The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Synbiotics Administration
2.3. Outcome Measurements
2.4. Participants’ Characteristic Data
2.5. Stress Assessment
2.6. HPA-Axis Assessment
2.7. Neuroinflammatory Cytokines Assessment
2.8. Tryptophan Metabolism Assessment
2.9. Gut Microbial Metabolites and Immunoglobulin Assessment
2.10. Statistical Analyses
3. Results
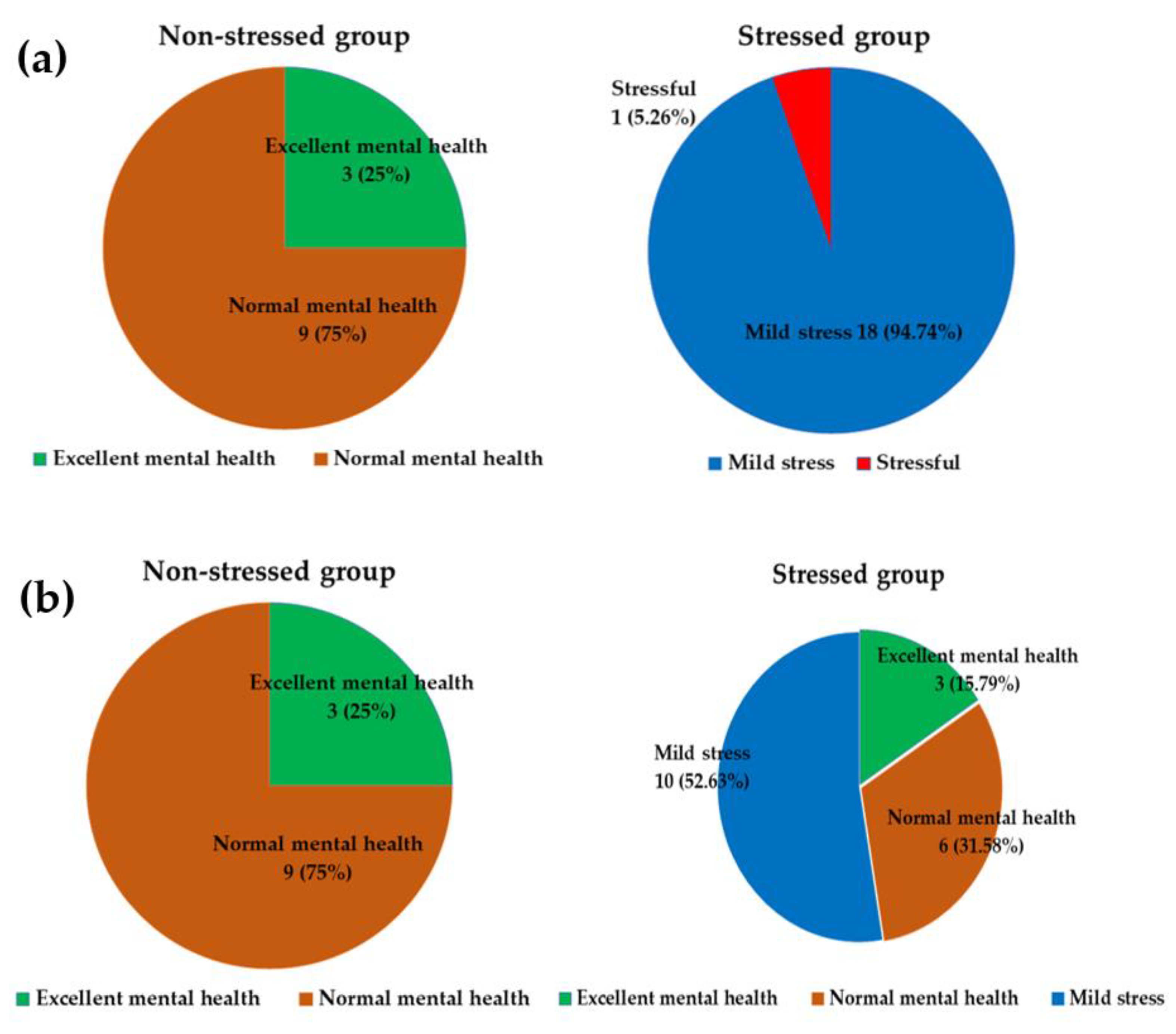
3.1. Characteristics of Participants
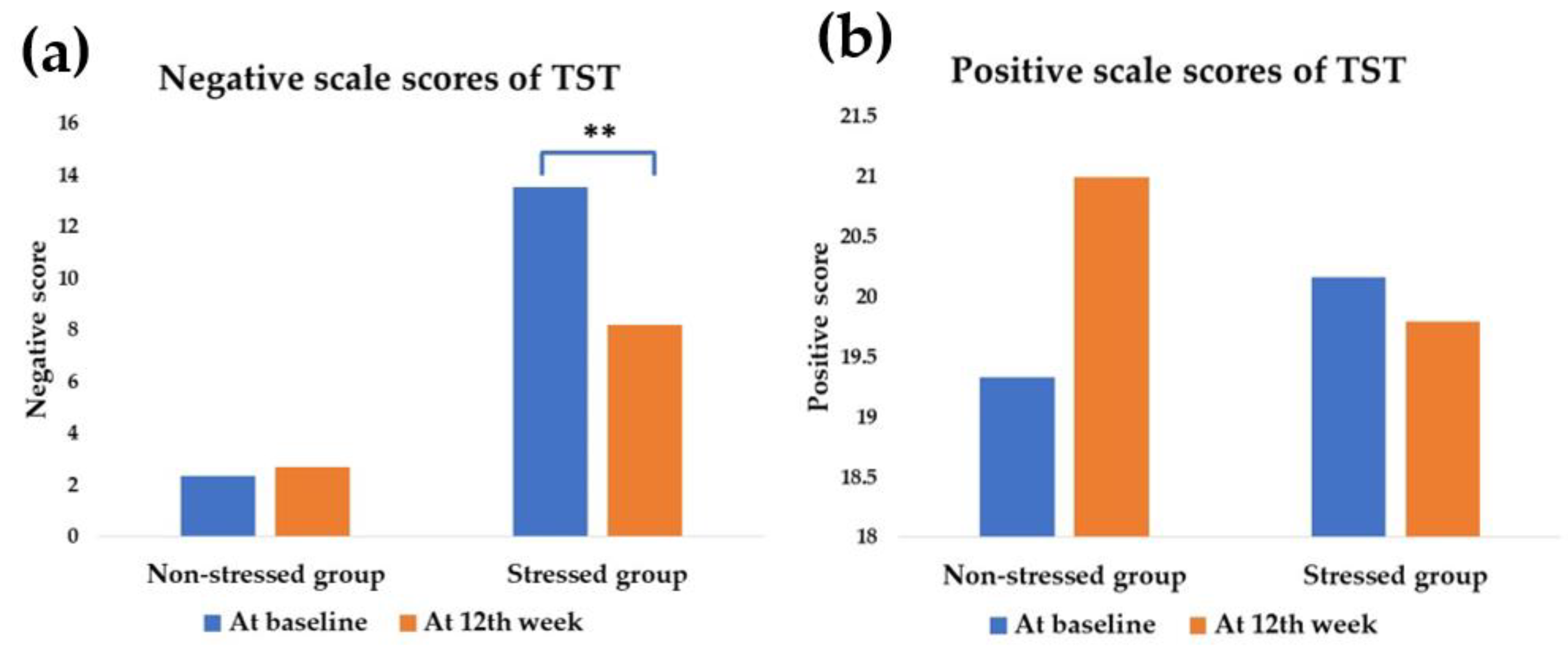
3.2. Effect of Synbiotics on Stress Status and Stress Score
3.3. Effect of Synbiotics Administration on HPA-Axis and Inflammatory Cytokines
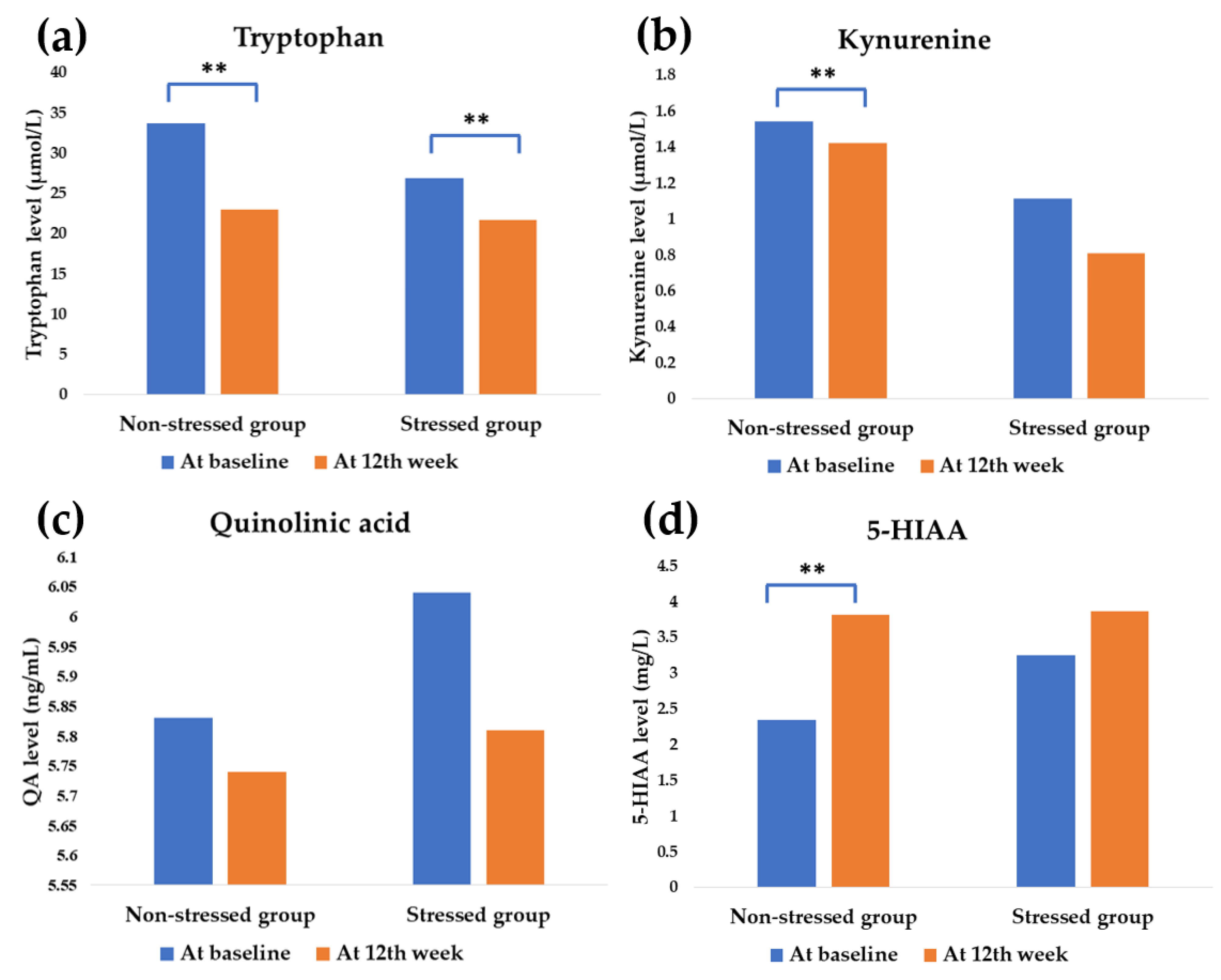
3.4. Effect of Synbiotics Administration on Tryptophan Metabolism
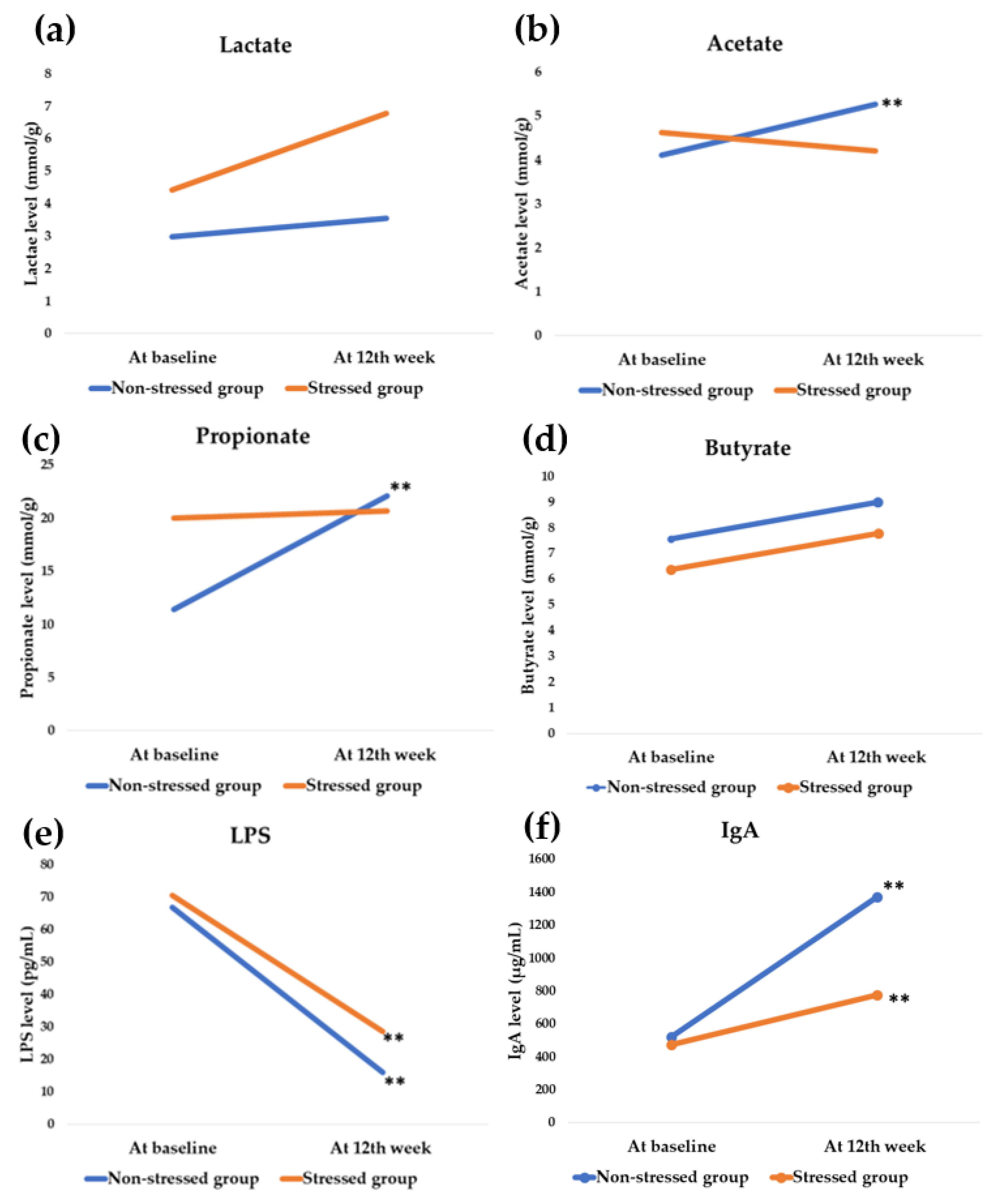
3.5. Effect of Synbiotics Administration on Gut Microbial Metabolites and Immunoglobulin
3.6. Differences in Pre- and Post-Administration Status among the Stressed and the Non-Stressed Groups
3.7. Gaussian Regression Analysis of the Outcomes at the End of the Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez, D.C.; Daundasekara, S.S.; Zvolensky, M.J.; Reitzel, L.R.; Maria, D.S.; Alexander, A.C.; Kendzor, D.E.; Businelle, M.S. Urban Stress Indirectly Influences Psychological Symptoms through Its Association with Distress Tolerance and Perceived Social Support among Adults Experiencing. Int. J. Environ. Res. Public Health 2020, 17, 5301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, L.; Yang, T.; Cottrell, R.; Peng, S.; Guo, W.; Jiang, S. The Impacts of Uncertainty Stress on Mental Disorders of Chinese College Students: Evidence From a Nationwide Study. Front. Psychol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Pentti, J.; Ferrie, J.E.; Batty, G.D.; Nyberg, S.T.; Jokela, M.; Virtanen, M.; Alfredsson, L.; Dragano, N.; I Fransson, E.; et al. Work stress and risk of death in men and women with and without cardiometabolic disease: A multicohort study. Lancet Diabetes Endocrinol. 2018, 6, 705–713. [Google Scholar] [CrossRef]
