Discriminant Analysis of Pu-Erh Tea of Different Raw Materials Based on Phytochemicals Using Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tea Samples
2.3. Determination of Phytochemicals
2.3.1. Water Extract (WE)
2.3.2. Total Phenolics (TPC)
2.3.3. Total Free Amino Acids (TFAAs)
2.3.4. Catechins Components and Caffeine
2.3.5. Theacrine
2.3.6. Free Amino Acids
2.4. Chemometric Analyses
3. Results and Discussion
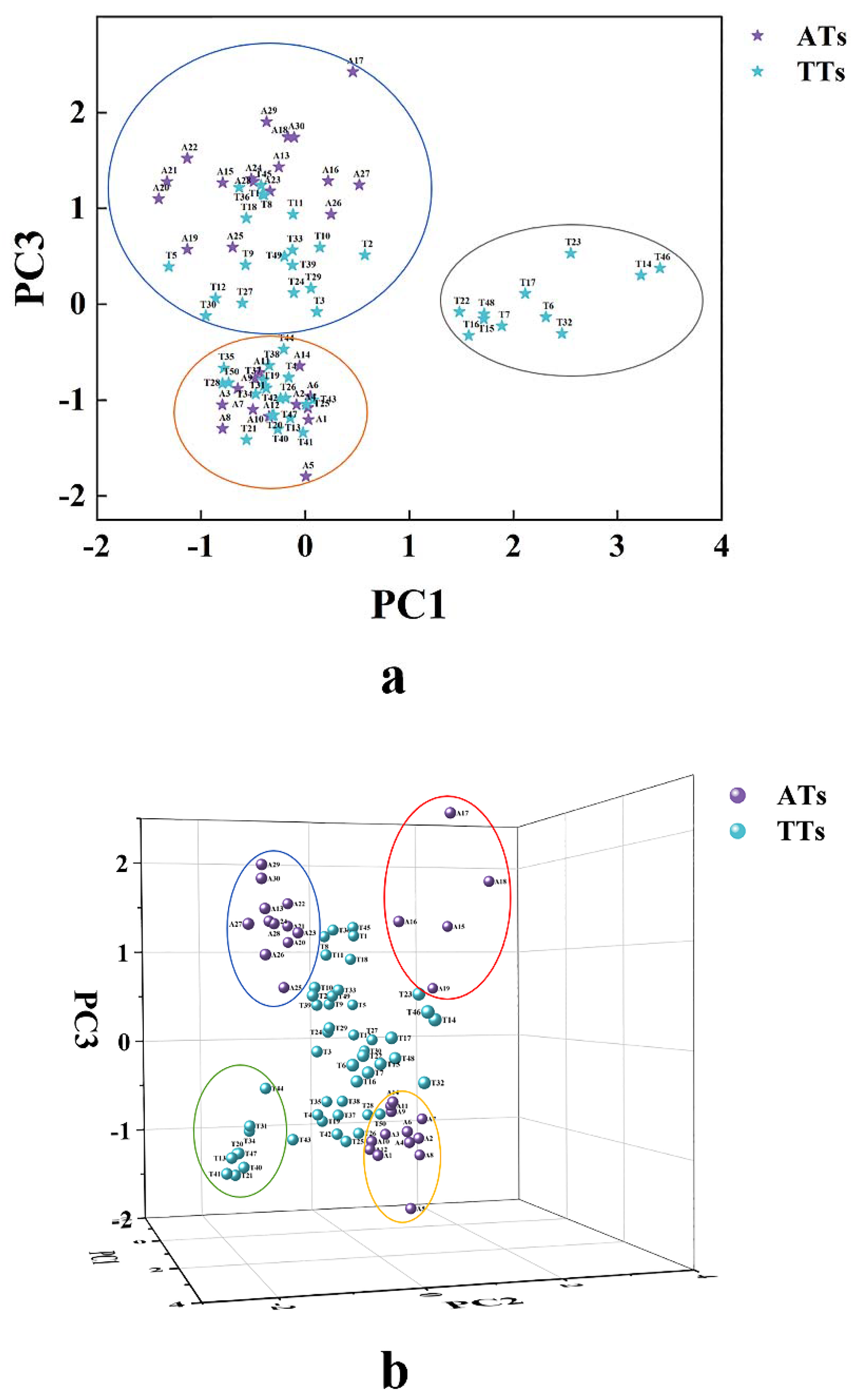
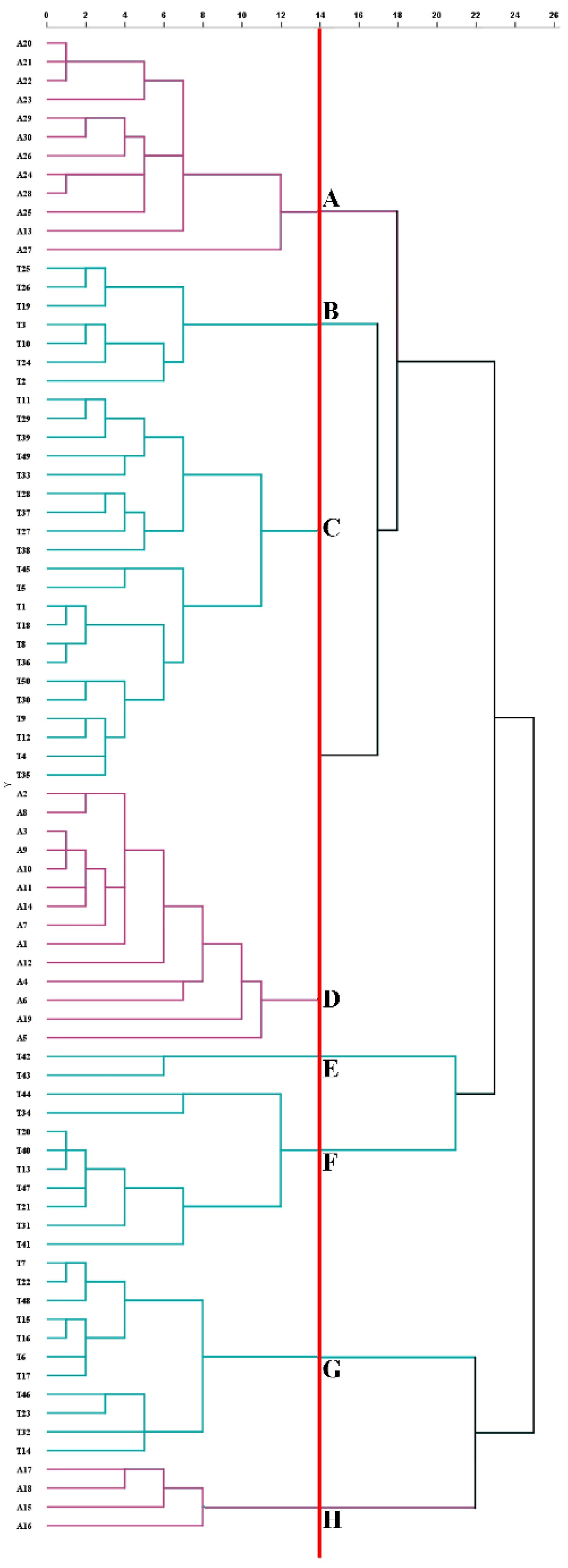
3.1. Feasibility of Phytochemicals to Classification of AT and TT
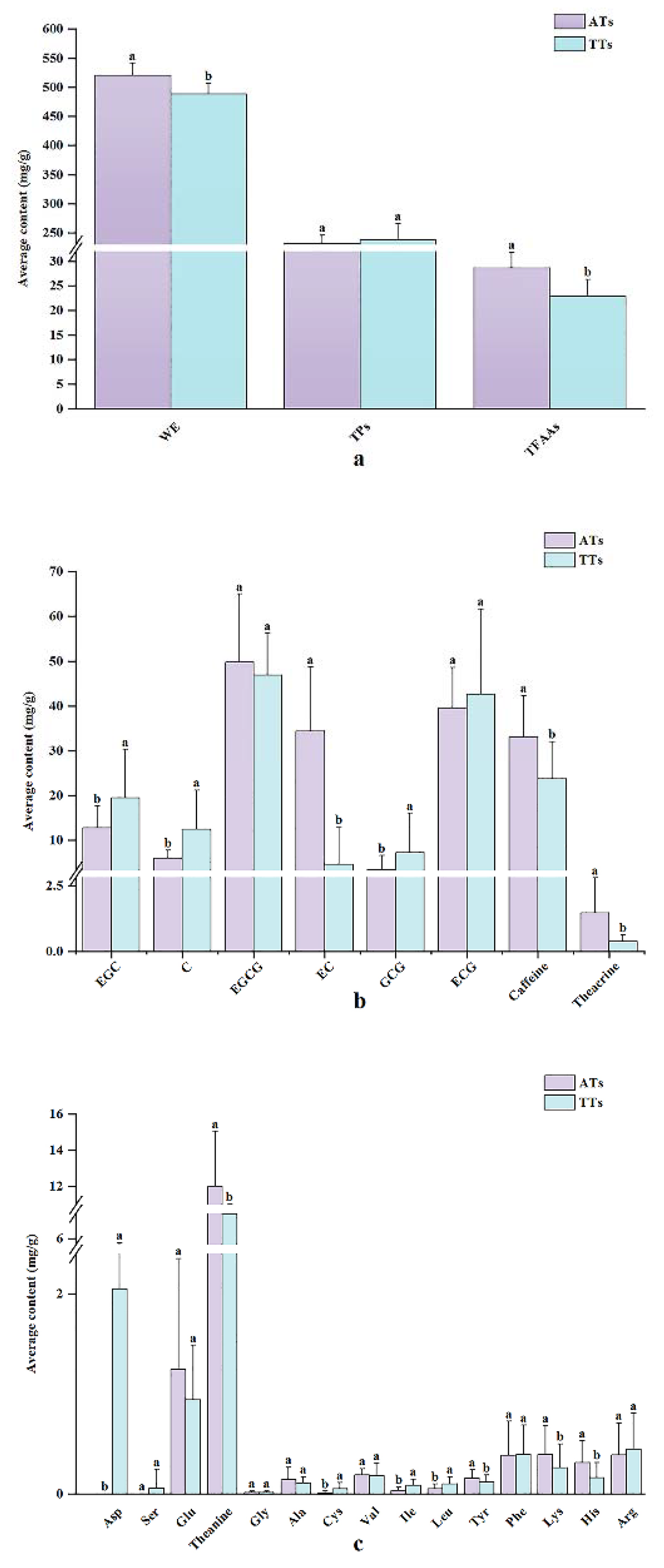
3.2. Comparison of Phytochemicals Differences Related with a Classification of AT and TT
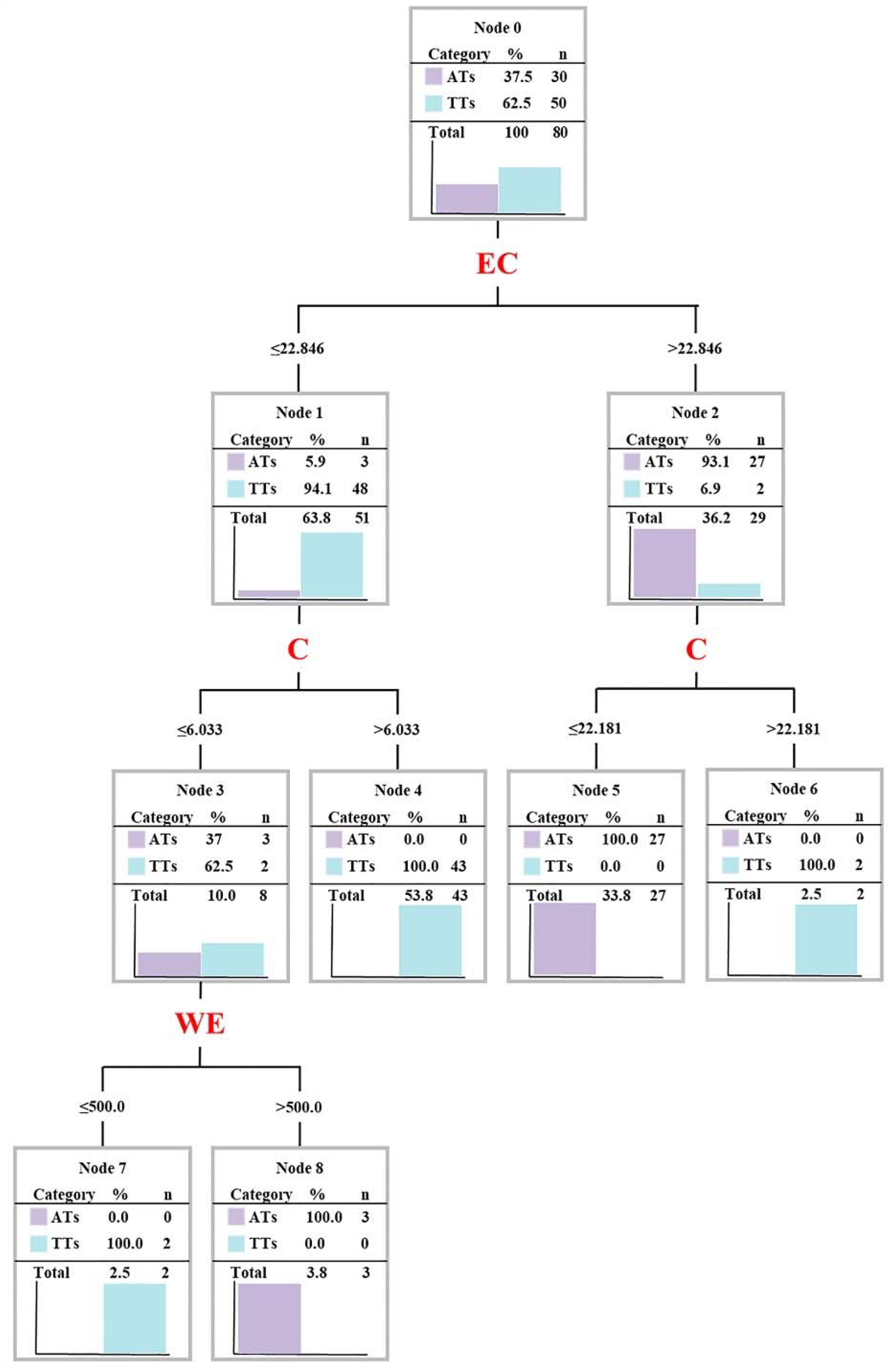
3.3. Establishment and Optimization of Discriminant Model of AT and TT
3.3.1. Establishing a Discriminant Model through SFDA
3.3.2. Optimizing Classification Model through DTA
3.3.3. Evaluating Classification Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; He, F.; Yang, W.; Yang, L.; Huang, S.; Mao, H.; Hou, Y.; Xiao, R. Pu-erh tea extraction alleviates intestinal inflammation in mice with flora disorder by regulating gut microbiota. Food Sci. Nutr. 2021, 9, 4883–4892. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Unachukwu, U.; Stepp, J.R.; Peters, C.M.; Long, C.; Kennelly, E. Pu-erh tea tasting in Yunnan, China: Correlation of drinkers’ perceptions to phytochemistry. J. Ethnopharmacol. 2010, 132, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, D.-H.; Guo, H.-J.; Sheng, C.-Y. Assessment of plant species diversity of ancient tea garden communities in Yunnan, Southwest of China. Agrofor. Syst. 2012, 87, 465–474. [Google Scholar] [CrossRef]
- Bao, X.; Dong, X.; Pan, S. A comparative study between wild ancient tea and tea garden tea. Zhejiang J. Agric. Sci. 2012, 24, 603–608. (In Chinese) [Google Scholar]
