Optimized Clarification Technology of Bayberry Juice by Chitosan/Sodium Alginate and Changes in Quality Characteristics during Clarification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Processing Methods
2.2.1. Chinese Bayberry Juice
2.2.2. Clarification by Individual Clarifiers
2.2.3. Clarification by Composite Clarifiers
2.3. Sedimentation Time and Final Turbidity Volume
2.4. Determination of Physicochemical Parameters
2.4.1. Protein Determination (Bradford Assay)
2.4.2. Total Soluble Solids and Total Carbohydrate Content
2.4.3. Tannin Content, Total Phenolic Content, Total Flavonoids Content and Total Anthocyanins Content
2.4.4. Pectin Content
2.4.5. Color Analysis
2.5. Determination of Aroma Using GC-MS
2.6. Statistics Data Analysis
3. Results and Discussion
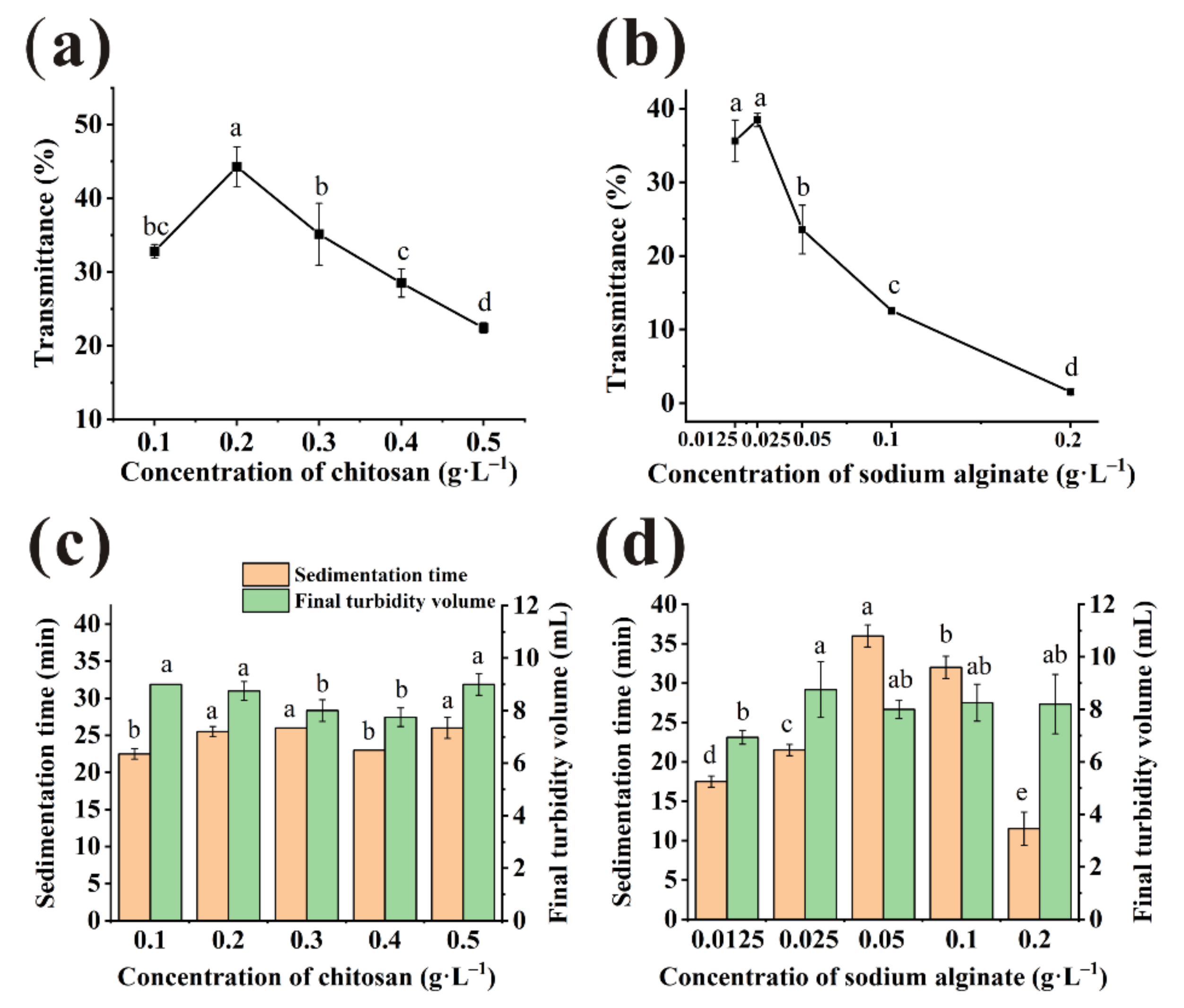
3.1. Effect of Individual Clarification
3.2. Effect of Composite Clarifiers
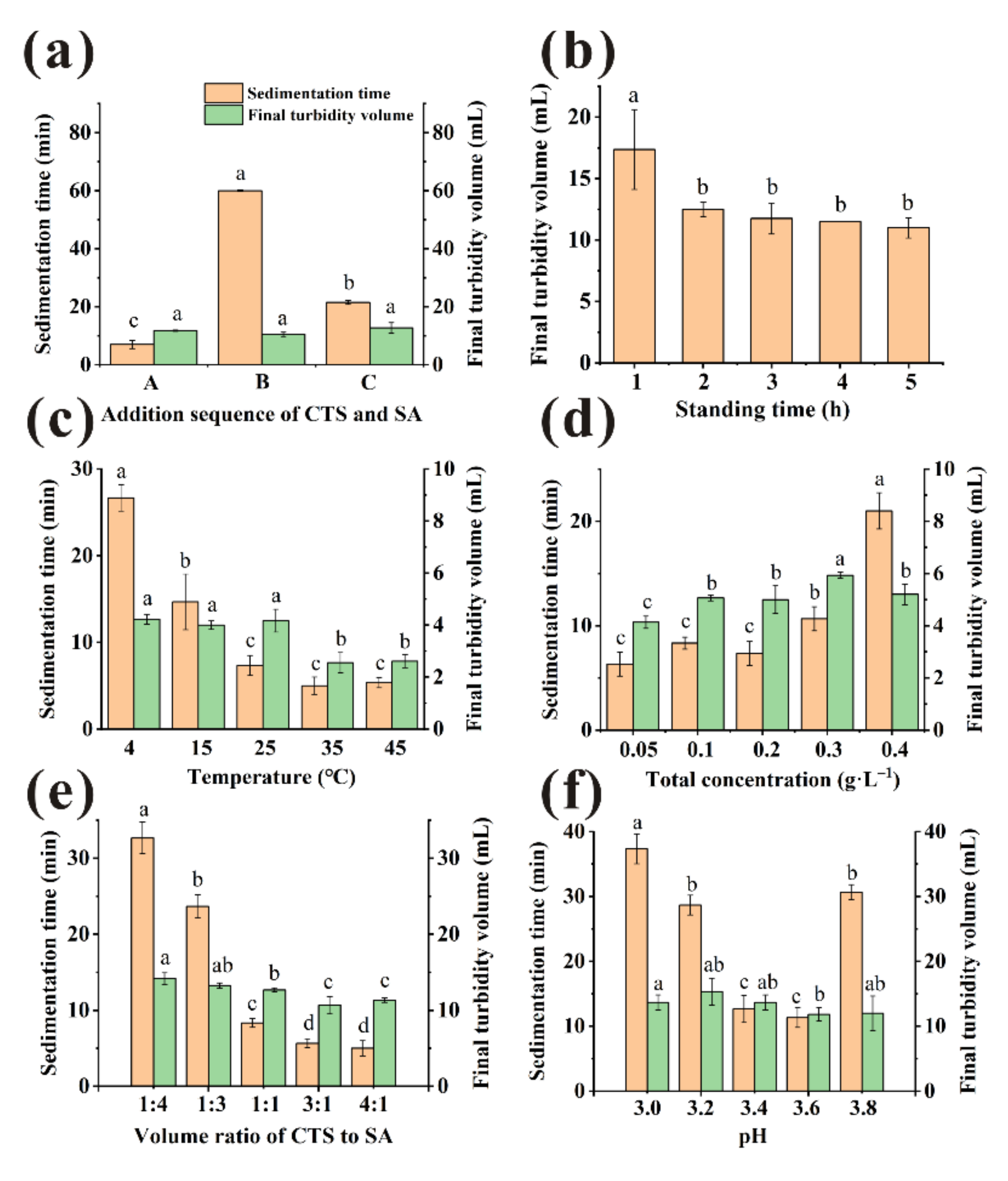
3.2.1. The Adding Sequence of Clarifiers
3.2.2. The Standing Time
3.2.3. The Temperature
3.2.4. The Total Concentration
3.2.5. The Volume Ratio of CTS and SA
3.2.6. The pH
3.2.7. Optimization of Clarification Condition by Orthogonal Experiment
3.3. Physicochemical Parameters
3.3.1. Total Carbohydrate and Soluble Solids Evaluation
3.3.2. Protein and Pectin Contents Analysis
3.3.3. Total Phenolic Content, Total Anthocyanins Content, Total Flavonoids Content, and Tannin Content
3.3.4. Color Analysis
3.4. GC-MS Analysis
3.4.1. Comparison of Volatile Flavor Compounds of Bayberry Juice Clarified with Different Methods
3.4.2. Determination of Key Odor Compounds Using ROAV Analysis
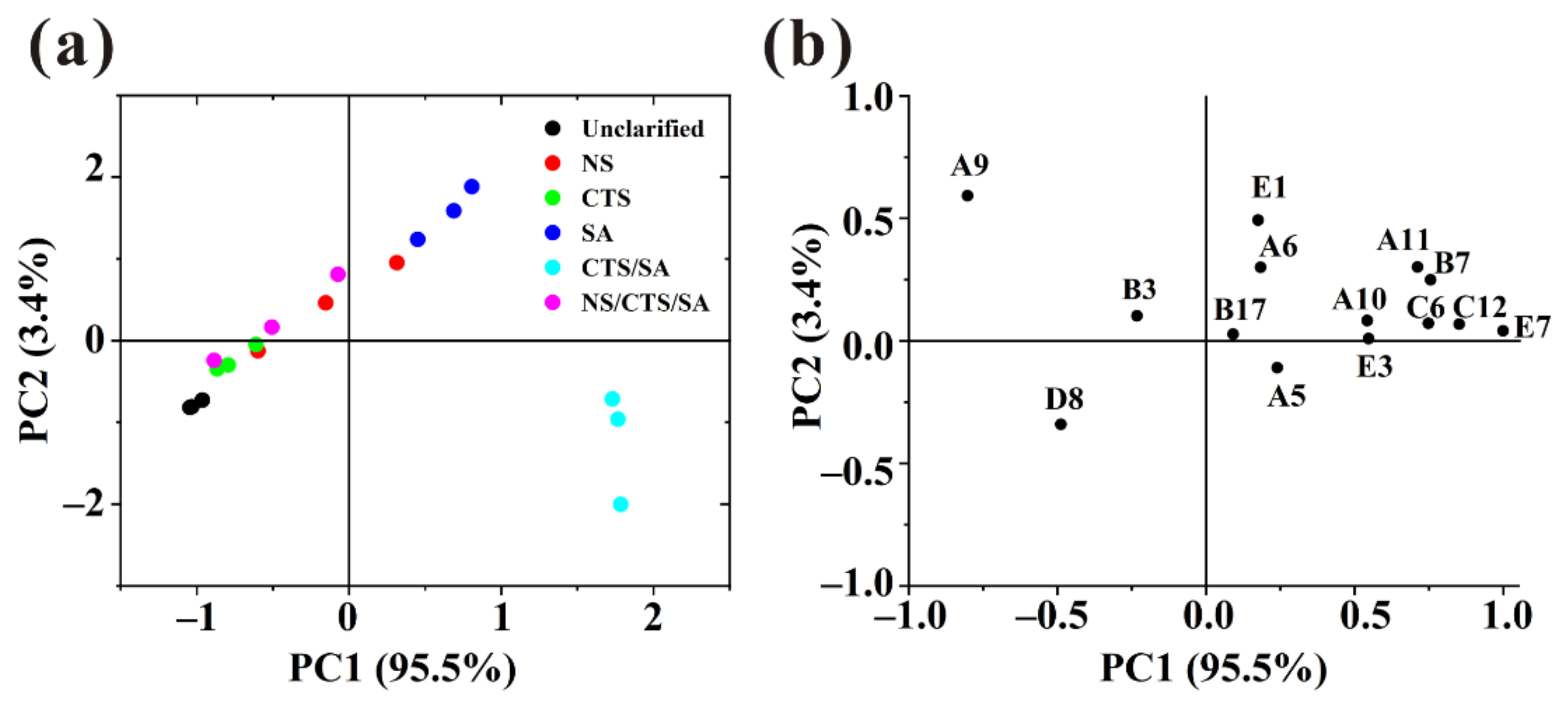
3.4.3. Principal Component Analysis (PCA) of Key Odor Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Shen, C.; Rao, J.; Wu, Q.; Wu, D.; Chen, K. The effect of indirect plasma-processed air pretreatment on the microbial loads, decay, and metabolites of Chinese bayberries. LWT 2021, 150, 111998. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Kalisz, S. Effects of various polysaccharide clarification agents and reaction time on content of polyphenolic compound, antioxidant activity, turbidity and colour of chokeberry juice. LWT 2018, 92, 347–360. [Google Scholar] [CrossRef]