- Holvoet, P. Stress in obesity and associated metabolic and cardiovascular disorders. Scientifica (Cairo) 2012, 2012, 205027. [Google Scholar] [CrossRef] [Green Version]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. (Encinitas) 2018, 17, 28–32. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Jury, J.; Perdue, M.H. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am. J. Physiol. Liver Physiol. 2007, 293, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Barouei, J.; Moussavi, M.; Hodgson, D.M. Effect of maternal probiotic intervention on HPA axis, immunity, and gut microbiota in a rat model of irritable bowel syndrome. PLoS ONE 2012, 7, e46051. [Google Scholar] [CrossRef] [Green Version]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented milk containing Lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Kesika, P.; Sivamaruthi, B.S.; Chaiyasut, C. Do Probiotics Improve the Health Status of Individuals with Diabetes Mellitus? A Review on Outcomes of Clinical Trials. Biomed Res. Int. 2019, 1531567. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, R.S.D.; Vieira-Coelho, M.A. Probiotics and prebiotics: Focus on psychiatric disorders—A systematic review. Nutr. Rev. 2020, 78, 437–450. [Google Scholar] [CrossRef]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Silk, D.B.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharmacol. 2021, 12, 712531. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2020, 39, 1395–1410. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Razeghi, S. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef] [Green Version]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phattharayuttawat, S.; Ngamthipwattana, T.; Sukhatungkha, K. The development of the Thai stress test. J. Psychiatr. Assoc. Thailand 2000, 45, 237–250. [Google Scholar]
- Ouwehand, A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Badawy, A.A.; Morgan, C.J. Rapid isocratic liquid chromatographic separation and quantification of tryptophan and six kynurenine metabolites in biological samples with ultraviolet and fluorimetric detection. Int. J. Tryptophan Res. 2010, 3, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Kotani, A.; Miyaguchi, Y.; Kohama, M.; Ohtsuka, T.; Shiratori, T.; Kusu, F. Determination of short-chain fatty acids in rat and human feces by high-performance liquid chromatography with electrochemical detection. Anal. Sci. 2009, 25, 1007–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, N.; Milesi, C.; Burn, O.; van den Bogert, B.; Nauta, A.; Hart, K.; Sowden, P.; Burnet, P.W.J.; Kadosh, K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021, 11, 8302. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Xia, C.; Wu, Z.; Zhong, Z.; Xu, Y.; Zeng, Y.; Liu, H.; Liu, R.; Liao, M. Fructooligosaccharides supplementation mitigated chronic stress-induced intestinal barrier impairment and neuroinflammation in mice. J. Funct. Foods 2020, 72, 104060. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S.; et al. Synbiotic Supplementation Improves Obesity Index and Metabolic Biomarkers in Thai Obese Adults: A Randomized Clinical Trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef]
- Bradburn, N.M.; Caplovitz, D. Correlates of Well-Being, Reports on Happiness; National Opinion Report Center, Aldine Publishing Company: Chicago, IL, USA, 1965; p. 8. [Google Scholar]
- Lee, E.-H. Review of the Psychometric Evidence of the Perceived Stress Scale. Asian Nurs. Res. 2012, 6, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Nilchaikovit, T.; Sukying, C.; Silpakit, C. Reliability and validity of the Thai version of the General Health Questionaire. J. Psychiatr. Assoc. Thailand 1996, 41, 2–17. [Google Scholar]
- Muhammad Umar Saeed, S.; Muhammad Anwar, S.; Majid, M. Quantification of Human Stress Using Commercially Available Single Channel EEG Headset. IEICE Trans. Inf. Syst. 2020, E100.D, 2241–2244. [Google Scholar] [CrossRef] [Green Version]
- Harvey, S.B.; Wadsworth, M.; Wessely, S.; Hotopf, M. The relationship between prior psychiatric disorder and chronic fatigue: Evidence from a national birth cohort study. Psychol. Med. 2008, 38, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roque, S.; Correia-Neves, M.; Mesquita, A.R.; Palha, J.A.; Sousa, N. Interleukin-10: A key cytokine in depression? Cardiovasc. Psychiatry Neurol. 2009, 2009, 187894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhees, J.L.; Tarr, A.J.; Wohleb, E.S.; Godbout, J.P.; Mo, X.; Sheridan, J.F.; Eubank, T.D.; Marsh, C.B. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE 2013, 8, e58488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, H.; Kanai, M.; Nakamura, T. Modulation of tryptophan metabolism, promotion of neurogenesis and alteration of anxiety-related behavior in tryptophan 2,3-dioxygenase-deficient mice. Int. J. Tryptophan Res. 2011, 4, 7–18. [Google Scholar] [CrossRef]
- Solvang, S.H.; Nordrehaug, J.E.; Aarsland, D.; Lange, J.; Ueland, P.M.; McCann, A.; Midttun, Ø.; Tell, G.S.; Giil, L.M. Kynurenines, neuropsychiatric symptoms, and cognitive prognosis in patients with mild dementia. Int. J. Tryptophan Res. 2019, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Bo, L.; Guojun, T.; Li, G. An Expanded Neuroimmunomodulation Axis: SCD83-Indoleamine 2,3-Dioxygenase-Kynurenine Pathway and Updates of Kynurenine Pathway in Neurologic Diseases. Front. Immunol. 2018, 9, 1363. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Caspani, G.; Kennedy, S.; Foster, J.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Cell Microbiol. 2019, 6, 454–681. [Google Scholar] [CrossRef]
- Hao, C.; Gao, Z.; Liu, X.; Rong, Z.; Jia, J.; Kang, K.; Guo, W.; Li, J. Intravenous administration of sodium propionate induces antidepressant or prodepressant effect in a dose dependent manner. Sci. Rep. 2020, 10, 19917. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltz, R.M.; Keirsey, J.; Kim, S.C.; Mackos, A.R.; Gharaibeh, R.Z.; Moore, C.C.; Xu, J.; Somogyi, A.; Bailey, M.T. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 533–540. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- van Eeden, W.A.; van Hemert, A.M.; Carlier, I.V.E.; Penninx, B.W.J.H.; Lamers, F.; Fried, E.I.; Schoevers, R.; Giltay, E.J. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl. Psychiatry 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Lekander, M.; Benson, S.; Schedlowski, M.; Engler, H. Sick for science: Experimental endotoxemia as a translational tool to develop and test new therapies for inflammation-associated depression. Mol. Psychiatry 2021, 26, 3672–3683. [Google Scholar] [CrossRef]
- Molina, M.L.; Guerrero, J.; Cidlowski, J.A.; Gatica, H.; Goecke, A. LPS regulates the expression of glucocorticoid receptor α and β isoforms and induces a selective glucocorticoid resistance in vitro. J. Inflamm. 2017, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Salehzadeh, M.; Soma, K.K. Glucocorticoid production in the thymus and brain: Immunosteroids and neurosteroids. Brain Behav. Immun. 2021, 18, 100352. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Spyrka, J.; Rachwalska, P.; Tadeusz, J.; Bugajski, J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol. Rep. 2013, 65, 1163–1175. [Google Scholar] [CrossRef]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal. Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Vogelzang, A.; Maruya, M.; Miyajima, M.; Murata, M.; Son, A.; Kuwahara, T.; Tsuruyama, T.; Yamada, S.; Matsuura, M.; et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018, 215, Jem.20180427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, A.C.; Carroll, D.; Evans, P.; Bosch, J.A.; Clow, A.; Hucklebridge, F.; Der, G. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle-aged and elderly. Brain Behav. Immun. 2006, 20, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanski, K.; Redmond, S.B. The effects of psychological and physical stressors on the secretion of immunoglobulin A in humans and mice. Bios 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Engeland, C.G.; Hugo, F.N.; Hilgert, J.B.; Nascimento, G.G.; Junges, R.; Lim, H.J.; Marucha, P.T.; Bosch, J.A. Psychological distress and salivary secretory immunity. Brain Behav. Immun. 2016, 52, 11–17. [Google Scholar] [CrossRef]


| Negative Scale Score (Sum of Item 1–12) | Positive Scale Score | ||||
|---|---|---|---|---|---|
| (Sum of Item 13–24) | |||||
| 12–36 | 9–11 | 6–8 | 3–5 | 0–2 | |
| 0–1 | 1 | 2 | 3 | 4 | 5 |
| 2–3 | 2 | 3 | 4 | 5 | 6 |
| 4–5 | 3 | 4 | 5 | 6 | 7 |
| 6–7 | 4 | 5 | 6 | 7 | 8 |
| 8–36 | 5 | 6 | 7 | 8 | 9 |
| Parameters | Non-Stressed Group (n = 12) | Stressed Group (n = 19) | p-Value * |
|---|---|---|---|
| Male, n (%) | 3 (25.00) | 8 (42.11) | 0.282 c |
| Female, n (%) | 9 (75.00) | 11 (57.89) | |
| Age (years) | 43.17 ± 3.01 | 45.79 ± 3.10 | 0.572 a |
| Weight, kg | 75.20 ± 4.28 | 76.75 ± 3.64 | 0.788 b |
| Body mass index (BMI), kg/m2 | 29.70 ± 1.59 | 29.36 ± 1.28 | 0.872 b |
| Blood urea nitrogen (BUN), mg/dL | 12.33 ± 1.19 | 13.32 ± 0.80 | 0.481 a |
| Creatinine clearance, mg/dL | 0.90 ± 0.11 | 0.87 ± 0.05 | 0.810 a |
| Aspartate aminotransferase (AST), IU/L | 25.17 ± 6.01 | 24.37 ± 5.62 | 0.776 a |
| Alanine aminotransferase (ALT), IU/L | 29.17 ± 9.04 | 27.63 ± 6.96 | 0.855 b |
| Smoking, n (%) | |||
| No | 12 (100.00) | 19 (100.00) | - |
| Yes | 0(0.00) | 0(0.00) | |
| Alcohol drinking, n (%) | |||
| No | 11 (91.67) | 13 (68.42) | 0.143 c |
| Yes | 1 (8.33) | 6 (31.58) |
| Parameters | Differences between Pre- and Post-Administration | ||
|---|---|---|---|
| Non-Stressed Group | Stressed Group | p-Value * | |
| TST scores | |||
| Negative scale scores # | 0.33 | −5.32 | 0.009 b** |
| Positive scale scores # | 0.33 | −5.32 | 0.009 b** |
| HPA-axis | |||
| Cortisol (ng/mL) | −89.48 | −40.34 | 0.491 b |
| DHEA-S (ng/mL) | 0.73 | −0.01 | 0.004 b** |
| Inflammatory cytokines | |||
| IL-10 (ng/mL) | 55.38 | 40.02 | 0.256 b |
| TNF-α (ng/mL) | 156.27 | 3.28 | <0.001 b** |
| Tryptophan metabolism | |||
| Tryptophan (µmol/L) | −10.7 | −5.20 | 0.029 b** |
| Kynurenine (µmol/L) | −0.12 | −0.30 | 0.919 b |
| QA (ng/mL) | −0.09 | −0.23 | 0.824 b |
| 5-HIAA (mg/L) | 1.47 | 0.61 | 0.626 b |
| Gut microbial metabolites & SCFAs | |||
| Lactate (mmol/g) | 0.55 | 2.34 | 0.351 b |
| Acetate (mmol/g) | 1.14 | 1.31 | 0.016 b** |
| Propionate (mmol/g) | 10.66 | 0.64 | 0.007 b** |
| Butyrate (mmol/g) | 1.42 | 1.39 | 0.208 b |
| Endotoxin | |||
| LPS (pg/mL) | −50.77 | −41.78 | 0.394 b |
| Immunoglobulin | |||
| IgA (µg/mL) | 846.57 | 299.37 | <0.001 b** |
| Parameters | Coefficient | 95% CI | p-Value * |
|---|---|---|---|
| TST score | |||
| Negative scale scores # | 2.39 | −3.06 to 7.84 | 0.372 |
| Positive scale scores # | 0.77 | −8.04 to 7.09 | 0.897 |
| HPA-axis | |||
| Cortisol (ng/mL) | −72.63 | −120.31 to −24.96 | 0.005 ** |
| DHEA-S (ng/mL) | −0.85 | −1.73 to 0.03 | 0.057 |
| Inflammatory cytokines | |||
| IL-10 (ng/mL) | −33.27 | −84.60 to 18.08 | 0.192 |
| TNF-α (ng/mL) | −79.85 | −176.28 to 16.58 | 0.100 |
| Tryptophan metabolism | |||
| Tryptophan (µmol/L) | 0.67 | −4.63 to 4.96 | 0.942 |