- Wang, T.; Li, X.; Yang, H.; Wang, F.; Kong, J.; Qiu, D.; Li, Z. Mass spectrometry-based metabolomics and chemometric analysis of Pu-erh teas of various origins. Food Chem. 2018, 268, 271–278. [Google Scholar] [CrossRef]
- Liang, M.; Xia, L.; Zhang, J.; Fang, C.; Chen, J.; Chen, L.; Sun, R. Study on quality comparison of old plant tea and tableland tea. J. Yunnan Agric. Univ. 2006, 4, 493–497. (In Chinese) [Google Scholar]
- Chowaniak, M.; Niemiec, M.; Zhu, Z.; Rashidov, N.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; Kuboń, M.; Fayzullo, S.; Mahmadyorzoda, U.; et al. Quality Assessment of Wild and Cultivated Green Tea from Different Regions of China. Molecules 2021, 26, 3620. [Google Scholar] [CrossRef]
- Gao, F.; Jiahua, L.I.; Zhao, P. Research and Analysis of Soil Nutrients Status of the Modern Tea Gardens and Ancient Tea Gardens in Pu’er City. J. Yunnan Agric. Univ. 2016, 31, 511–518. (In Chinese) [Google Scholar] [CrossRef]
- Yang, G.; Wang, X.; Xie, J. Analysis of the Relationship between Soil Nutrients and Tea Main Quality Components of Ancient Tea Arboretum and Modern Tea Garden in Yunnan Province. J. Tea Sci. 2015, 35, 574–582. [Google Scholar]
- Lin, X.; Li, Q.; He, L.; Lan, S.; Lin, T.; Liu, H. Application of Heavy Rare Earth Element Fingerprints in Discrimination of Pu’er Old Plant Tea and Tableland Tea. Mod. Food Sci. Technol. 2013, 29, 2921–2925. [Google Scholar]
- Sarker, S.D.; Nahar, L. Applications of High-Performance Liquid Chromatography in the Analysis of Herbal Products. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 405–425. [Google Scholar]
