Flavour Compensation Role of Yeast Strains in Reduced-Salt Dry Sausages: Taste and Odour Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Yeast Strains
2.2. Preparation of Dry Sausages
2.3. Analysis of Physical, Microbial, and Quality Characteristics of Dry Sausages
2.4. Electronic Nose Analysis
2.5. Electronic Tongue Analysis
2.6. Volatile Compound Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physical, Microbial, and Quality Characteristic Analysis
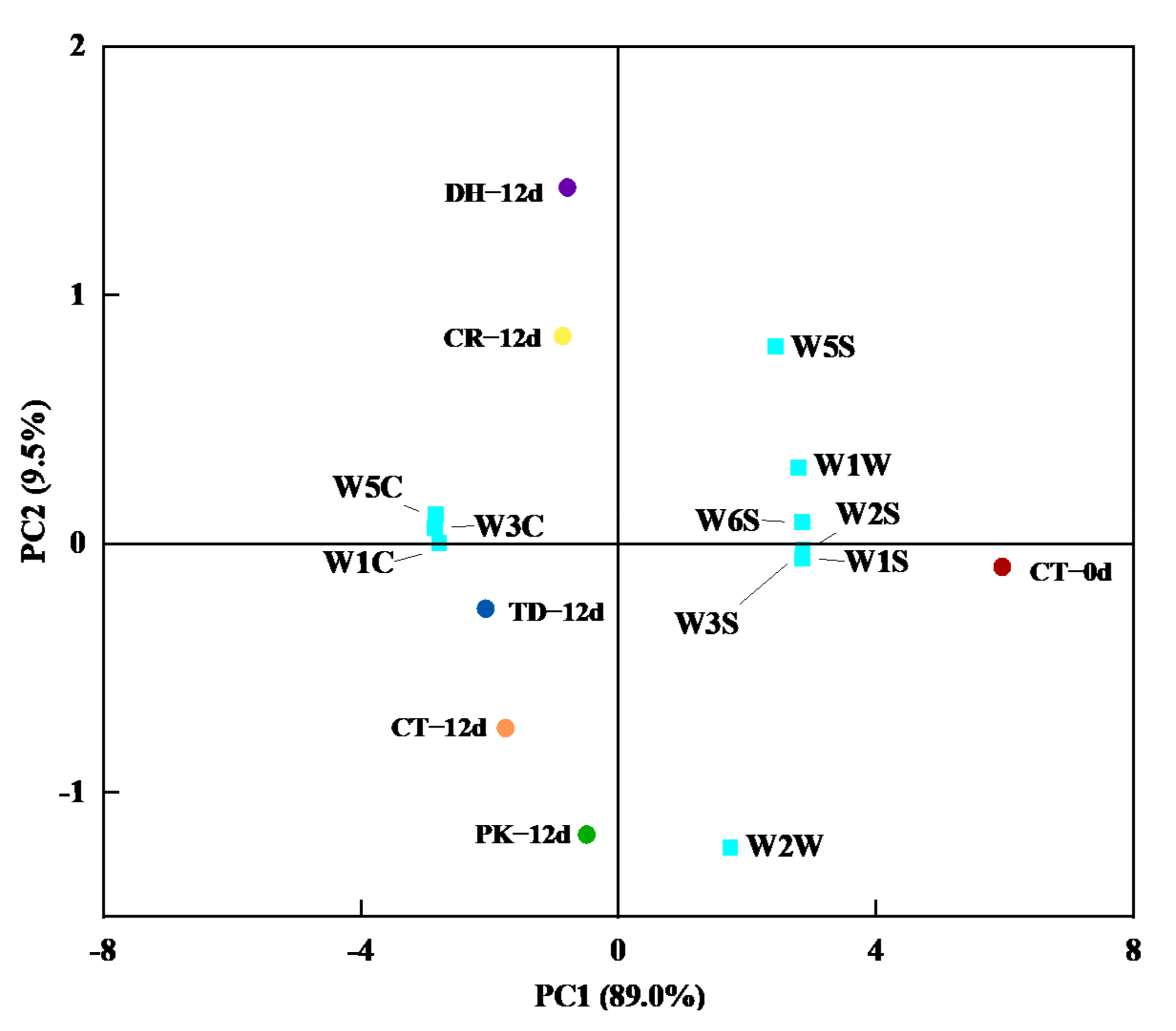
3.2. Electronic Nose Analysis
3.3. Electronic Tongue Analysis
3.4. Volatile Compound Analysis
3.5. PLS-DA of Volatile Compounds
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, M.J. Inclusion of seaweeds as healthy approach to formulate new low-salt meat products. Curr. Opin. Food Sci. 2021, 40, 20–25. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.B.; Han, Y.R.; Ge, G.; Zhao, M.M.; Sun, W.Z. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocoll. 2019, 96, 36–42. [Google Scholar] [CrossRef]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ Brit. Med. J. 2007, 334, 1–8. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Jenner, K.H.; MacGregor, G.A. WASH-World action on salt and health. Kidney Int. 2010, 78, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilaqueo, M.; Duizer, L.; Aguilera, J.M. The morphology of salt crystals affects the perception of saltiness. Food Res. Int. 2015, 76, 675–681. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Campagnol, P.C.B.; dos Santos, B.A.; Wanger, R.; Terra, N.N.; Pollonio, M.A.R. The effect of yeast extract addition on quality of fermented sausages at low NaCl content. Meat Sci. 2011, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.X.; Hu, Y.Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B.H. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, L.; Zhang, H.; Wang, Y.; Chen, Q.; Kong, B.H. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT Food Sci. Technol. 2020, 124, 109061. [Google Scholar] [CrossRef]
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Corral, S.; Belloch, C.; López-Díez., J.J.; Salvador, A.; Flores, M. Yeast inoculation as a strategy to improve the physico-chemical and sensory properties of reduced salt fermented sausages produced with entire male fat. