Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacterial Strain
2.2. Activation and Preparation of Bacterial Culture
2.3. Preparation of Capsules Loaded with Lactobacillus gasseri
2.4. Lactobacillus gasseri Viability
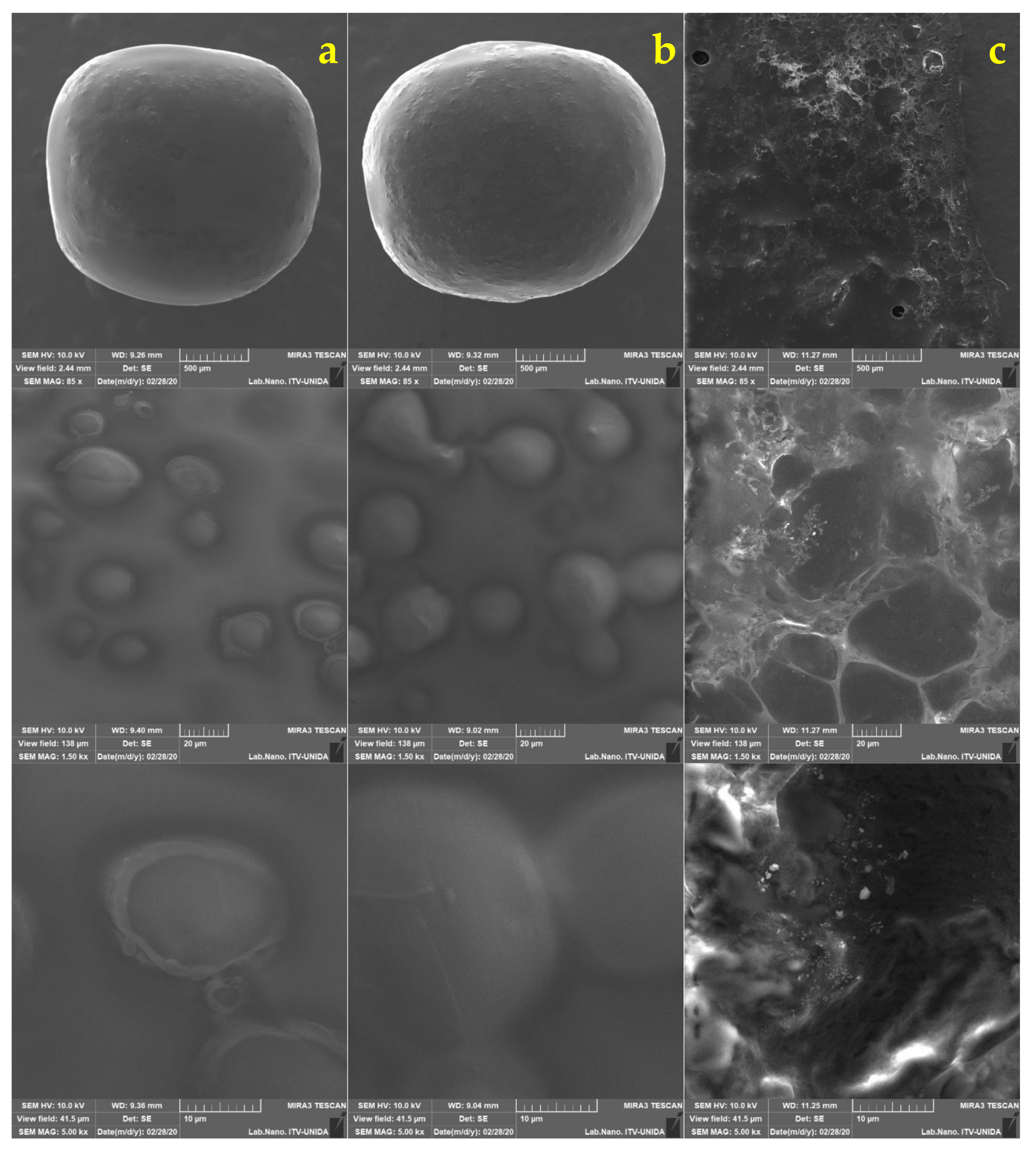
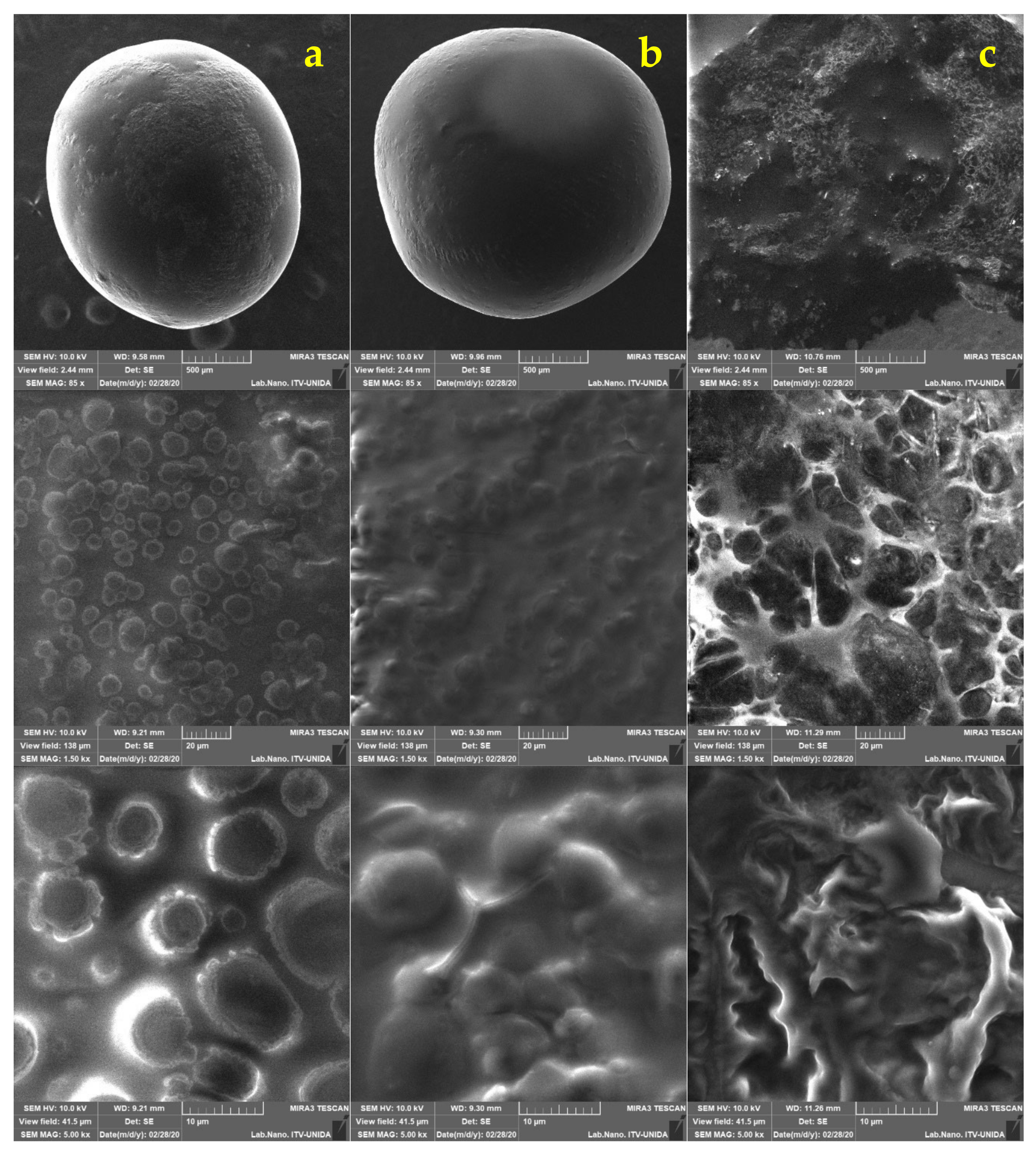
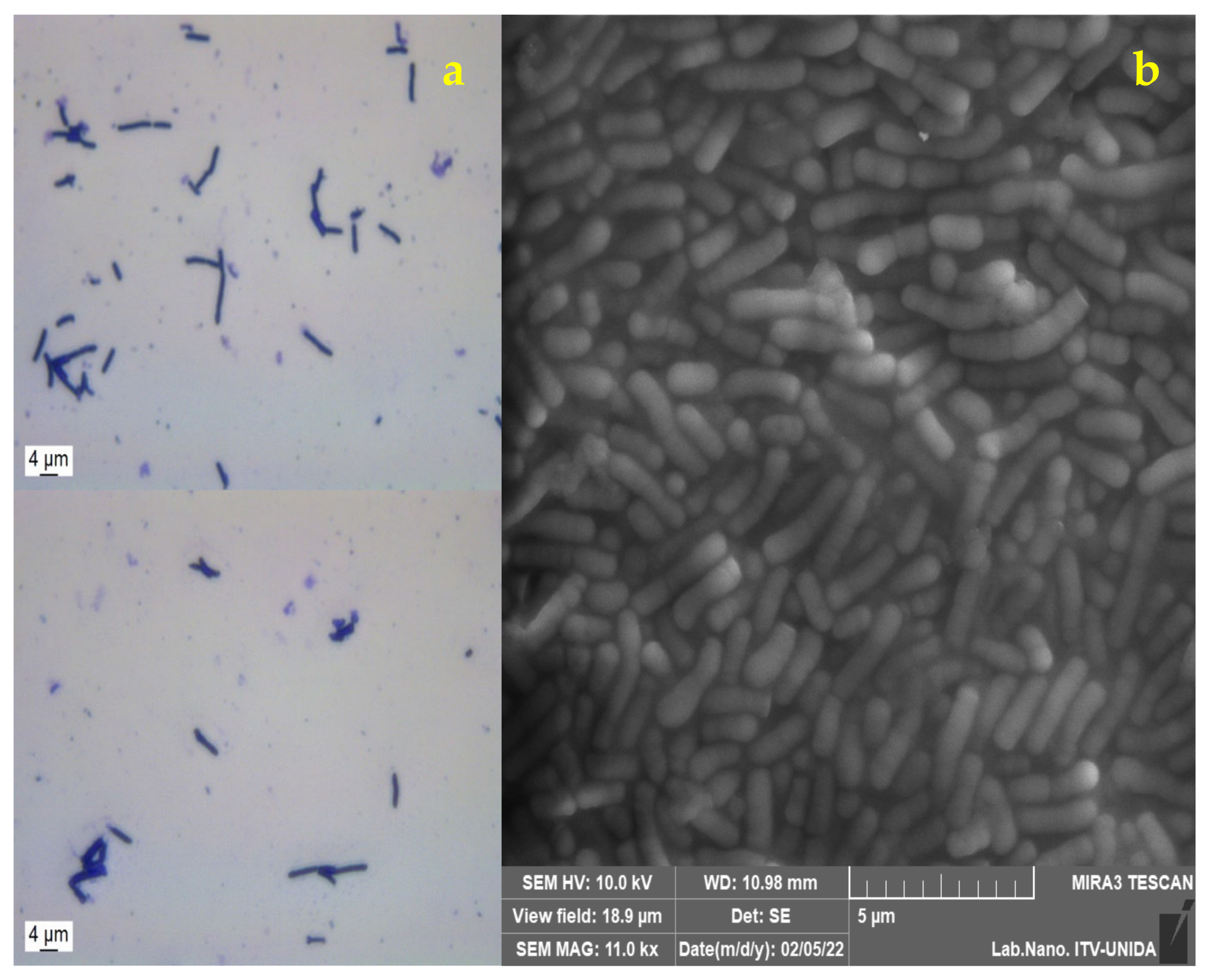
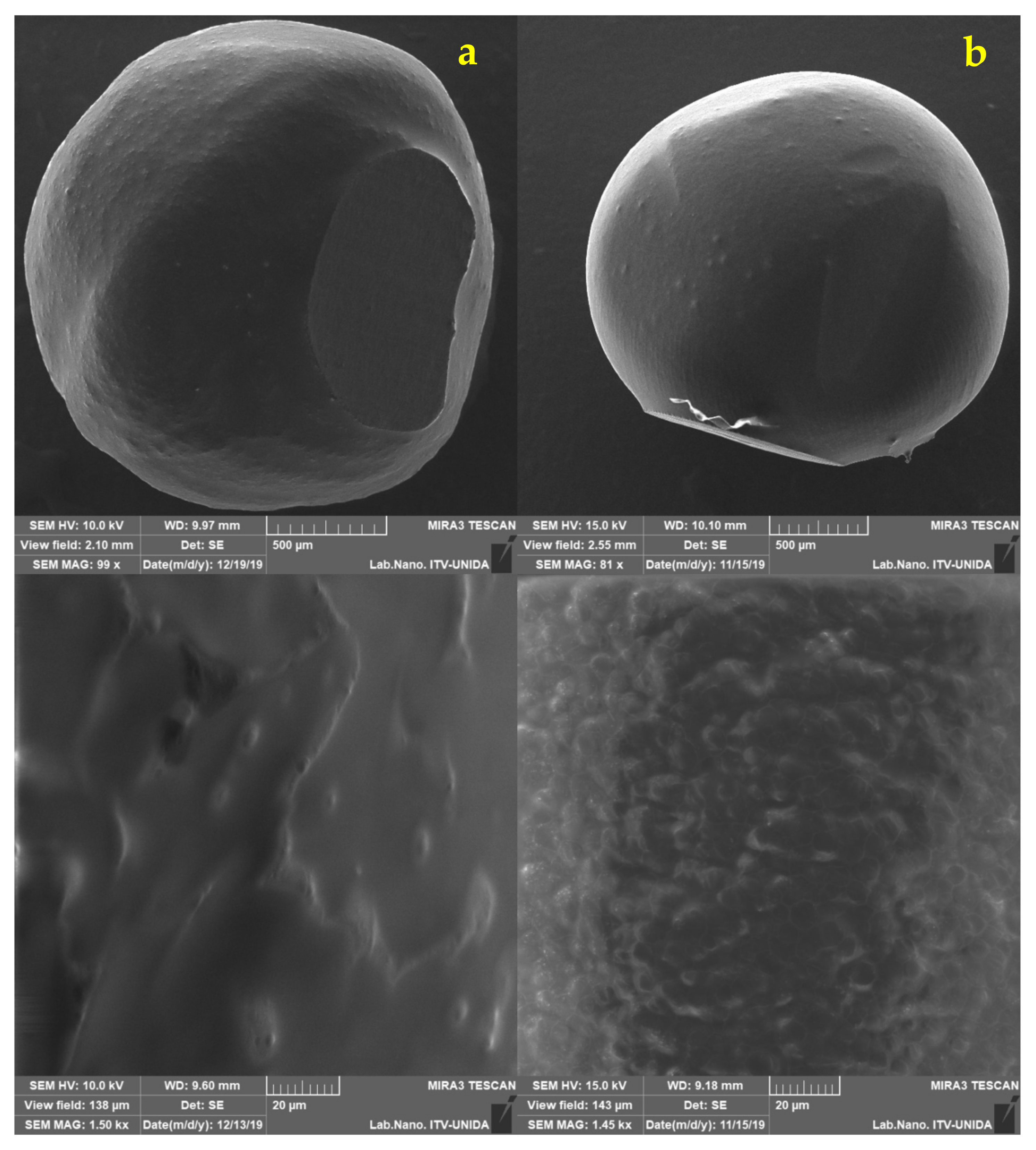
2.5. Morphological Analysis of Free and Encapsulated Lactobacillus gasseri
2.6. Viability of Lactobacillus gasseri Encapsulated in Juice
2.7. Viability of Free and Encapsulated Lactobacillus gasseri in Simulated Gastrointestinal Conditions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Viability of Capsules Loaded with Lactobacillus gasseri
3.2. Morphological Analysis of Lactobacillus gasseri
3.3. Viability of Encapsulated Lactobacillus gasseri in Apple Juice
3.4. Viability of Free and Encapsulated Lactobacillus gasseri under Simulated Gastrointestinal Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jędrusek-Golińska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Michałowska, A.; Szymandera-Buszka, K. Recent progress in the use of functional foods for older adults: A narrative review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Functional foods: Concepts and application to inulin and oligofructose. Br. J. Nutr. 2002, 87, 139–143. [Google Scholar] [CrossRef]