- Pinelo, M.; Zeuner, B.; Meyer, A.S. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food Bioprod. Process. 2010, 88, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Tastan, O.; Baysal, T. Clarification of pomegranate juice with chitosan: Changes on quality characteristics during storage. Food Chem. 2015, 180, 211–218. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Ye, X.; Liu, N.; Chen, J. Turbidity, antioxidant compounds, color, and dynamics of clarification of bayberry juice using various polysaccharide-based clarifying agents. J. Food Process. Preserv. 2019, 43, e13980. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, W.; Liu, F.; Chen, R. Removal of difenoconazole and nitenpyram by composite calcium alginate beads during apple juice clarification. Chemosphere 2021, 286, 131813. [Google Scholar] [CrossRef]
- Diao, Y.; Yu, X.; Zhang, C.; Jing, Y. Optimisation of the clarification of kiwifruit juice with tannic acid-modified chitosan. Czech J. Food Sci. 2021, 39, 189–196. [Google Scholar] [CrossRef]
- Konovalova, I.N.; Stepanova, N.V.; Vasilevskii, P.B.; Bereza, I.G. Intensification of the Flotation Treatment of Water with Natural Polyelectrolytes. Russ. J. Appl. Chem. 2001, 74, 449–454. [Google Scholar] [CrossRef]
- Qin, Y.; Cai, L.; Feng, D.; Shi, B.; Liu, J.; Zhang, W.; Shen, Y. Combined use of chitosan and alginate in the treatment of wastewater. J. Appl. Polym. Sci. 2007, 104, 3581–3587. [Google Scholar] [CrossRef]
- Lv, J.; Meng, Y.; Shi, Y.; Li, Y.; Chen, J.; Sheng, F. Properties of epsilon-polylysine·HCl/high-methoxyl pectin polyelectrolyte complexes and their commercial application. J. Food Process. Preserv. 2019, 44, e14320. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J. Hazard. Mater. 2021, 403, 124054. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Sudha, P.N.; Anil, S. Removal of the heavy metal ion chromiuim(VI) using Chitosan and Alginate nanocomposites. Int. J. Biol. Macromol. 2017, 104, 1459–1468. [Google Scholar] [CrossRef]
