Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Grape Sampling and In-Field Measurements
2.3. Optical Sensor and Indices
2.4. Chemical Analysis
2.5. Analysis of Total Polyphenols, Anthocyanins, and Flavonoids
2.6. Antioxidant Activity
2.7. HPLC-DAD Anthocyanin Analysis
2.8. Statistical Analysis
3. Results
3.1. Analysis of Ripeness
3.2. Polyphenols and Antioxidant Acitivy
3.3. Anthocyanin Profile
3.4. Changes in Cluster Fluorescence during Maturation
3.5. Relationship between Destructive and Fluorescent Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krstic, M. Growing Quality Grapes to Winery Specification: Quality Measurement and Management Options for Grapegrowers; Winetitles: Adelaide, Australia, 2003. [Google Scholar]
- Nogales-Bueno, J.; Hernández-Hierro, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J. Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: A preliminary approach. Food Chem. 2014, 152, 586–591. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meléndez, E.; Ortiz, M.; Sarabia, L.; Íñiguez, M.; Puras, P. Modelling phenolic and technological maturities of grapes by means of the multivariate relation between organoleptic and physicochemical properties. Anal. Chim. Acta 2013, 761, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Xu, Y.; Simon, J.E.; Welch, C.; Wightman, J.D.; Ferruzzi, M.G.; Ho, L.; Passinetti, G.M.; Wu, Q. Survey of polyphenol constituents in grapes and grape-derived products. J. Agric. Food Chem. 2011, 59, 10586–10593. [Google Scholar] [CrossRef]
- Vinas, P.; Campillo, N.; Martínez-Castillo, N.; Hernández-Córdoba, M. Solid-phase microextraction on-fiber derivatization for the analysis of some polyphenols in wine and grapes using gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 1279–1284. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of Vis/NIR spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. Fresh Prod. 2009, 1, 35–41. [Google Scholar]
- Tuccio, L.; Remorini, D.; Pinelli, P.; Fierini, E.; Tonutti, P.; Scalabrelli, G.; Agati, G. Rapid and non-destructive method to assess in the vineyard grape berry anthocyanins under different seasonal and water conditions. Aust. J. Grape Wine Res. 2011, 17, 181–189. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Malegori, C.; Civelli, R.; Guidetti, R. Wavelength selection with a view to a simplified handheld optical system to estimate grape ripeness. Am. J. Enol. Vitic. 2014, 65, 117–123. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Understanding variability in winegrape production systems 2. Within vineyard variation in quality over several vintages. Aust. J. Grape Wine Res. 2005, 11, 33–42. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Moise, N.; Agati, G.; Latouche, G.; Ghozlen, N.B.; Meyer, S. New portable optical sensors for the assessment of winegrape phenolic maturity based on berry fluorescence. J. Food Compos. Anal. 2008, 21, 650–654. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kopecký, J.; Riederer, M.; Pfündel, E.E. UV screening by phenolics in berries of grapevine (Vitis vinifera). Funct. Plant Biol. 2003, 30, 1177–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Ghozlen, N.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 10, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.A.; Pfündel, E.E. Origins of non-linear and dissimilar relationships between epidermal UV absorbance and UV absorbance of extracted phenolics in leaves of grapevine and barley. Plant Cell Environ. 2005, 28, 580–590. [Google Scholar] [CrossRef]
- Agati, G.; Meyer, S.; Matteini, P.; Cerovic, Z.G. Assessment of anthocyanins in grape (Vitis vinifera L.) berries using a noninvasive chlorophyll fluorescence method. J. Agric. Food Chem. 2007, 55, 1053–1061. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.; Goovaerts, P.; Tardaguila, J. Assessment of the spatial variability of anthocyanins in grapes using a fluorescence sensor: Relationships with vine vigour and yield. Precis. Agric. 2012, 13, 457–472. [Google Scholar] [CrossRef]
- Savi, S.; Poni, S.; Moncalvo, A.; Frioni, T.; Rodschinka, I.; Arata, L.; Gatti, M. Destructive and optical non-destructive grape ripening assessment: Agronomic comparison and cost-benefit analysis. PLoS ONE 2019, 14, e0216421. [Google Scholar] [CrossRef]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Pedrós, R.; Goulas, Y.; Jacquemoud, S.; Louis, J.; Moya, I. FluorMODleaf: A new leaf fluorescence emission model based on the PROSPECT model. Remote Sens. Environ. 2010, 114, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Ben Ghozlen, N.; Moise, N.; Latouche, G.; Martinon, V.; Mercier, L.; Besancon, E.; Cerovic, Z. Assessment of grapevine maturity using a new portable sensor: Non-destructive quantification of anthocyanins. J. Int. Sci. Vigne Vin 2010, 44, 1–8. [Google Scholar]