- Kneifel, W. Functional foods with lactic acid bacteria: Probiotics-Prebiotics-Nutraceuticals. Biotechnol. Prog. 2000, 17, 101–107. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Betoret, N.; Puente, L.; Díaz, M.J.; Pagán, M.J.; García, M.J.; Gras, M.L.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Senadeera, S.S.; Prasanna, P.H.P.; Jayawardana, N.W.I.A.; Gunasekara, D.C.S.; Senaddera, P.; Chandrasekara, A. Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon 2018, 4, e00955. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.K. Bacteria, beneficial: Probiotic lactic acid bacteria: An overview. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L., McNamara, J., Eds.; Elsevier: Oxford, UK, 2022; Volume 4, pp. 32–33. [Google Scholar]
- Mulaw, G.; Tessema, T.S.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019, 2019, 7179514. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Mahboubi, M. Lactobacillus gasseri as a functional food and its role in obesity. Int. J. Med. Rev. 2019, 6, 59–64. [Google Scholar] [CrossRef]
- Francl, A.L.; Thongaram, T.; Miller, M.J. The PTS transporters of Lactobacillus gasseri ATCC 33323. BMC Microbiol. 2010, 10, 77. [Google Scholar] [CrossRef]
- Jurado-Gámez, H.; Martínez-Benavides, J.; Romero-Benavides, D.A.; Morillo-Garcés, J.A.; Orbes-Villacorte, A.E.; Mesías-Pantoja, L.N. Cinética de fermentación, pruebas de desafío in vitro y efecto de inhibición de Lactobacillus gasseri ATCC 19992. Vet. Zootec. 2016, 10, 72–89. [Google Scholar] [CrossRef]