- dos Santos, P.M.; Filho, E.P.; Rodriguez-Saona, L. Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem. 2013, 138, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Tomazzoli, M.M.; Neto, R.D.P.; Moresco, R.; Westphal, L.; Zeggio, A.R.S.; Specht, L.; Costa, C.; Rocha, M.; Maraschin, M. Discrimination of Brazilian propolis according to the seasoning using chemometrics and machine learning based on UV-Vis scanning data. J. Integr. Bioinform. 2015, 12, 15–26. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Kim, J.; Park, H.-J.; Liu, K.-H.; Lee, C.H. Application of Metabolomics in the Analysis of Manufacturing Type of Pu-erh Tea and Composition Changes with Different Postfermentation Year. J. Agric. Food Chem. 2009, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, C.; Wu, T.; Xu, C.; Wang, J.; Xia, T. Classification of raw Pu-erh teas with different storage time based on characteristic compounds and effect of storage environment. LWT 2020, 133, 109914. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, B.; Ma, C.; Xu, C.; Wang, J.; Wang, Z.; Yin, D.; Xia, T. Classification of Pu-erh ripened teas and their differences in chemical constituents and antioxidant capacity. LWT 2021, 153, 112370. [Google Scholar] [CrossRef]
- Sun, H.; Lu, W.; Gao, B. Non-targeted detection of butter adulteration using pointwise UHPLC-ELSD and UHPLC-UV fin-gerprints with chemometrics. Food Chem. 2021, 356, 129604. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Detection of camellia oil adulteration using chemometrics based on fatty acids GC fingerprints and phytosterols GC–MS fingerprints. Food Chem. 2021, 352, 129422. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Wu, M.-C.; Chien, H.-J.; Wang, W.-C.; Kuo, C.-Y.; Lai, C.-C. Honey proteomic signatures for the identification of honey adulterated with syrup, producing country, and nectar source using SWATH-MS approach. Food Chem. 2021, 354, 129590. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV–vis, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2019, 164, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Y.; Zhang, S.; Luo, L.; Zeng, L. Effect of brewing conditions on phytochemicals and sensory profiles of black tea infusions: A primary study on the effects of geraniol and β-ionone on taste perception of black tea infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Yue, C.-N.; Tong, H.-R. Analysis of taste characteristics and identification of key chemical components of fifteen Chinese yellow tea samples. J. Food Sci. Technol. 2020, 58, 1378–1388. [Google Scholar] [CrossRef]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors Affecting the Caffeine and Polyphenol Contents of Black and Green Tea Infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef]
- Magné, C.; Larher, F. High sugar content of extracts interferes with colorimetric determination of amino acids and free proline. Anal. Biochem. 1992, 200, 115–118. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Liao, C.; Chen, L.; Wang, J.; Zeng, L. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chem. 2018, 269, 24–34. [Google Scholar] [CrossRef]
- Li, H.; Fang, K.; Qin, D.; Jiang, X.; Wang, Q.; Pan, C.; Li, B.; Wang, Q.; Wu, H. Comparative Transcriptome Analysis Reveals Putative Genes Responsible for High Theacrine Content in Kucha (Camellia Kucha (Chang et Wang) Chang). Trop. Plant Biol. 2021, 14, 82–92. [Google Scholar] [CrossRef]
- Lu, M.; Han, J.; Zhu, B.; Jia, H.; Yang, T.; Wang, R.; Deng, W.-W.; Zhang, Z.-Z. Significantly increased amino acid accumulation in a novel albino branch of the tea plant (Camellia sinensis). Planta 2018, 249, 363–376. [Google Scholar] [CrossRef]
- Rodríguez, L.C.; Pérez-Castaño, E.; Ruiz-Samblás, C. Quality performance metrics in multivariate classification methods for qualitative analysis. TrAC Trends Anal. Chem. 2016, 80, 612–624. [Google Scholar] [CrossRef]
