Agavins Impact on Gastrointestinal Tolerability-Related Symptoms during a Five-Week Dose-Escalation Intervention in Lean and Obese Mexican Adults: Exploratory Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
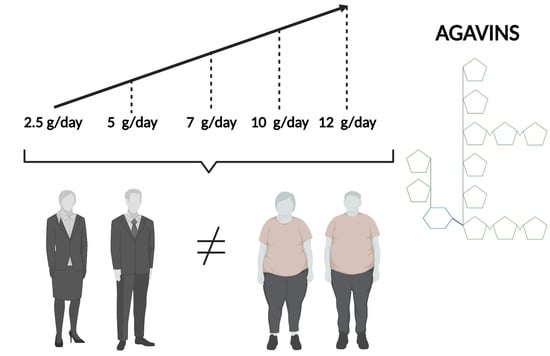
2.2. Experimental Design
2.3. Tolerability-Related Gastrointestinal Symptoms, Appetite and Satiety Subjective Assessment
2.4. Body Weight and Body Composition Determination
2.5. Metabolic Markers Assessment
2.6. Statistical Analysis
3. Results
3.1. Tolerability-Related GI Symptoms, and Subjective Appetite and Satiety Ratings
3.2. Quantification of Metabolic Markers and Body Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 November 2020).
- GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 77, 13–27. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. In Brief to the State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020; pp. 17–18. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.A.; Ordovas, J.M.; Martínez, J.A. Contribution of macronutrients to obesity: Implications for precision nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320. [Google Scholar] [CrossRef]
- Cani, P.D. Severe obesity and gut microbiota: Does bariatric surgery really reset the system? Gut 2019, 68, 5–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deehan, E.C.; Walter, J. The fiber gap and the disappearing gut microbiome: Implications for human nutrition. Trends Endocrinol. Metab. 2016, 27, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Mancilla-Margalli, N.A.; López, M.G. Water-soluble carbohydrates and fructans structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef]
- Huazano-García, A.; López, M.G. Agavins reverse the metabolic disorders in overweight mice through the increment of short chain fatty acids and hormones. Food Funct. 2015, 6, 3720–3727. [Google Scholar] [CrossRef]
- Huazano-García, A.; Shin, H.; López, M.G. Modulation of gut microbiota of overweight mice by agavins and their association with body weight loss. Nutrients 2017, 9, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huazano-García, A.; Silva-Adame, M.B.; Vázquez-Martínez, J.; Gastelum-Arellanez, A.; Sánchez-Segura, L.; López, M.G. Highly-branched neo-fructans (agavins) attenuate metabolic endotoxemia and low-grade inflammation in association with gut microbiota modulation on high-fat diet-fed mice. Foods 2020, 9, 1792. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D.; Doligale, J.L.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Gastrointestinal tolerance and utilization of agave inulin by healthy adults. Food Funct. 2014, 5, 1142–1149. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.E.; Parnell, J.A.; Eksteen, B.; Raman, M.; Bomhof, M.R.; Rioux, K.P.; Madsen, K.L.; Reimer, R.A. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: A randomized controlled trial protocol. BMC Gastroenterol. 2015, 15, 169. [Google Scholar] [CrossRef] [Green Version]
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Windey, K.; Verbeke, K.; Broekaert, W.F. Tolerance and the effect of high doses of wheat bran extract, containing arabinoxylan-oligosaccharides, and oligofructose on faecal output: A double-blind, randomized, placebo-controlled, cross-over trial. J. Nutr. Sci. 2014, 3, e49. [Google Scholar] [CrossRef] [Green Version]
- Klosterbuer, A.S.; Hullar, M.A.J.; Li, F.; Traylor, E.; Lampe, J.W.; Thomas, W.; Slavin, J.L. Gastrointestinal effects of resistant starch, soluble maize fibre and pullulan in healthy adults. Br. J. Nutr. 2013, 110, 1068–1074. [Google Scholar] [CrossRef]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal tolerance of chicory inulin products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Korczak, R.; Kamil, A.; Fleige, L.; Donovan, S.M.; Slavin, J.L. Dietary fiber and digestive health in children. Nutr. Rev. 2017, 75, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health implications of dietary fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Costabile, A.; Bustillo, A.G.R.; Gibson, G.R. A randomised, double-blind, cross-over study investigating the prebiotic effect of agave fructans in healthy human subjects. J. Nutr. Sci. 2015, 4, e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Márquez-Aguirre, A.L.; Camacho-Ruíz, R.M.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; González-Ávila, M.; Gálvez-Gastélum, F.J.; Díaz-Martínez, N.E.; Ortuño-Sahagún, D. Fructans from Agave tequilana with a lower degree of polymerization prevent weight gain, hyperglycemia and liver steatosis in high-fat diet-induced obese mice. Plant. Foods Hum. Nutr. 2016, 71, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Camberos, E.; Barragán-Álvarez, C.P.; Diaz-Martinez, N.E.; Rathod, V.; Flores-Fernández, J.M. Effects of agave fructans (Agave tequilana Weber var. azul) on body fat and serum lipids in obesity. Plant. Foods Hum. Nutr. 2018, 73, 34–39. [Google Scholar] [CrossRef]
- Stewart, M.L.; Nikhanj, S.D.; Timm, D.A.; Thomas, W.; Slavin, J.L. Evaluation of the effect of four fibers on laxation, gastrointestinal tolerance and serum markers in healthy humans. Ann. Nutr. Metab. 2010, 56, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Hess, J.R.; Birkett, A.M.; Thomas, W.; Slavin, J.L. Effects of short-chain fructooligosaccharides on satiety responses in healthy men and women. Appetite 2011, 56, 128–134. [Google Scholar] [CrossRef]
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Evenepoel, P.; Hamer, H.; Houben, E.; Windey, K.; Welling, G.W.; et al. Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: A double-blind, randomised, placebo-controlled, cross-over trial. Br. J. Nutr. 2012, 108, 2229–2242. [Google Scholar] [CrossRef] [Green Version]
- Azpiroz, F.; Molne, L.; Mendez, S.; Nieto, A.; Manichanh, C.; Mego, A.; Accarino, A.; Santos, J.; Sailer, M.; Theis, S.; et al. Effect of chicory-derived inulin on abdominal sensations and bowel motor function. J. Clin. Gastroenterol. 2017, 51, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azpiroz, F.; Malagelada, J.R. Abdominal bloating. Gastroenterology 2005, 129, 1060–1078. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Damen, B.; Broekaert, W.F.; Verbeke, K.; Delcour, J.A.; Courtin, C.M. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 2016, 7, 167–190. [Google Scholar] [CrossRef]