- Selle, K.; Klaenhammer, R.T. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Miyoshi, M.; Ogawa, A.; Sakai, F.; Kadooka, Y. Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high-fat diet. J. Nutr. Sci. 2016, 5, e23. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Vallejo-Castillo, V. Probiotic encapsulation. Afr. J. Microbiol. Res. 2013, 7, 4743–4753. [Google Scholar]
- Chun, H.; Kim, C.H.; Cho, Y.H. Microencapsulation of Lactobacillus plantarum DKL 109 using external ionic gelation method. Korean J. Food Sci. Anim. Resour. 2014, 34, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Holkem, T.E.; Raddatz, C.G.; Barin, S.J.; Moraes Flores, M.E.; Muller, I.E.; Codevilla, F.C.; Jacob-Lopez, E.; Ferreira Grosso, R.C.; Menezes, R.C. Production of microcapsules containing Bifidobacterium BB-12 by emulsification/internal. LWT Food Sci. Technol. 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar]
- Ramos, P.E.; Cerqueria, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2017, 58, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Jiménez, L.; Betoret, E.; Betoret, N.; Patrignani, F.; Barrera, C.; Seguí, L.; Lanciotti, R.; Rosa, M.D. High pressures homogenization (HPH) to microencapsulate L. salivarius spp. Salivarius in mandarin juice. Probiotic survival and in vitro digestion. J. Food Eng. 2018, 240, 43–48. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of homogenization techniques on reducing the size of microcapsules and the survival of probiotic bacteria therein. J. Food Sci. 2009, 74, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Braschi, G.; Betoret, E.; Reinheimer, J.A.; Lanciotti, R. Microencapsulation of functional strains by high pressure homogenization for