- Yeung, K.Y.; Ruzzo, W.L. Principal component analysis for clustering gene expression data. Bioinformatics 2001, 17, 763–774. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, O.; Fu, H.; Ouyang, L.; Gong, X.; Meng, P.; Wang, Z.; Dai, M.; Guo, X.; Wang, Y. UPLC–Q-TOF/MS-based untargeted metabolomics coupled with chemometrics approach for Tieguanyin tea with seasonal and year variations. Food Chem. 2019, 283, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, B.; Ma, C.; Zheng, C.; Zhou, B.; Guo, G.; Xia, T. Region identification of Xinyang Maojian tea using UHPLC-Q-TOF/MS-based metabolomics coupled with multivariate statistical analyses. J. Food Sci. 2021, 86, 1681–1691. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.-J.; Ho, C.-T.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2018, 67, 5423–5436. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, J.; Wang, L.; Wu, D.; Qi, D.; Sha, Y.; Liu, B. Identification of Volatile Components in Tea Infusions by Headspace–Programmed Temperature Vaporization–Gas Chromatography–Mass Spectrometry (HS–PTV–GC–MS) with Chemometrics. Anal. Lett. 2020, 53, 1902–1918. [Google Scholar] [CrossRef]
- Ouyang, S.-H.; Zhai, Y.-J.; Wu, Y.-P.; Xie, G.; Wang, G.-E.; Mao, Z.-F.; Hu, H.-H.; Luo, X.-H.; Sun, W.-Y.; Liang, L.; et al. Theacrine, a Potent Antidepressant Purine Alkaloid from a Special Chinese Tea, Promotes Adult Hippocampal Neurogenesis in Stressed Mice. J. Agric. Food Chem. 2021, 69, 7016–7027. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-R.; Kuo, P.-C.; Li, Y.-C.; Jhuo, C.-F.; Hsu, W.-L.; Tzen, J.T. Theacrine and strictinin, two major ingredients for the anti-influenza activity of Yunnan Kucha tea. J. Ethnopharmacol. 2020, 262, 113190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Quickly distinguishing ancient tea from terrace tea. Mod. Agric. 2018, 8, 99. (In Chinese) [Google Scholar]
- Gao, S.-L.; Hu, S.-S.; He, P.; Feng, K.; Pan, R.-Y.; Zhang, S.; Guo, B.; Lee, T.-C.; Lin, J.-K. Effects of reducing chemical fertilizer on the quality components of Tieguanyin tea leaves. IOP Conf. Ser. Earth Environ. Sci. 2020, 559. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Hu, X.; Li, J.; Hu, J.; Liu, R.; Deng, Z.-Y. Effects of Fertilizing with N, P, Se, and Zn on Regulating the Element and Functional Component Contents and Antioxidant Activity of Tea Leaves Planted in Red Soil. J. Agric. Food Chem. 2014, 62, 3823–3830. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Zheng, X.; Zhuo, C.; Hou, Q.; Yao, L.; Wang, X.; Wang, J.; Ni, W. Foliar application of glycinebetaine and Zn fertilizer improves both the apparent and functional qualities of albino tea [Camellia sinensis (L.) O. Kuntze]. Food Funct. 2021. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Liu, J. Enhanced semi-supervised local Fisher discriminant analysis for face recognition. Futur. Gener. Comput. Syst. 2012, 28, 244–253. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ma, X. Study on specificity of colon carcinoma-associated serum markers and establishment of SVM prediction model. Saudi J. Biol. Sci. 2017, 24, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, X.; Jiang, Y.; Caffin, N.; D’Arcy, B.; Singanusong, R.; Xu, Y. Compositional analysis of teas from Australian su-permarkets. Food Chem. 2006, 94, 115–122. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zhou, J.; He, W.; Zeng, J.; Jiang, Y.; Cheng, H. Catechin contents in tea (Camellia sinensis) as affected by cultivar and environment and their relation to chlorophyll contents. Food Chem. 2011, 125, 44–48. [Google Scholar] [CrossRef]