- Vacio-Muro, K.J.; Lozano-Álvarez, J.A.; Sánchez-González, M.N.; Vela, N.A.C.; Torres-Ramírez, E.; Jáuregui-Rincón, J. Remoción de contaminantes del nejayote con alginato y quitosano. Rev. Int. Contam. Ambient. 2020, 36, 497–515. [Google Scholar] [CrossRef]
- Matter, I.A.; Darwesh, O.M.; Eida, M.F. Harvesting of microalgae Scenedesmus obliquus using chitosan-alginate dual flocculation system. Biosci. Res. 2018, 15, 540–548. [Google Scholar]
- Son, Y.-J.; Hwang, I.-K.; Nho, C.; Kim, S.; Kim, S. Determination of Carbohydrate Composition in Mealworm (Tenebrio molitor L.) Larvae and Characterization of Mealworm Chitin and Chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, L.; Han, X.; Huang, X.; Ding, X. Optimization of Simultaneous Ultrasonic-Assisted Extraction of Total Flavonoids and Tannins from Mulberry Leaves. Food Sci. 2015, 36, 44–48. [Google Scholar]
- Capistrán-Carabarin, A.; Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods 2019, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Chen, J.; Chen, S.; Xia, Q.; Liu, D.; Ye, X. Sensory evaluation, physicochemical properties and aroma-active profiles in a diverse collection of Chinese bayberry (Myrica rubra) cultivars. Food Chem. 2016, 212, 374–385. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Han, B.; Wu, W.; Zhao, Q.; Wei, C.; Liu, W. Comprehensive analysis of volatile compounds in cold-pressed safflower seed oil from Xinjiang, China. Food Sci. Nutr. 2019, 8, 903–914. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, S.; Wang, S.; Xue, Y.; Jiang, L.; Liu, L. Optimization of Protein Removal from Soybean Whey Wastewater Using Chitosan Ultrafiltration. J. Food Process Eng. 2016, 40, e12370. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, H.; Zhou, W.; Liu, Y.; Chen, P.; Ruan, R. Enhanced Harvesting of Chlorella vulgaris Using Combined Flocculants. Appl. Biochem. Biotechnol. 2016, 180, 791–804. [Google Scholar] [CrossRef]