a potential use in fermented milk. Int. Food Res. J. 2017, 97, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of Chitosan-Alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, W.; Song, Y.; Zhou, S.F. Improvement and metabolomics-based analysis of d-lactic acid production from agro-industrial wastes by Lactobacillus delbrueckii submitted to adaptive laboratory evolution. J. Agric. Food Chem. 2020, 68, 7660–7669. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of various encapsulating materials on the stability of probiotic bacteria. J. Food Sci. 2009, 74, 100–107. [Google Scholar] [CrossRef]
- Poletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Wagner, R.; De Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophilus when encapsulated with the prebiotics rice bran, inulin and Hi-Maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Ladislau, H.F.L.; de Farias, T.G.S.; Soares, B.L.M.; Madeios, J.A.D.C.; Melo, N.F.C.B.; Stamford-Arnaud, T.M.; Stamford, T.C.M.; Stamford, T.L.M. The effect of Co-Encapsulation of Lactobacillus rhamnosus GG ATCC 53103 with inulin on alginate/chitosan matrix: The viability in fermented soy blend and simulated digestive system. Int. J. Food Sci. Technol. 2021, 56, 5395–5401. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and Co-Extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- Zazzali, I.; Calvo, T.R.A.; Ruíz-Henestrosa, V.M.P.; Santagapita, P.R.; Perullini, M. Effects of pH, extrusion tip size and storage protocol on the structural properties of Ca (II) alginate beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef]
- Dorożyński, P.P.; Kulinowski, P.; Mendyk, A.; Młynarczyk, A.; Jachowicz, R. Novel application of MRI technique combined with Flow-Through cell dissolution apparatus as supportive discriminatory test for evaluation of controlled release formulations. AAPS J. 2010, 11, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2018, 25, 120–129. [Google Scholar] [CrossRef]
- Olivares, A.; Soto, C.; Caballero, E.; Altamirano, C. Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage. Electron. J. Biotechnol. 2019, 42, 42–48. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Barthet, V.J. Canola: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, W.C., Corke, H., Seethamaraman, K., Faubion, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1–4, pp. 237–241. [Google Scholar]
- Ortakci, F.; Sert, S. Stability of free and encapsulated Lactobacillus acidophilus ATCC 4356 in yogurt and in an artificial human gastric digestion system. J. Dairy Sci. 2012, 95, 6918–6925. [Google Scholar] [CrossRef]
- Moghanjougi, Z.M.; Bari, M.R.; Khaledabad, M.A.; Amiri, S.; Almasi, H. Microencapsulation of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis BB-12 in pectin and sodium alginate: A comparative study on viability, stability, and structure. Food Sci. Nutr. 2021, 9, 5103–5111. [Google Scholar] [CrossRef]
- Hugues-Ayala, A.M.; Sarabia-Sainz, J.A.I.; González-Rios, H.; Vázquez-Moreno, L.; Ramos-Clamont Montfort, G. Airbrush encapsulation of Lactobacillus rhamnosus GG in dry microbeads of alginate coated with regular buttermilk proteins. LWT Food Sci. Technol. 2020, 117, 108639. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Altermann, E.; Goh, Y.J.; Tallon, R.; Sanozky-Dawes, R.B.; Pfeiler, E.A.; O’Flaherty, S.; Buck, B.L.; Dobson, A.; Duong, T.; et al. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl. Environ. Microbiol. 2008, 74, 4610–4625. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Allan-Wojtas, P.; Holley, R.A. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006, 71, 20–24. [Google Scholar] [CrossRef]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Basti, A.A.; Ansari, F. Viability of microencapsulated and non-microencapsulated Lactobacilli in a commercial beverage. Biotechnol. Rep. 2020, 25, e00432. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pranteda, M.L.; Poncelet, D.; Náder-Macías, M.E.; Arcos, A.; Aguilera, M.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A. Stability of Lactobacilli encapsulated in various microbial polymers. J. Biosci. Bioeng. 2012, 113, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Sousa, S.; Gomes, A.M.; Pintado, M.M.; Silva, J.P.; Costa, P.; Amaral, M.H.; Rocha-Santos, T.; Freitas, A.C. Storage stability of Lactobacillus paracasei as free cells or encapsulated in Alginate-Based microcapsules in low pH fruit juices. Food Bioprocess Technol. 2012, 5, 2748–2757. [Google Scholar] [CrossRef]