- Wen, B.; Ren, S.; Zhang, Y.; Duan, Y.; Shen, J.; Zhu, X.; Wang, Y.; Ma, Y.; Zou, Z.; Fang, W. Effects of geographic locations and topographical factors on secondary metabolites distribution in green tea at a regional scale. Food Control. 2019, 110, 106979. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia Kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Xu, J.-K.; Kurihara, H.; Zhao, L.; Yao, X.-S. Theacrine, a special purine alkaloid with sedative and hypnotic properties from Cammelia assamica var. kucha in mice. J. Asian Nat. Prod. Res. 2007, 9, 665–672. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zheng, X.; Li, J.; Ye, C.; Song, X. Theacrine, a purine alkaloid with anti-inflammatory and analgesic ac-tivities. Fitoterapia 2010, 81, 627–631. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, S.-R.; Sun, K.; Wang, X.-H.; Jiang, J.-L.; Luo, L.-Y.; Zeng, L. Characterization of bitter taste theacrine in Pu-erh tea. J. Food Compos. Anal. 2021, 106, 104331. [Google Scholar] [CrossRef]

| Raw Materials | Sources | Counts | Codes 1 |
|---|---|---|---|
| AT | Banpen, Laobanzhang, Hekai, Xinbanzhang, Laoman’e | 30 | A1~A30 |
| TT | Nannuo, Bulang, Mensong, Lincang, Lancang, Xiding, Gelanghe, Menla, Dali | 50 | T1~T50 |
| True Classification 1 | |||
|---|---|---|---|
| Type 1 | Type 2 | ||
| Predictive classification | Type 1 | TP | TN |
| Type 2 | FP | FN | |
| PCs | Eigenvalue | Percentage of Variance (%) | Cumulative Percentage of Variance (%) |
|---|---|---|---|
| PC1 | 6.381 | 24.542 | 24.542 |
| PC2 | 5.030 | 19.348 | 43.889 |
| PC3 | 2.928 | 11.261 | 55.151 |
| PC4 | 2.534 | 9.745 | 64.896 |
| PC5 | 1.745 | 6.711 | 71.607 |
| PC6 | 1.205 | 4.635 | 76.242 |
| PC7 | 1.069 | 4.110 | 80.352 |
| Numbers | Variables | Statistics | df1 | df2 | Significance |