- Cerovic, Z.G.; Goutouly, J.P.; Hilbert, G.; Destrac-Irvine, A.; Martinon, V.; Moise, N. Mapping winegrape quality attributes using portable fluorescence-based sensors. Frutic 2009, 9, 301–310. [Google Scholar]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J. Proposal of an index for the objective evaluation of the colour of red table grapes. Food Res. Int. 1995, 28, 373–377. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Almela, L.; Muñoz, J.A.; Hidalgo, V.; Carreño, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res. Int. 1998, 31, 667–672. [Google Scholar] [CrossRef]
- Gambacorta, G.; Antonacci, D.; La Gatta, M.; Faccia, M.; La Gatta, B.; Pati, S.; Coletta, A.; La Notte, E. Phenolic composition of Aglianico and Nero di Troia grapes and wines as affected by cover cropping and irrigation. Ital. J. Food Sci. 2011, 23, 381. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Coletta, A.; Trani, A.; Faccia, M.; Punzi, R.; Dipalmo, T.; Crupi, P.; Antonacci, D.; Gambacorta, G. Influence of viticultural practices and winemaking technologies on phenolic composition and sensory characteristics of Negroamaro red wines. Int. J. Food Sci. 2013, 48, 2215–2227. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.M. Analysis of several phenolic compounds with potential antioxidant properties in grape extracts and wines by high-performance liquid chromatography-photodiode array detection without sample preparation. J. Chromatogr. A 2000, 881, 461–469. [Google Scholar] [CrossRef]
- Brar, H.S.; Singh, Z.; Swinny, E. Dynamics of anthocyanin and flavonol profiles in the ‘Crimson Seedless’ grape berry skin during development and ripening. Sci. Hortic. 2008, 117, 349–356. [Google Scholar] [CrossRef] [Green Version]
- De la Cruz, A.A.; Hilbert, G.; Rivière, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.C.; Delrot, S.; Mérillon, J.M. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC–MS and LC–NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Measuring the color of table grapes. Color Res. Appl. 1996, 21, 50–54. [Google Scholar] [CrossRef]
- Crupi, P.; Palattella, D.; Corbo, F.; Clodoveo, M.L.; Masi, G.; Caputo, A.R.; Battista, F.; Tarricone, L. Effect of pre-harvest inactivated yeast treatment on the anthocyanin content and quality of table grapes. Food Chem. 2021, 337, 128006. [Google Scholar] [CrossRef]
- Brouillard, R.; Figueiredo, P.; Elhabiri, M.; Dangles, O. Molecular interactions of phenolic compounds in relation to the colour of fruit and vegetables. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, F.A., Robins, R.J., Eds.; Oxford Science Publications: Oxford, UK, 1997; pp. 29–49. [Google Scholar]
- Pérez-Álvarez, E.P.; Molina, D.I.; Vivaldi, G.A.; García-Esparza, M.J.; Lizama, V.; Álvarez, I. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: I. Water relations, vine performance and grape composition. Agric Water Manag. 2021, 248, 106772. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Mizuno, A.; Kiso, K.; Hashizume, K. Effect of shading on proanthocyanidin biosynthesis in grape berry. J. Jpn. Soc. Hort. Sci. 2007, 76, 112–119. [Google Scholar] [CrossRef] [Green Version]
- De la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Crupi, P.; Coletta, A.; Anna Milella, R.; Perniola, R.; Gasparro, M.; Genghi, R.; Antonacci, D. HPLC-DAD-ESI-MS Analysis of Flavonoid Compounds in 5 Seedless Table Grapes Grown in Apulian Region. J. Food Sci. 2012, 77, C174–C181. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Santalla, M.; Masa, A. Environmental and genetic variation of phenolic compounds in grapes (Vitis vinifera) from northwest Spain. J. Agric. Sci. 2009, 147, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Winemaking-Stabilization, Aging Chemistry and Biochemistry; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Admane, N.; Genovese, F.; Altieri, G.; Tauriello, A.; Trani, A.; Gambacorta, G.; Verrastro, V.; Di Renzo, G.C. Effect of ozone or carbon dioxide pre-treatment during long-term storage of organic table grapes with modified atmosphere packaging. LWT 2018, 98, 170–178. [Google Scholar] [CrossRef]
- Macheix, J.; Fleuriet, A.; Billot, J. The main phenolics of fruits. In Fruit Phenolics, 1st ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 1–103. [Google Scholar]
- Milella, R.A.; Antonacci, D.; Crupi, P.; Incampo, F.; Carrieri, C.; Semeraro, N.; Colucci, M. Skin extracts from 2 Italian table grapes (Italia and Palieri) inhibit tissue factor expression by human blood mononuclear cells. J. Food Sci. 2012, 77, H154–H159. [Google Scholar] [CrossRef]
- Coletta, A.; Berto, S.; Crupi, P.; Cravero, M.C.; Tamborra, P.; Antonacci, D.; Daniele, P.G.; Prenesti, E. Effect of viticulture practices on concentration of polyphenolic compounds and total antioxidant capacity of Southern Italy red wines. Food Chem. 2014, 152, 467–474. [Google Scholar] [CrossRef]
- Heier, A.; Blaas, W.; Droß, A.; Wittkowski, R. Anthocyanin analysis by HPLC/Esi-MS. Am. J. Enol. Vitic. 2002, 53, 78–86. [Google Scholar]
- Ryan, J.M.; Revilla, E. Anthocyanin composition of Cabernet Sauvignon and Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Anthocyanin transformation in Cabernet Sauvignon wine during aging. J. Agric. Food Chem. 2003, 51, 7989–7994. [Google Scholar] [CrossRef]
- Liang, Z.; Owens, C.L.; Zhong, G.Y.; Cheng, L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Faccia, M.; Trani, A.; Gambacorta, G. Application of abscisic acid (S-ABA) and sucrose to improve colour, anthocyanin content and antioxidant activity of cv. Crimson Seedless grape berries. Aust. J. Grape Wine Res. 2015, 21, 18–29. [Google Scholar] [CrossRef]
- Bahar, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Lurie, S.; Lichter, A. Auto-fluorescence for analysis of ripening in Thompson Seedless and colour in Crimson Seedless table grapes. Aust. J. Grape Wine Res. 2012, 18, 353–359. [Google Scholar] [CrossRef]
- Bramley, R.; Le Moigne, M.; Evain, S.; Ouzman, J.; Florin, L.; Fadaili, E.; Hinze, C.; Cerovic, Z. On-the-go sensing of grape berry anthocyanins during commercial harvest: Development and prospects. Aust. J. Grape Wine Res. 2011, 17, 316–326. [Google Scholar] [CrossRef]
- Agati, G.; D’Onofrio, C.; Ducci, E.; Cuzzola, A.; Remorini, D.; Tuccio, L.; Lazzini, F.; Mattii, G. Potential of a multiparametric optical sensor for determining in situ the maturity components of red and white Vitis vinifera wine grapes. J. Agric. Food Chem. 2013, 61, 12211–12218. [Google Scholar] [CrossRef]
- Ferrandino, A.; Pagliarani, C.; Carlomagno, A.; Novello, V.; Schubert, A.; Agati, G. Improved fluorescence-based evaluation of flavonoid in red and white winegrape cultivars. Aust. J. Grape Wine Res. 2017, 23, 207–214. [Google Scholar] [CrossRef]
- Pinelli, P.; Romani, A.; Fierini, E.; Agati, G. Prediction models for assessing anthocyanins in grape berries by fluorescence sensors: Dependence on cultivar, site and growing season. Food Chem. 2018, 244, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre-and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [Green Version]
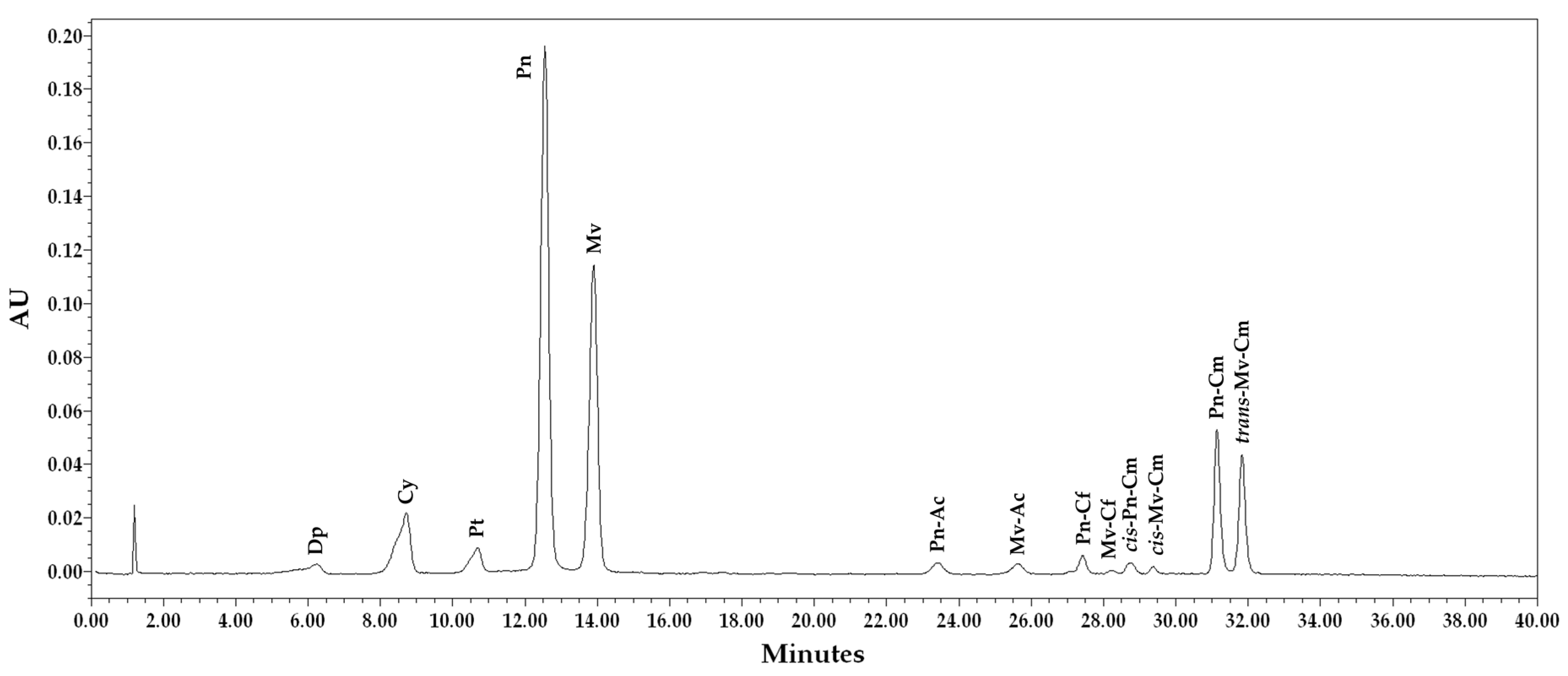
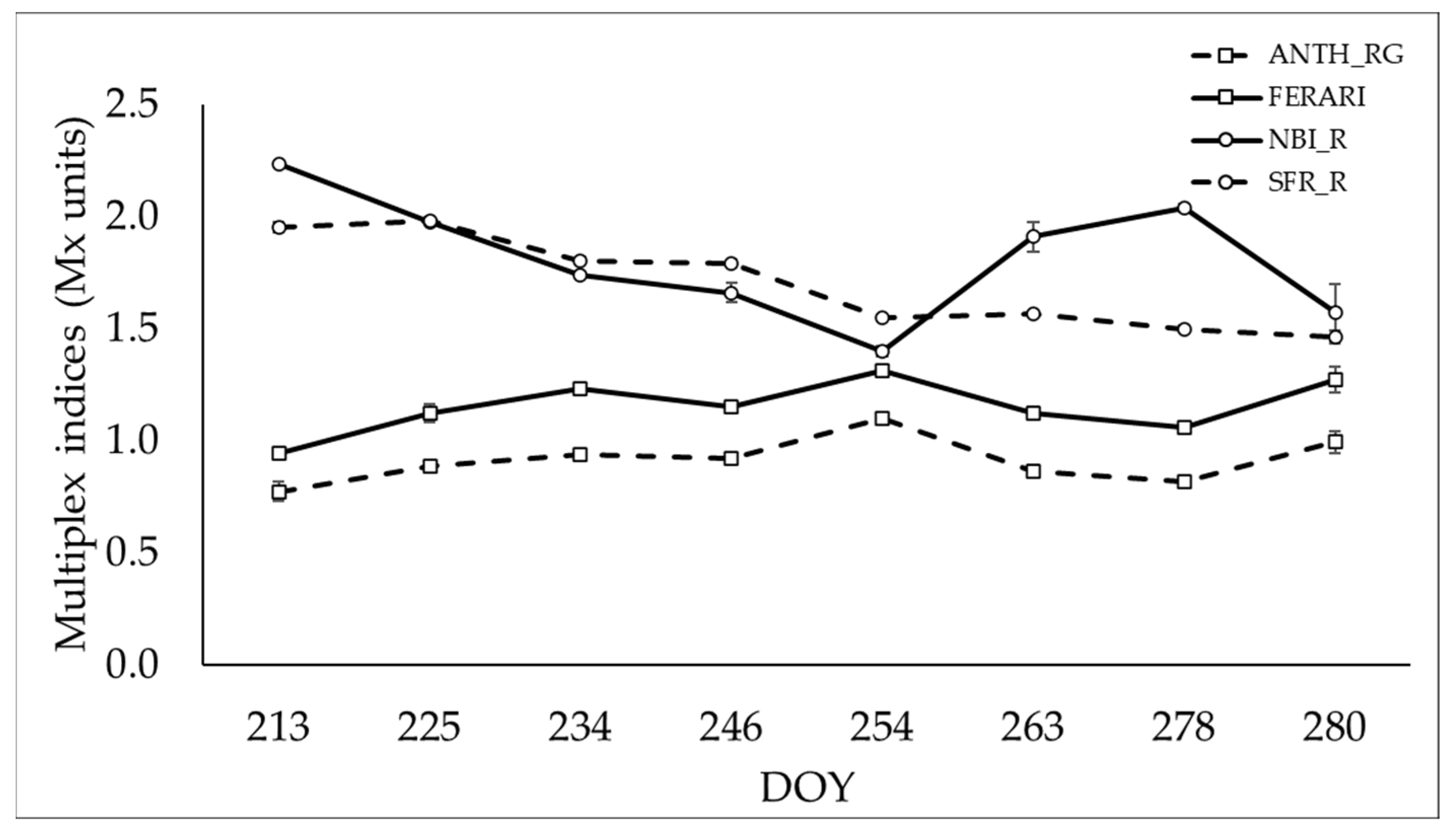
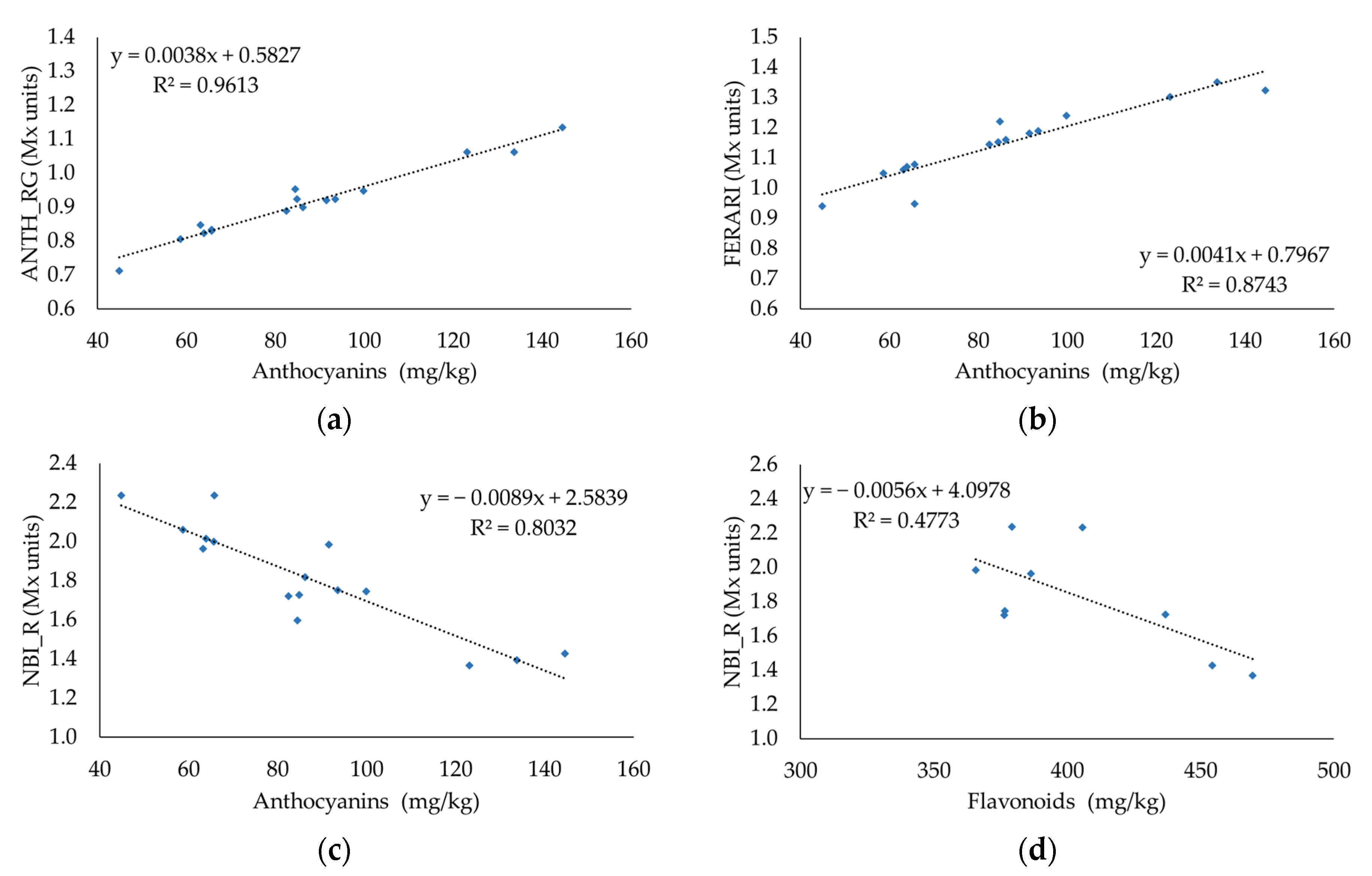
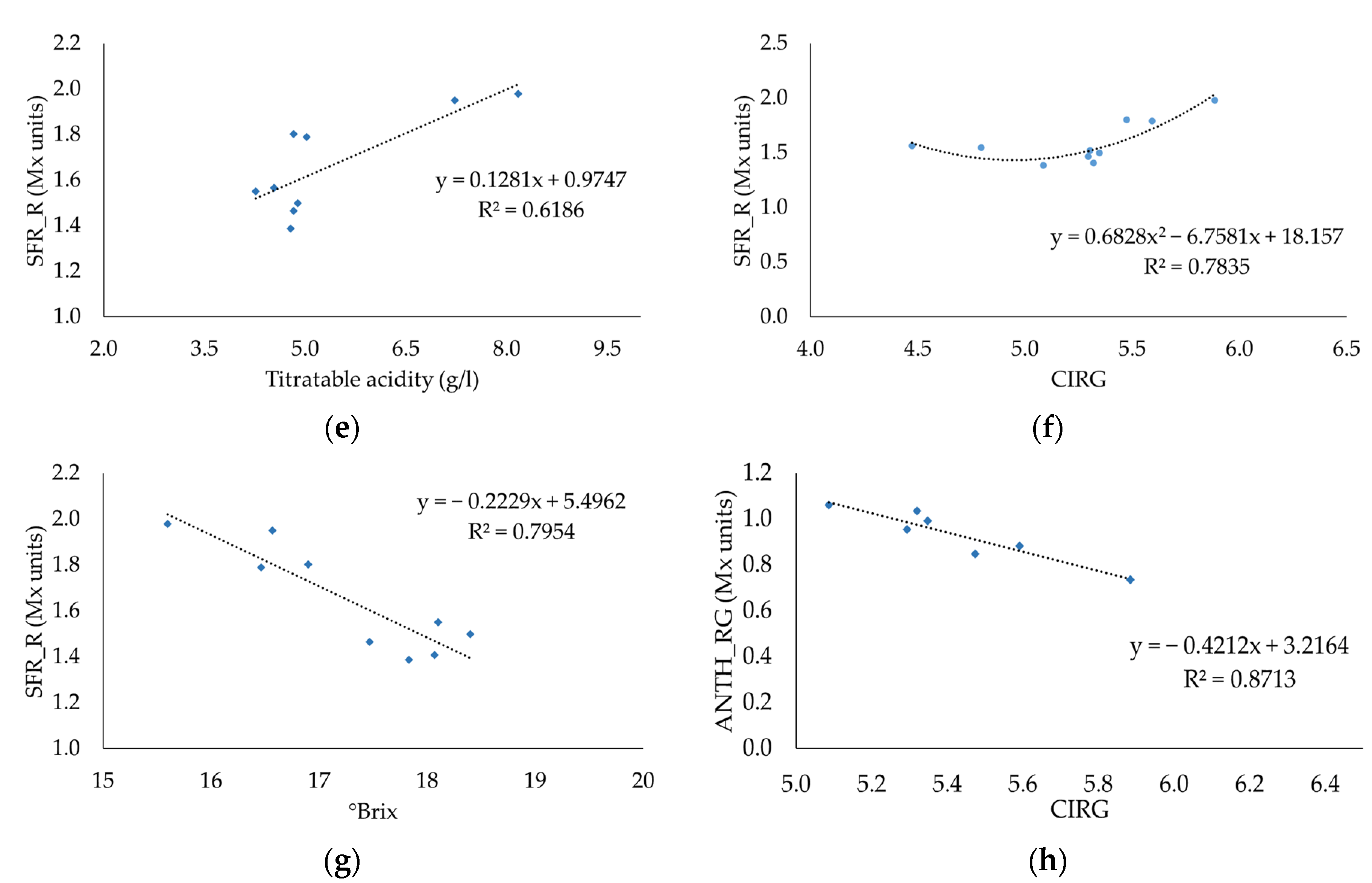
| Quality Parameters | Sample | Maturation Time (DOY) | ||||
|---|---|---|---|---|---|---|
| 246 | 254 | 263 | 278 | 280 (Harvest) | ||
| pH | SB1 | A 3.35 d ± 0.01 * | A 3.51 b ± 0.01 | A 3.56 a ± 0.01 | A 3.51 b ± 0.01 | B 3.47 c ± 0.01 |
| SB2 | A 3.34 c ± 0.01 | A 3.49 b ± 0.02 | B 3.44 b ± 0.02 | B 3.45 b ± 0.01 | A 3.61 a ± 0.01 | |
| TSS (°Brix) | SB1 | A 16.6 b ± 0.1 | A 16.9 b ± 0.2 | A 18.1 a ± 0.1 | A 18.4 a ± 0.2 | A 17.8 a ± 0.1 |
| SB2 | B 15.6 cd ± 0.3 | A 16.5 bc ± 0.5 | B 14.8 d ± 0.3 | B 17.5 ab ± 0.1 | A 18.1 a ± 0.2 | |
| Titratable acidity (g/L) | SB1 | B 7.23 a ± 0.58 | A 4.83 b ± 0.03 | B 4.26 c ± 0.15 | A 4.89 b ± 0.19 | A 4.78 b ± 0.08 |
| SB2 | A 8.18 a ± 0.09 | A 5.03 b ± 0.30 | A 4.54 b ± 0.04 | A 4.83 b ± 0.05 | A 4.94 b ± 0.10 | |
| CIRG | SB1 | B 5.30 a ± 0.07 | A 5.47 a ± 0.32 | A 4.80 b ± 0.14 | A 5.35 a ± 0.14 | A 5.09 ab ± 0.16 |
| SB2 | A 5.89 a ± 0.21 | A 5.59 ab ± 0.19 | B 4.47 c ± 0.08 | A 5.29 b ± 0.08 | A 5.32 b ± 0.20 | |
| Parameters | Sample | Maturation Time (DOY) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 213 | 225 | 234 | 246 | 254 | 263 | 278 | 280 (Harvest) | ||
| Total polyphenols (mg/kg) | SB1 | B 715.3 b ± 9.2 * | B 911.7 a ± 56.6 | A 669.2 b ± 44.9 | A 846.9 ab ± 95.5 | A 746.5 b ± 33.9 | A 710.1 b ± 30.0 | A 813.8 ab ± 59.1 | B 737.5 b ± 54.9 |
| SB2 | A 819.9 b ± 40.7 | A 995.9 a ± 16.2 | A 684.0 c ± 16.2 | A 913.6 ab ± 89.0 | A 731.4 bc ± 54.6 | B 586.3 d ± 6.8 | A 785.6 b ± 36.1 | A 851.4 ab ± 43.4 | |
| Flavonoids (mg/kg) | SB1 | A 405.7 c ± 7.4 | A 542.6 a ± 35.1 | A 386.4 c ± 38.4 | A 480.8 ab ± 55.2 | A 436.8 b ± 9.3 | A 376.4 c ± 22.4 | A 469.4 b ± 36.0 | A 468.7 b ± 28.0 |
| SB2 | B 379.3 b ± 11.2 | B 485.0 a ± 13.7 | A 365.7 b ± 17.9 | A 473.7 a ± 20.8 | B 376.5 b ± 34.5 | B 289.8 c ± 1.7 | A 454.4 a ± 24.8 | A 508.8 a ± 33.0 | |
| Anthocyanins (mg/kg) | SB1 | B 44.8 e ± 7.4 | A 86.2 bc ± 7.5 | B 63.2 d ± 14.6 | A 84.9 bc ± 14.3 | A 82.4 c ± 1.6 | A 63.9 d ± 0.1 | B 93.4 b ± 6.4 | B 123.0 a ± 4.1 |
| SB2 | A 65.7 d ± 11.4 | B 65.6 d ± 9.7 | A 91.5 c ± 10.8 | A 99.9 c ± 1.6 | A 84.5 c ± 19.2 | B 58.6 d ± 2.1 | A 133.7 b ± 4.3 | A 144.6 a ± 1.7 | |
| Antioxidant activity | |||||||||
| ABTS (µM/g) | SB1 | A 3.6 a ± 0.1 | A 3.5 a ± 0.1 | A 2.7 b ± 0.2 | A 2.0 c ± 0.1 | A 2.9 b ± 0.2 | A 3.7 a ± 0.1 | A 4.1 a ± 0.6 | A 3.4 a ± 0.2 |
| SB2 | B 2.9 c ± 0.1 | A 3.3 b ± 0.1 | B 2.1 d ± 0.1 | A 2.1 d ± 0.1 | B 1.9 d ± 0.1 | B 3.1 bc ± 0.1 | A 3.7 a ± 0.2 | A 3.8 a ± 0.2 | |
| DPPH (µM/g) | SB1 | A 0.9 c ± 0.1 | A 1.2 c ± 0.2 | A 1.7 b ± 0.1 | A 1.6 b ± 0.1 | A 1.8 b ± 0.1 | A 2.0 ab ± 0.1 | A 2.4 a ± 0.4 | A 2.2 ab ± 0.3 |
| SB2 | A 1.1 d ± 0.1 | A 1.2 d ± 0.1 | B 1.4 cd ± 0.1 | A 1.6 c ± 0.1 | B 1.2 d ± 0.1 | A 2.0 b ± 0.1 | A 2.1 b ± 0.1 | A 2.5 a ± 0.1 | |
| Anthocyanins | Sample | Maturation Time (DOY) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 213 | 225 | 234 | 246 | 254 | 263 | 278 | 280 (Harvest) | ||
| Dp | SB1 | B 0.5 d ± 0.2 * | A 2.0 a ± 0.2 | B 1.0 c ± 0.1 | B 1.4 b ± 0.2 | A 1.7 ab ± 0.1 | A 0.7 cd ± 0.2 | B 0.3 d ± 0.1 | A 1.2 bc ± 0.1 |
| SB2 | A 1.4 c ± 0.1 | A 1.9 b ± 0.3 | A 1.7 bc ± 0.3 | A 2.7 a ± 0.4 | B 0.7 d ± 0.2 | A 0.5 d ± 0.2 | A 1.5 bc ± 0.5 | A 1.4 c ± 0.1 | |
| Cy | SB1 | B 2.2 d ± 0.4 | A 2.9 cd ± 0.4 | A 1.9 d ± 0.3 | A 2.6 cd ± 0.7 | A 3.1 c ± 0.4 | A 1.2 e± 0.1 | A 10.6 b ± 0.1 | A 14.9 a ± 0.3 |
| SB2 | A 5.9 b ± 1.5 | A 2.5 c ± 0.2 | A 2.4 c ± 0.5 | A 2.2 c ± 0.1 | B 1.0 d ± 0.3 | B 0.7 d ± 0.1 | B 5.3 b ± 1.7 | B 8.8 a ± 0.6 | |
| Pt | SB1 | B 1.0 d ± 0.2 | A 2.5 a ± 0.2 | B 1.3 cd ± 0.2 | B 2.1 ab ± 0.2 | A 2.2 a ± 0.1 | A 1.6 c ± 0.1 | A 1.3 cd ± 0.2 | B 1.9 b ± 0.1 |
| SB2 | A 2.4 b ± 0.1 | A 2.3 b ± 0.3 | A 2.4 b ± 0.3 | A 3.5 a ± 0.5 | B 0.9 c ± 0.3 | B 0.3 d ± 0.2 | A 2.0 b ± 0.6 | A 2.5 b ± 0.1 | |
| Pn | SB1 | B 16.4 cd ± 3.9 | A 21.5 c ± 2.3 | A 21.5 c ± 4.5 | A 19.5 cd ± 5.0 | A 16.3 d ± 0.1 | A 11.4 e ± 0.1 | A 31.7 b ± 1.3 | B 37.5 a ± 0.5 |
| SB2 | A 34.2 b ± 3.3 | B 15.4 d ± 1.6 | A 26.9 bc ± 4.8 | A 16.7 d ± 0.9 | B 7.1 e ± 2.1 | A 10.0 e ± 2.1 | B 22.6 c ± 3.2 | A 42.4 a ± 0.5 | |
| Mv | SB1 | B 10.5 c ± 1.5 | A 23.0 a ± 1.3 | B 15.8 bc ± 3.7 | B 23.1 a ± 2.4 | A 21.0 ab ± 2.1 | A 15.6 bc ± 0.8 | A 11.5 c ± 1.8 | B 17.4 b ± 2.1 |
| SB2 | A 23.0 b ± 2.1 | A 19.7 bc ± 3.0 | A 24.1 b ± 2.9 | A 32.5 a ± 1.6 | B 8.4 c ± 2.5 | A 14.1 c ± 2.4 | A 15.8 c ± 5.0 | A 24.7 b ± 0.9 | |
| Pn-Ac | SB1 | A 0.2 c ± 0.1 | A 0.3 d ± 0.1 | A 0.4 c ± 0.1 | A 0.7 b ± 0.1 | A 0.8 b ± 0.1 | A 0.6 bc ± 0.1 | A 0.8 b ± 0.1 | A 1.1 a ± 0.1 |
| SB2 | A 0.7 b ± 0.3 | A 0.6 b ± 0.2 | A 0.5 c ± 0.1 | A 0.9 b ± 0.1 | B 0.4 c ± 0.1 | B 0.3 c ± 0.1 | A 0.9 ab ± 0.3 | A 1.2 a ± 0.1 | |
| Mv-Ac | SB1 | B 0.3 c ± 0.1 | A 0.8 ab ± 0.1 | B 0.5 bc ± 0.1 | B 0.8 ab ± 0.2 | A 0.9 a ± 0.1 | A 0.9 a ± 0.1 | B 0.4 ab ± 0.1 | B 0.6 b ± 0.1 |
| SB2 | A 0.8 bc ± 0.3 | A 0.6 c ± 0.1 | A 1.0 b ± 0.1 | A 1.5 a ± 0.1 | B 0.3 d ± 0.1 | A 0.7 c ± 0.1 | A 0.8 bc ± 0.2 | A 1.1 b ± 0.1 | |
| Pn-Cf | SB1 | B 0.1 e ± 0.1 | A 0.7 cd ± 0.1 | A 0.3 de ± 0.1 | A 0.5 d ± 0.1 | A 0.9 c ± 0.1 | A 0.5 cd ± 0.1 | A 1.3 b ± 0.1 | A 1.6 a ± 0.1 |
| SB2 | A 0.6 bc ± 0.3 | A 0.7 b ± 0.1 | A 0.4 c ± 0.1 | A 0.7 b ± 0.1 | B 0.3 b ± 0.1 | A 0.7 b ± 0.1 | A 1.0 ab ± 0.3 | B 1.2 a ± 0.1 | |
| Mv-Cf | SB1 | A 0.3 ab ± 0.1 | nd | A 0.1 b ± 0.1 | A 0.2 ab ± 0.1 | nd | A 0.4 a ± 0.1 | nd | nd |