| Kynurenine (µmol/L) | −0.26 | −0.77 to 0.24 | 0.289 |
| QA (ng/mL) | −0.18 | −1.06 to 0.70 | 0.670 |
| 5-HIAA (mg/L) | 0.65 | −1.17 to 2.47 | 0.466 |
| Gut microbial metabolites & SCFAs | |||
| Lactate (mmol/g) | 3.71 | −1.32 to 8.75 | 0.139 |
| Acetate (mmol/g) | −1.19 | −2.18 to 0.16 | 0.082 |
| Propionate (mmol/g) | −1.07 | −9.69 to 7.55 | 0.798 |
| Butyrate (mmol/g) | −1.17 | −9.87 to 7.53 | 0.782 |
| Endotoxin | |||
| LPS (pg/mL) | 4.25 | −26.21 to 34.72 | 0.774 |
| Immunoglobulin | |||
| IgA (µg/mL) | −554.95 | −1037.47 to −72.43 | 0.026 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalitsuradej, E.; Sirilun, S.; Sittiprapaporn, P.; Sivamaruthi, B.S.; Pintha, K.; Tantipaiboonwong, P.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Chaiyasut, C. The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study. Foods 2022, 11, 759. https://doi.org/10.3390/foods11050759
Lalitsuradej E, Sirilun S, Sittiprapaporn P, Sivamaruthi BS, Pintha K, Tantipaiboonwong P, Khongtan S, Fukngoen P, Peerajan S, Chaiyasut C. The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study. Foods. 2022; 11(5):759. https://doi.org/10.3390/foods11050759
Chicago/Turabian StyleLalitsuradej, Ekasit, Sasithorn Sirilun, Phakkharawat Sittiprapaporn, Bhagavathi Sundaram Sivamaruthi, Komsak Pintha, Payungsak Tantipaiboonwong, Suchanat Khongtan, Pranom Fukngoen, Sartjin Peerajan, and Chaiyavat Chaiyasut. 2022. "The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study" Foods 11, no. 5: 759. https://doi.org/10.3390/foods11050759
APA StyleLalitsuradej, E., Sirilun, S., Sittiprapaporn, P., Sivamaruthi, B. S., Pintha, K., Tantipaiboonwong, P., Khongtan, S., Fukngoen, P., Peerajan, S., & Chaiyasut, C. (2022). The Effects of Synbiotics Administration on Stress-Related Parameters in Thai Subjects—A Preliminary Study. Foods, 11(5), 759. https://doi.org/10.3390/foods11050759