|---|---|---|---|---|---|
| 1 | EC | 117.094 | 1 | 58 | 1.54 × 10−15 |
| 2 | C | 118.649 | 2 | 57 | 4.81 × 10−21 |
| 3 | Theanine | 106.949 | 3 | 56 | 3.67 × 10−23 |
| 4 | WE | 94.932 | 4 | 55 | 5.10 × 10−24 |
| 5 | EGC | 88.306 | 5 | 54 | 9.73 × 10−25 |
| 6 | Theacrine | 83.408 | 6 | 53 | 3.24 × 10−25 |
| 7 | Ala | 77.816 | 7 | 52 | 2.74 × 10−25 |
| 8 | Arg | 80.700 | 8 | 51 | 3.10 × 10−26 |
| Verification Modes | True Classification | Performance Index (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ATs | TTs | Accuracy | Precision | Recall | F-Score | |||
| Predictive classification | Training set | ATs | 10 | 0 | 100 | 100 | 100 | 100 |
| TTs | 0 | 10 | ||||||
| Model set (LOO) | ATs | 20 | 0 | 100 | 100 | 100 | 100 | |
| TTs | 0 | 40 | ||||||
| DTA | ATs | 30 | 0 | 100 | 100 | 100 | 100 | |
| TTs | 0 | 50 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-R.; Shi, Y.; Jiang, J.-L.; Luo, L.-Y.; Zeng, L. Discriminant Analysis of Pu-Erh Tea of Different Raw Materials Based on Phytochemicals Using Chemometrics. Foods 2022, 11, 680. https://doi.org/10.3390/foods11050680
Zhang S-R, Shi Y, Jiang J-L, Luo L-Y, Zeng L. Discriminant Analysis of Pu-Erh Tea of Different Raw Materials Based on Phytochemicals Using Chemometrics. Foods. 2022; 11(5):680. https://doi.org/10.3390/foods11050680
Chicago/Turabian StyleZhang, Shao-Rong, Yu Shi, Jie-Lin Jiang, Li-Yong Luo, and Liang Zeng. 2022. "Discriminant Analysis of Pu-Erh Tea of Different Raw Materials Based on Phytochemicals Using Chemometrics" Foods 11, no. 5: 680. https://doi.org/10.3390/foods11050680
APA StyleZhang, S.-R., Shi, Y., Jiang, J.-L., Luo, L.-Y., & Zeng, L. (2022). Discriminant Analysis of Pu-Erh Tea of Different Raw Materials Based on Phytochemicals Using Chemometrics. Foods, 11(5), 680. https://doi.org/10.3390/foods11050680