- Sun, J.; Qin, L.; Li, G.; Kang, Y. Effect of hydraulic conditions on flocculation performances and floc characteristics in Chinese herbal extracts by chitosan and chitosan hydrochloride. Chem. Eng. J. 2013, 225, 641–649. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; He, J.; Li, Q. Novel strategy for controlling colloidal instability during the flocculation pretreatment of landfill leachate. Chemosphere 2021, 287, 132051. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Zhang, M.; Fang, Z.X.; Sun, J.C.; Wang, Y.Q. How to improve bayberry (Myrica rubra Sieb. et Zucc.) juice flavour quality: Effect of juice processing and storage on volatile compounds. Food Chem. 2014, 151, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, Y.; Zhan, Y.; He, J.; Zhu, S. Effect of high pressure processing on the stability of anthocyanin, ascorbic acid and color of Chinese bayberry juice during storage. J. Food Eng. 2013, 119, 701–706. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Fu, R.-J.; Yao, K.; Jia, D.-Y.; He, Q.; Chi, Y.-L. Clarification effect of collagen hydrolysate clarifier on chrysanthemum beverage. LWT 2018, 91, 70–76. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Simultaneous adsorption for cationic and anionic dyes using chitosan/electrospun sodium alginate nanofiber composite sponges. Carbohydr. Polym. 2022, 276, 118728. [Google Scholar] [CrossRef]
- Devi, M.P.; Sekar, M.; Chamundeswari, M.; Moorthy, A.; Krithiga, G.; Murugan, N.S.; Sastry, T.P. A novel wound dressing material—fibrin–chitosan–sodium alginate composite sheet. Bull. Mater. Sci. 2012, 35, 1157–1163. [Google Scholar] [CrossRef] [Green Version]
- Guibal, E.; Van Vooren, M.; Dempsey, B.A.; Roussy, J. A Review of the Use of Chitosan for the Removal of Particulate and Dissolved Contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Zeng, D.; Xiao, G.; Xu, Y.; Zou, B.; Wu, J.; Yu, Y. Protein and polyphenols involved in sediment formation in cloudy litchi juice. Food Sci. Biotechnol. 2019, 28, 945–953. [Google Scholar] [CrossRef]
- Belgheisi, S.; Kenari, R.E. Improving the qualitative indicators of apple juice by Chitosan and ultrasound. Food Sci. Nutr. 2019, 7, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Dai, J.; Wei, X.; Zhong, Y.; Liu, X.; Guo, D.; Wang, L.; Huang, Y.; Zhang, C.; Liu, Y.; et al. Characterization of recombinant GRIP32 as a novel haze protein for protein-polyphenol haze models and prevention of haze formation with polysaccharides in the models. LWT 2020, 136, 110317. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Du, A.W.; Sun, J. Effect of Fining and Filtration on the Haze Formation in Bayberry (Myrica rubra Sieb. et Zucc.) Juice. J. Agric. Food Chem. 2007, 55, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Erkan-Koç, B.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Effects of various protein- and polysaccharide-based clarification agents on antioxidative compounds and colour of pomegranate juice. Food Chem. 2015, 184, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, B.; Wang, S.; Guo, K.; Shen, X.; Yue, Q.; Xu, X. Synthesis, characterization and flocculation performance of a novel sodium alginate-based flocculant. Carbohydr. Polym. 2020, 248, 116790. [Google Scholar] [CrossRef]
- Campbell, J.; Sarkhosh, A.; Habibi, F.; Gajjar, P.; Ismail, A.; Tsolova, V.; El-Sharkawy, I. Evaluation of Biochemical Juice Attributes and Color-Related Traits in Muscadine Grape Population. Foods 2021, 10, 1101. [Google Scholar] [CrossRef]
- Schmidt, H.D.O.; Rockett, F.C.; Ebert, G.; Sartori, G.V.; Rezzadori, K.; Rodrigues, R.C.; Rios, A.D.O.; Manfroi, V. Effect of enzymatic treatments and microfiltration on the physicochemical quality parameters of feijoa (Acca sellowiana) juice. Int. J. Food Sci. Technol. 2021, 56, 4983–4994. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; He, L.; Xie, J.; Chen, C.; Tian, H. Characterization of aroma compounds in bayberry juice by sensory evaluation and gas chromatography–mass spectrometry. J. Food Meas. Charact. 2020, 14, 505–513. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Characterization of aroma-active volatiles in three Chinese bayberry (Myrica rubra) cultivars using GC–MS–olfactometry and an electronic nose combined with principal component analysis. Food Res. Int. 2015, 72, 8–15. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, K.; Ni, H.; Li, T.; Li, L.J.; Li, Q.B.; Chen, F. Aroma enhancement of instant green tea infusion using β-glucosidase and β-xylosidase. Food Chem. 2020, 315, 126287. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Effect of fat content on aroma generation during processing of dry fermented sausages. Meat Sci. 2011, 87, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Sellami, I.; Mall, V.; Schieberle, P. Changes in the Key Odorants and Aroma Profiles of Hamlin and Valencia Orange Juices Not from Concentrate (NFC) during Chilled Storage. J. Agric. Food Chem. 2018, 66, 7428–7440. [Google Scholar] [CrossRef]
- Sun, B.; Chen, H. Edible Flavoring Method, 3rd ed.; Chemical Industry Press: Beijing, China, 2003; pp. 28–40. [Google Scholar]

| Experimental Number | Volume Ratios of CTS to SA(A) | Total Concentration(B)/g·L−1 | Incubation Temperature(C)/°C | Transmittance/% |
|---|---|---|---|---|
| 1 | 3 (3:1) | 3 (0.2) | 1 (15) | 25.17 ± 1.80 |
| 2 | 1 (1:3) | 2 (0.1) | 3 (35) | 38.37 ± 3.72 |
| 3 | 3 | 1 (0.05) | 3 | 12.06 ± 1.25 |
| 4 | 1 | 3 | 2 (25) | 49.79 ± 0.87 |
| 5 | 2 (1:1) | 3 | 3 | 85.47 ± 0.68 |
| 6 | 3 | 2 | 2 | 41.76 ± 1.76 |
| 7 | 2 | 2 | 1 | 83.70 ± 1.02 |
| 8 | 2 | 1 | 2 | 76.23 ± 0.15 |
| 9 | 1 | 1 | 1 | 58.54 ± 2.25 |
| K1 | 146.71 | 146.84 | 167.42 | |
| K2 | 245.41 | 163.84 | 167.79 | |
| K3 | 78.99 | 160.43 | 135.90 | |
| k1 | 48.90 | 48.95 | 55.81 | |
| k2 | 81.80 | 54.61 | 55.93 | |
| k3 | 26.33 | 53.48 | 45.30 | |
| R | 55.47 | 5.67 | 10.63 |
| Samples | Soluble Solids (°Brix) | Total Carbohydrate (mg/mL) | Protein Content (mg/L) | Pectin Content (mg/L) |
|---|---|---|---|---|
| Unclarified | 7.93 ± 0.06 a | 93.48 ± 2.42 a | 478.03 ± 16.08 a | 198.48 ± 3.11 a |
| NS | 7.77 ± 0.06 b | 84.11 ± 1.05 b | 325.04 ± 3.91 b | 179.78 ± 14.00 b |
| CTS | 7.80 ± 0.00 b | 84.81 ± 3.17 b | 138.25 ± 1.09 c | 46.67 ± 7.62 d |
| SA | 7.60 ± 0.00 c | 93.23 ± 2.84 a | 71.12 ± 1.20 d | 70.21 ± 6.02 d |
| CTS/SA | 7.60 ± 0.00 c | 84.85 ± 0.30 b | 9.06 ± 11.08 e | 27.96 ± 1.56 e |
| NS/CTS/SA | 7.60 ± 0.00 c | 88.76 ± 1.20 ab | 76.4 ± 10.02 d | 56.57 ± 4.67 d |
| Samples | Total Phenolic Content (mg/L) | Total Anthocyanins Content (mg/L) | Total Flavonoids Content (mg/L) | Tannin Content (mg/L) |
|---|---|---|---|---|
| Unclarified | 868.94 ± 37.62 a | 85.36 ± 2.99 a | 556.40 ± 11.90 a | 194.78 ± 3.83 a |
| NS | 726.48 ± 6.81 b | 80.37 ± 3.04 b | 445.21 ± 12.98 b | 158.41 ± 17.34 b |
| CTS | 481.00 ± 5.36 c | 75.49 ± 0.21 c | 156.75 ± 6.94 c | 109.33 ± 2.09 c |
| SA | 470.89 ± 3.65 c | 69.30 ± 1.77 d | 133.79 ± 4.35 d | 113.52 ± 25.42 c |
| CTS/SA | 467.09 ± 1.95 c | 77.54 ± 0.99 bc | 124.36 ± 7.51 d | 87.99 ± 16.90 c |
| NS/CTS/SA | 465.93 ± 2.59 c | 70.10 ± 0.80 d | 92.35 ± 12.56 e | 84.71 ± 10.87 c |
| Samples | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Unclarified | 30.65 ± 0.03 e | 40.44 ± 0.026 d | 15.37 ± 0.04 b | – |
| NS | 35.06 ± 0.09 d | 41.46 ± 0.04 c | 17.40 ± 0.06 a | 4.96 ± 0.05 d |
| CTS | 42.21 ± 0.11 c | 43.45 ± 0.04 a | 12.77 ± 0.18 d | 12.23 ± 0.14 c |
| SA | 45.80 ± 0.69 b | 40.86 ± 0.75 d | 14.01 ± 0.80 c | 15.24 ± 0.73 b |
| CTS/SA | 50.26 ± 0.11 a | 42.56 ± 0.13 b | 12.42 ± 0.13 de | 19.94 ± 0.11 a |
| NS/CTS/SA | 50.61 ± 0.24 a | 41.60 ± 0.20 c | 12.03 ± 0.22 e | 20.27 ± 0.26 a |
| ROAV | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serial Number | Compound | Odor Descriptor a | Aroma Threshold b (μg/kg) | Unclarified | NS | CTS | SA | CTS/SA | NS/ CTS/SA |
| A5 | Nonanal | sweet, green, fruity | 1 | 0.296 | 0.218 | 0.200 | 0.293 | 0.384 | 0.393 |
| A6 | (E)-2-Octenal | fatty | 3 | 0.329 | 0.508 | 0.688 | 0.552 | 0.482 | 0.365 |
| A9 | (E)-2-Nonenal | green, cucumber | 0.08 | 100.000 | 100.000 | 100.000 | 100.000 | 81.678 | 100.000 |
| A10 | 2,4-Nonadienal | deep-fried | 0.05 | 1.901 | 1.903 | 1.927 | 2.881 | 2.989 | 2.168 |
| A11 | (E,E)-2,4-Nonadienal | fatty, pungent | 0.09 | 0.000 | 4.251 | 5.110 | 8.702 | 9.582 | 7.055 |
| B3 | 2-Heptanol | mushroom, earthy | 0.07 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.241 |
| B7 | 3,7-Dimethyl-1,6-Octadien-3-ol | flowery | 6 | 0.044 | 0.061 | 0.078 | 0.112 | 0.117 | 0.088 |
| B17 | (E,Z)-3,6-Nonadien-1-ol | green, fresh | 3 | 0.086 | 0.051 | 0.046 | 0.119 | 0.121 | 0.139 |
| C6 | Hexanoic acid, ethyl ester | fruity, cucumber | 1 | 0.044 | 0.073 | 0.000 | 0.099 | 0.125 | 0.052 |
| C12 | Dihydro-5-pentyl-2(3H)-furanone | sweet, spice | 25 | 0.045 | 0.116 | 0.098 | 0.180 | 0.251 | 0.170 |
| D8 | Caryophyllene | woody | 64 | 0.147 | 0.026 | 0.004 | 0.007 | 0.004 | 0.006 |
| E1 | 1-Penten-3-one | pungent, train oil-like | 1 | 0.132 | 0.123 | 0.166 | 0.196 | 0.136 | 0.132 |
| E3 | 2-Pentylfuran | fruity, green | 6 | 0.025 | 0.044 | 0.130 | 0.110 | 0.135 | 0.086 |
| E7 | beta-Damascenone | sweet, green | 0.002 | 24.85 | 32.31 | 43.351 | 77.321 | 100.000 | 49.250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Lv, J.; Ju, C.; Wang, Y.; Zhu, Y.; Chen, J. Optimized Clarification Technology of Bayberry Juice by Chitosan/Sodium Alginate and Changes in Quality Characteristics during Clarification. Foods 2022, 11, 671. https://doi.org/10.3390/foods11050671
Wu A, Lv J, Ju C, Wang Y, Zhu Y, Chen J. Optimized Clarification Technology of Bayberry Juice by Chitosan/Sodium Alginate and Changes in Quality Characteristics during Clarification. Foods. 2022; 11(5):671. https://doi.org/10.3390/foods11050671
Chicago/Turabian StyleWu, Andi, Jimin Lv, Changxin Ju, Yiwen Wang, Yanyun Zhu, and Jianchu Chen. 2022. "Optimized Clarification Technology of Bayberry Juice by Chitosan/Sodium Alginate and Changes in Quality Characteristics during Clarification" Foods 11, no. 5: 671. https://doi.org/10.3390/foods11050671
APA StyleWu, A., Lv, J., Ju, C., Wang, Y., Zhu, Y., & Chen, J. (2022). Optimized Clarification Technology of Bayberry Juice by Chitosan/Sodium Alginate and Changes in Quality Characteristics during Clarification. Foods, 11(5), 671. https://doi.org/10.3390/foods11050671