- Shah, N.P.; Ding, W.K.; Fallourd, M.J.; Leyer, G. Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. J. Food Sci. 2010, 75, 278–282. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Gallardo Lopez, A.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Hill, C.; Gahan, C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmed, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.G.; Cezarino, E.C.; Michelon, M.; Sato, A.C.K. Symbiotic microencapsulation to enhance Lactobacillus acidophilus survival. LWT Food Sci. Technol. 2018, 89, 503–509. [Google Scholar] [CrossRef]
- Zeashan, M.; Afzaal, M.; Saeed, F.; Ahmen, A.; Tufail, T.; Ahmed, A.; Anjum, M.F. Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions. Food Sci. Nutr. 2020, 8, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ji, Y.R.; Lee, S.; Choi, M.J.; Cho, Y. Microencapsulation of probiotic Lactobacillus acidophilus KBLl409 by extrusion technology to enhance survival under simulated intestinal and Freeze-Drying conditions. J. Microbiol. Biotechnol. 2019, 29, 721–730. [Google Scholar] [CrossRef] [PubMed]
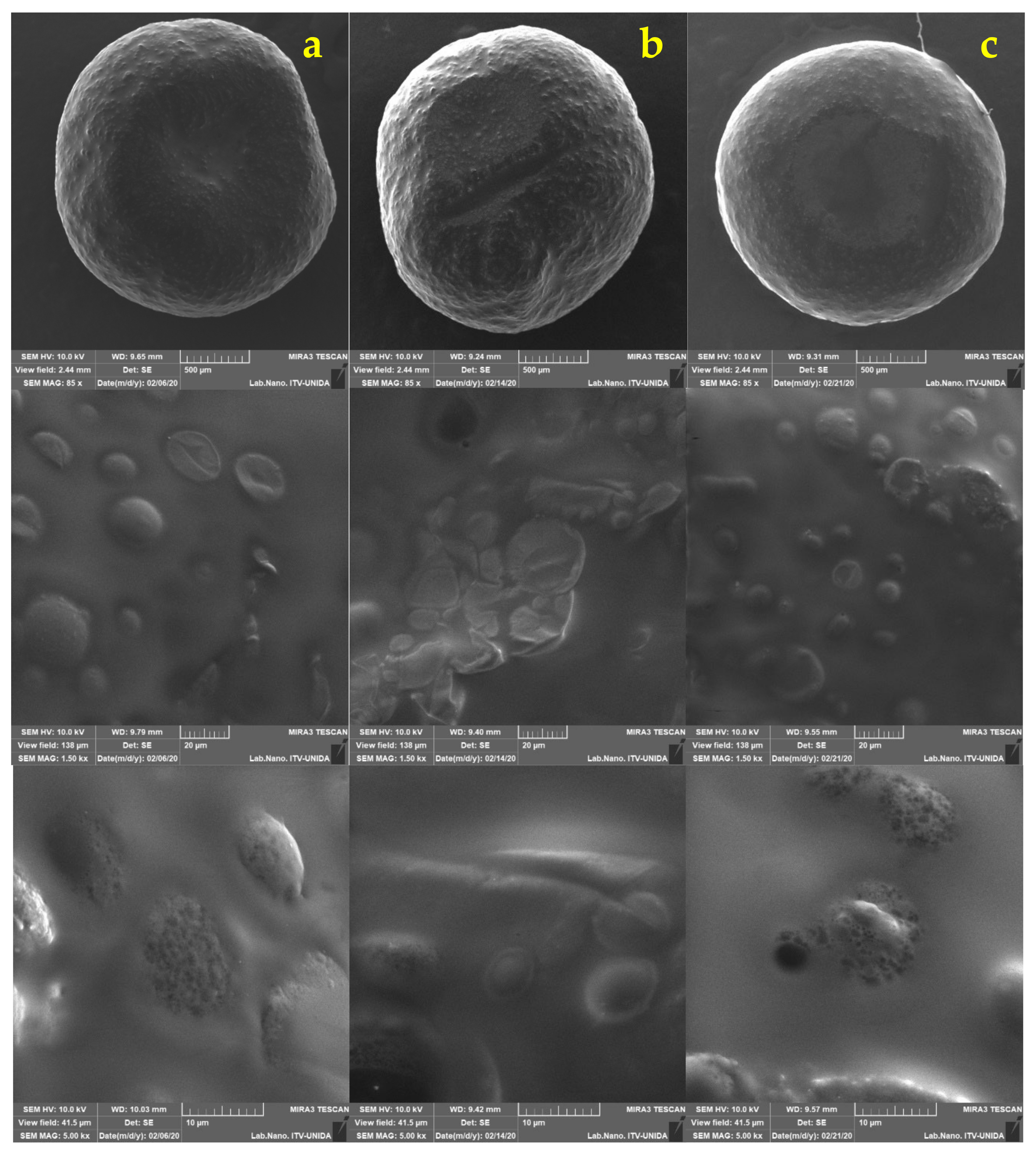
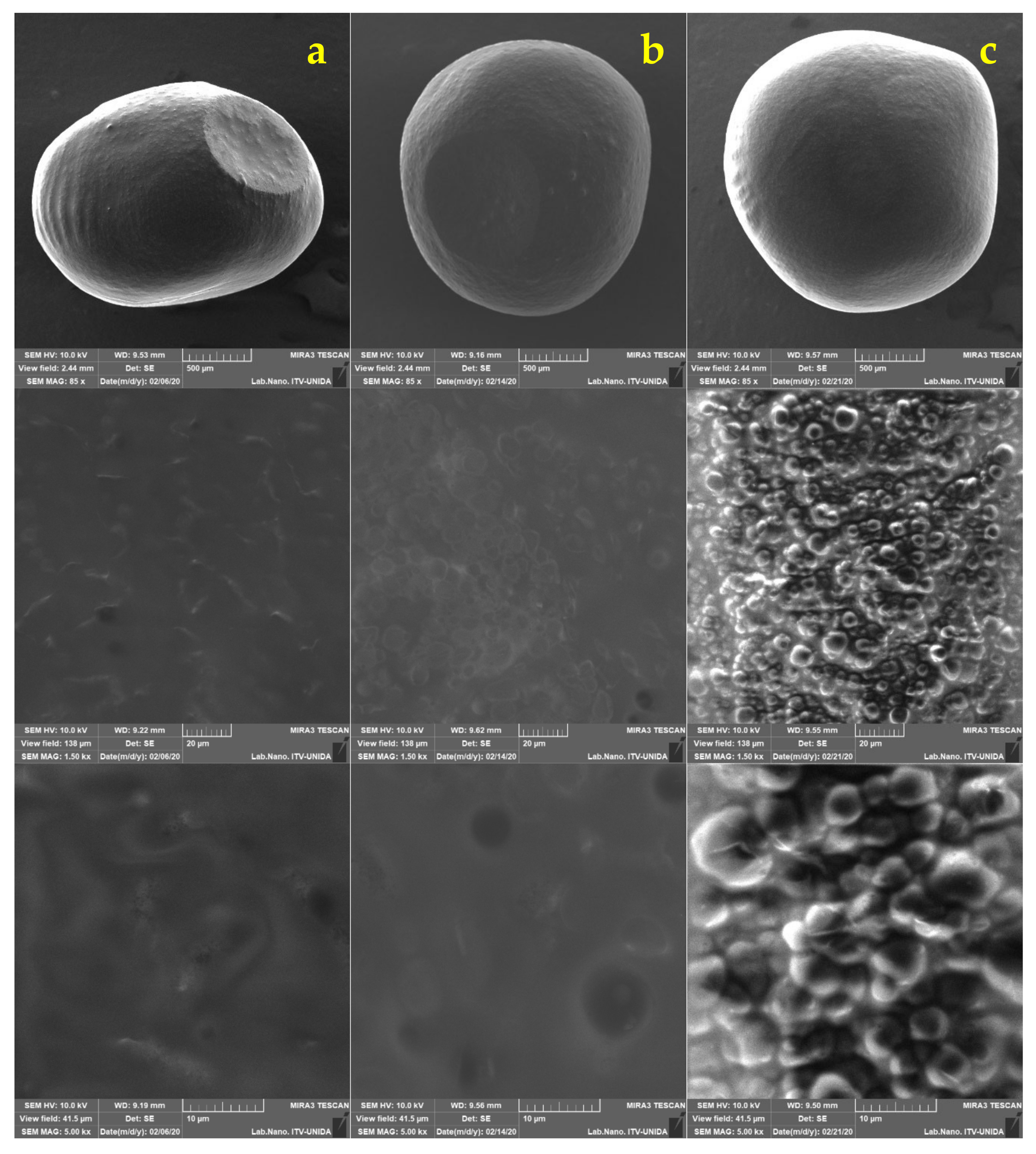
| Encapsulation Method | Maximum Feret Diameter (mm) | Minimum Feret Diameter (mm) | Circularity |
|---|---|---|---|
| ALG UT | 3.284 ± 0.304 a | 2.915 ± 0.196 a | 0.885 ± 0.092 a |
| ALG AM | 2.856 ± 0.123 b | 2.599 ± 0.122 b | 0.854 ± 0.019 b |
| Method | log CFU/mL (N0) | log CFU/mL (N) | Viability (%) |
|---|---|---|---|
| ALG AM | 5.771 ± 0.326 a | 4.497 ± 0.075 b | 77 |
| ALG UT | 4.151 ± 0.213 a | 2.304 ± 0.04 b | 55.43 |
| Free LG | 6.7 ± 0.023 a | 4.232 ± 0.089 b | 63.10 |
| Method | Initial log CFU/mL (N0) | Final log CFU/mL (N) | Viability (%) |
|---|---|---|---|
| ALG AM | 4.961 ± 0.243 a | 4.962 ± 0.166 a | 100 |
| ALG UT | 4.895 ± 0.025 a | 3.875 ± 0.087 b | 79.14 |
| Free LG | 7.09 ± 0.014 a | No growth b | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Pérez, A.; Romero-Chapol, O.O.; Castillo-Olmos, A.G.; García, H.S.; Suárez-Quiroz, M.L.; Singh, J.; Figueroa-Hernández, C.Y.; Viveros-Contreras, R.; Cano-Sarmiento, C. Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice. Foods 2022, 11, 740. https://doi.org/10.3390/foods11050740
Varela-Pérez A, Romero-Chapol OO, Castillo-Olmos AG, García HS, Suárez-Quiroz ML, Singh J, Figueroa-Hernández CY, Viveros-Contreras R, Cano-Sarmiento C. Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice. Foods. 2022; 11(5):740. https://doi.org/10.3390/foods11050740
Chicago/Turabian StyleVarela-Pérez, Abigail, Oscar O. Romero-Chapol, Ana G. Castillo-Olmos, Hugo S. García, Mirna L. Suárez-Quiroz, Jaspreet Singh, Claudia Y. Figueroa-Hernández, Rubí Viveros-Contreras, and Cynthia Cano-Sarmiento. 2022. "Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice" Foods 11, no. 5: 740. https://doi.org/10.3390/foods11050740
APA StyleVarela-Pérez, A., Romero-Chapol, O. O., Castillo-Olmos, A. G., García, H. S., Suárez-Quiroz, M. L., Singh, J., Figueroa-Hernández, C. Y., Viveros-Contreras, R., & Cano-Sarmiento, C. (2022). Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice. Foods, 11(5), 740. https://doi.org/10.3390/foods11050740