| SB2 | A 0.3 b ± 0.1 | nd | A 0.2 b ± 0.1 | A 0.2 b ± 0.1 | A 0.1 b ± 0.1 | A 0.8 a ± 0.2 | nd | A 0.3 b ± 0.1 | |
| Cis-Pn-Cm | SB1 | B 0.2 b ± 0.1 | A 0.6 a ± 0.1 | A 0.3 b ± 0.1 | B 0.6 a ± 0.1 | A 0.6 a ± 0.1 | A 0.6 a ± 0.1 | A 0.6 a ± 0.1 | A 0.7 a ± 0.1 |
| SB2 | A 0.6 bc ± 0.2 | A 0.6 b ± 0.1 | A 0.5 bc ± 0.1 | A 1.0 a ± 0.1 | A 0.4 b ± 0.1 | B 0.3 c ± 0.1 | A 0.8 ab ± 0.2 | A 0.9 ab ± 0.1 | |
| cis-Mv-Cm | SB1 | A 0.1 a ± 0.1 | B 0.2 a ± 0.1 | A 0.2 a ± 0.1 | B 0.3 a ± 0.1 | A 0.3 a ± 0.1 | A 0.3 a ± 0.1 | A 0.1 a ± 0.1 | B 0.2 a ± 0.1 |
| SB2 | A 0.3 b ± 0.1 | A 0.3 b ± 0.1 | A 0.2 b ± 0.1 | A 0.6 a ± 0.1 | B 0.1 b ± 0.1 | A 0.2 bc ± 0.1 | A 0.3 b ± 0.1 | A 0.4 ab ± 0.1 | |
| Pn-Cm | SB1 | B 1.5 f ± 0.3 | A 2.9 e ± 0.2 | B 2.7 e ± 0.8 | B 3.7 cd ± 0.9 | A 4.5 c ± 0.1 | A 3.7 d ± 0.1 | A 6.0 b ± 0.1 | B 6.9 a ± 0.1 |
| SB2 | A 5.6 b ± 1.3 | A 2.9 c ± 0.4 | A 3.9 bc ± 0.6 | A 5.0 b ± 0.3 | B 2.5 c ± 0.7 | B 2.8 c ± 0.2 | A 5.7 b ± 1.8 | A 9.2 a ± 0.2 | |
| trans-Mv-Cm | SB1 | B 1.2 e ± 0.1 | A 3.9 c ± 0.2 | B 3.5 cd ± 0.9 | B 6.2 ab ± 0.9 | A 6.8 a ± 0.9 | A 5.5 b ± 0.1 | B 2.5 d ± 0.4 | B 3.9 c ± 0.7 |
| SB2 | A 4.7 cd ± 2.1 | A 3.8 cd ± 0.7 | A 5.4 c ± 0.5 | A 9.9 a ± 0.3 | B 2.9 d ± 0.9 | A 5.1 c ± 0.7 | A 5.3 c ± 1.5 | A 7.3 b ± 0.2 | |
| Total | SB1 | B 34.5 d ± 5.8 | A 61.5 bc ± 4.8 | A 49.4 cd ± 10.9 | B 61.6 bc ± 10.6 | A 59.0 c ± 2.7 | A 42.8 d ± 1.3 | A 67.0 b ± 4.0 | B 87.7 a ± 2.4 |
| SB2 | A 80.5 b ± 15.3 | A 51.3 c ± 7.0 | A 69.5 b ± 9.9 | A 77.3 b ± 4.5 | B 25.1 e ± 7.5 | B 36.6 d ± 0.9 | A 61.8 bc ± 19.7 | A 101.6 a ± 0.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamie, N.; Tarricone, L.; Verrastro, V.; Natrella, G.; Faccia, M.; Gambacorta, G. Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods. Foods 2022, 11, 663. https://doi.org/10.3390/foods11050663
Hamie N, Tarricone L, Verrastro V, Natrella G, Faccia M, Gambacorta G. Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods. Foods. 2022; 11(5):663. https://doi.org/10.3390/foods11050663
Chicago/Turabian StyleHamie, Najwane, Luigi Tarricone, Vincenzo Verrastro, Giuseppe Natrella, Michele Faccia, and Giuseppe Gambacorta. 2022. "Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods" Foods 11, no. 5: 663. https://doi.org/10.3390/foods11050663
APA StyleHamie, N., Tarricone, L., Verrastro, V., Natrella, G., Faccia, M., & Gambacorta, G. (2022). Assessment of “Sugranineteen” Table Grape Maturation Using Destructive and Auto-Fluorescence Methods. Foods, 11(5), 663. https://doi.org/10.3390/foods11050663








