ± 0.35 a | 1.92 ± 0.03 c |
| VOCs | BJY | LH | AA |
|---|---|---|---|
| Hydrocarbons | |||
| (1-Hexadecylheptadecyl)-Cyclohexane | 2.13 ± 0.01 | — | — |
| 1,3-Bis(1,1-dimethylethyl)-Benzene | 12.72 ± 0.05 | 17.68 ± 0.00 | 0.70 ± 0.01 |
| 2-Methylhexacosane | — | — | 2.70 ± 0.30 |
| (2,3-Dimethyldecyl)-Benzene | 2.27 ± 0.01 | — | — |
| 2,4-dimethyl-1-Decene | — | 3.82 ± 0.00 | — |
| 2,4-Dimethyl-Eicosane, | — | — | 0.39 ± 0.01 |
| 2,6,10,14-Tetramethyl-Pentadecane | — | — | 11.19 ± 0.03 |
| 2-Methyl-Dodecane | — | 2.45 ± 0.01 | — |
| 2-Methyl-Hexadecane | — | — | 6.74 ± 0.13 |
| 2-Methyltetracosane | 2.53 ± 0.02 | 2.72 ± 0.16 | — |
| 3-Methyl-Heptadecane | — | — | 5.06 ± 0.51 |
| 8-Heptyl-Pentadecane | — | — | 2.63 ± 0.18 |
| 8-methyl-1-Undecene | — | 2.37 ± 0.00 | — |
| 8-Methyl-Heptadecane | — | — | 1.60 ± 0.04 |
| Decyl-Cyclohexane | — | — | 3.85 ± 0.04 |
| Decyl-Cyclopentane | — | — | 1.18 ± 0.01 |
| Undecyl-Cyclohexane | — | — | 2.19 ± 0.03 |
| Dodecylcyclohexane | — | — | 2.03 ± 0.11 |
| Eicosane | — | 0.74 ± 0.00 | 7.59 ± 4.28 |
| Heneicosane | 13.36 ± 0.66 | 6.32 ± 0.40 | 19.05 ± 0.59 |
| Heptadecane | — | — | 6.66 ± 0.36 |
| Hentriacontane | — | 5.60 ± 0.12 | — |
| Hexadecane | — | — | 4.90 ± 0.99 |
| Octadecane | — | — | 3.73 ± 0.08 |
| Squalane | — | — | 6.00 ± 0.88 |
| Tetradecane | 1.28 ± 0.01 | 3.99 ± 0.01 | 0.82 ± 0.01 |
| Alcohols | |||
| 1-Dodecanol | 2.74 ± 0.00 | 4.18 ± 0.00 | — |
| 1-Octen-3-ol | 9.46 ± 0.04 | 14.62 ± 0.35 | — |
| 2-(2-Ethoxyethoxy)-Ethanol | 13.76 ± 2.33 | 6.93 ± 0.02 | — |
| 2-(Hexadecyloxy)-Ethanol | 2.74 ± 0.00 | — | — |
| 2-methyl-1-Decanol | — | 2.30 ± 0.07 | — |
| 2-Methyl-1-Hexadecanol | 2.67 ± 0.12 | — | — |
| 5-Methyl-2-(1-methylethyl)-1-Hexanol | — | 4.28 ± 0.07 | — |
| Octahydro-4a(2H)-Naphthalenemethanol | — | — | 0.41 ± 0.01 |
| Phenols | |||
| 2,4-Bis(1,1-dimethylethyl)-enol | 8.92 ± 0.08 | 13.36 ± 0.03 | 0.06 ± 0.01 |
| Aldehydes | |||
| Dodecanal | 2.28 ± 0.01 | 1.38 ± 0.06 | — |
| Hexadecanal | 0.17 ± 0.01 | 2.31 ± 0.05 | 2.45 ± 0.10 |
| Nonanal | — | 1.84 ± 0.01 | — |
| Pentadecanal | 0.12 ± 0.00 | — | 2.92 ± 0.01 |
| Tridecanal | 7.64 ± 0.10 | — | — |
| Tetradecanal | 2.04 ± 0.01 | — | — |
| Esters | |||
| [1,1′-Bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | 2.21 ± 0.01 | — | — |
| 2-Hexyldecyl propionate | — | — | 3.55 ± 0.00 |
| 2-Thiopheneacetic acid, oct-3-en-2-yl ester | — | — | 1.59 ± 0.00 |
| 9-Hexadecenoic acid, 9-hexadecenyl ester | 5.37 ± 0.01 | 3.11 ± 0.01 | — |
| Docosanoic acid nonyl ester | 5.59 ± 0.00 | — | — |
| VOCs | BJY | LH | AA | |||
|---|---|---|---|---|---|---|
| Quantity | Relative Content | Quantity | Relative Content | Quantity | Relative Content | |
| Hydrocarbons | 6 | 34.29% | 9 | 45.68% | 19 | 89.02% |
| Alcohols | 6 | 31.36% | 5 | 32.32% | 1 | 0.41% |
| Phenols | 1 | 8.92% | 1 | 13.36% | 1 | 0.06% |
| Aldehydes | 5 | 12.25% | 3 | 5.53% | 2 | 5.37% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yan, W.; Fu, Y.; Wang, L.; Lv, X.; Dai, R.; Li, X.; Jia, F. The Use of Electronic Nose in the Quality Evaluation and Adulteration Identification of Beijing-You Chicken. Foods 2022, 11, 782. https://doi.org/10.3390/foods11060782
Chen J, Yan W, Fu Y, Wang L, Lv X, Dai R, Li X, Jia F. The Use of Electronic Nose in the Quality Evaluation and Adulteration Identification of Beijing-You Chicken. Foods. 2022; 11(6):782. https://doi.org/10.3390/foods11060782
Chicago/Turabian StyleChen, Jingru, Wenjie Yan, Yu Fu, Liang Wang, Xueze Lv, Ruitong Dai, Xingmin Li, and Fei Jia. 2022. "The Use of Electronic Nose in the Quality Evaluation and Adulteration Identification of Beijing-You Chicken" Foods 11, no. 6: 782. https://doi.org/10.3390/foods11060782
APA StyleChen, J., Yan, W., Fu, Y., Wang, L., Lv, X., Dai, R., Li, X., & Jia, F. (2022). The Use of Electronic Nose in the Quality Evaluation and Adulteration Identification of Beijing-You Chicken. Foods, 11(6), 782. https://doi.org/10.3390/foods11060782