- Manichanh, C.; Eck, A.; Varela, E.; Roca, J.; Clemente, J.C.; González, A.; Knights, D.; Knight, R.; Estrella, S.; Hernandez, C.; et al. Anal gas evacuation and colonic microbiota in patients with flatulence: Effect of diet. Gut 2014, 63, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, M.T.; Graff, L.A.; Avery, L.; Palatnick, C.; Parnerowski, K.; Targownik, L.E. Gastrointestinal symptoms before and during menses in healthy women. BMC Womens Health 2014, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Judkins, T.C.; Dennis-Wall, J.C.; Sims, S.M.; Colee, J.; Langkamp-Henken, B. Stool frequency and form and gastrointestinal symptoms differ by day of the menstrual cycle in healthy adult women taking oral contraceptives: A prospective observational study. BMC Womens Health 2020, 20, 136. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Lean (BMI a 18.5–24.9 kg/m2) | Obese (BMI ≥ 30 kg/m2) | |
|---|---|---|---|
| Demographics | n | 16 | 22 |
| Male, n (%) | 8 (50) | 6 (27) | |
| Female, n (%) | 8 (50) | 16 (73) | |
| Age (years) | 38. 8 ± 10.4 | 41.4 ± 8.9 | |
| Clinical variables | BMI (kg/m2) | 23.7 ± 1.3 | 33.01 ± 3.5 |
| Bodyweight (kg) | 63.19 ± 8.1 | 86.96 ± 12.7 | |
| Relative fat mass (kg) | 29.47 ± 5.5 | 42.26 ± 5.8 | |
| Absolute fat mass (kg) | 18.50 ± 2.7 | 36.79 ± 7.8 | |
| Fat-free mass (kg) | 44.96 ± 8.1 | 50.17 ± 8.7 | |
| Skeletal muscle mass (kg) | 20.32 ± 4.3 | 23.04 ± 5.1 | |
| Total body water (L) | 32.9 ± 5.7 | 37.15 ± 6.4 | |
| Extracellular water (L) | 13.69 ± 2.2 | 16.42 ± 2.4 | |
| Fasting glucose (mg/dL) | 83.06 ± 7.8 | 84.45 ± 11.7 | |
| Triglycerides (mg/dL) | 134.6 ± 64.1 | 158.1 ± 84.1 | |
| CHOL-T b (mg/dL) | 201.6 ± 38.6 | 175.1 ± 33.1 | |
| HDL-C c (mg/dL) | 51.17 ± 11.2 | 41.36 ± 10.2 | |
| LDL-C d (mg/dL) | 123.8 ± 31.2 | 102.1 ± 22.6 | |
| VLDL-C e (mg/dL) | 26.96 ± 12.8 | 31.64 ± 16.8 |
| L-AG a | L-PL b | O-AG c | O-PL d | |
|---|---|---|---|---|
| Total energy (kcal) | 1367 ± 127.4 | 1286.2 ± 69.9 | 1938 ± 217.2 | 1623.5 ± 175.8 |
| Carbohydrates (%) | 44.7 ± 3.9 | 43 ± 1.9 | 47.1 ± 3.3 | 51.4 ± 4.5 |
| Proteins (%) | 20.3 ± 1.2 | 23 ± 2.7 | 17.1 ± 1.4 | 18.8 ± 1.9 |
| Fat (%) | 35 ± 3.9 | 34.1 ± 2.7 | 35.9 ± 3.3 | 29.8 ± 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Adame, M.B.; Martínez-Alvarado, A.; Martínez-Silva, V.A.; Samaniego-Méndez, V.; López, M.G. Agavins Impact on Gastrointestinal Tolerability-Related Symptoms during a Five-Week Dose-Escalation Intervention in Lean and Obese Mexican Adults: Exploratory Randomized Clinical Trial. Foods 2022, 11, 670. https://doi.org/10.3390/foods11050670
Silva-Adame MB, Martínez-Alvarado A, Martínez-Silva VA, Samaniego-Méndez V, López MG. Agavins Impact on Gastrointestinal Tolerability-Related Symptoms during a Five-Week Dose-Escalation Intervention in Lean and Obese Mexican Adults: Exploratory Randomized Clinical Trial. Foods. 2022; 11(5):670. https://doi.org/10.3390/foods11050670
Chicago/Turabian StyleSilva-Adame, María Blanca, Arlen Martínez-Alvarado, Víctor Armando Martínez-Silva, Virginia Samaniego-Méndez, and Mercedes G. López. 2022. "Agavins Impact on Gastrointestinal Tolerability-Related Symptoms during a Five-Week Dose-Escalation Intervention in Lean and Obese Mexican Adults: Exploratory Randomized Clinical Trial" Foods 11, no. 5: 670. https://doi.org/10.3390/foods11050670
APA StyleSilva-Adame, M. B., Martínez-Alvarado, A., Martínez-Silva, V. A., Samaniego-Méndez, V., & López, M. G. (2022). Agavins Impact on Gastrointestinal Tolerability-Related Symptoms during a Five-Week Dose-Escalation Intervention in Lean and Obese Mexican Adults: Exploratory Randomized Clinical Trial. Foods, 11(5), 670. https://doi.org/10.3390/foods11050670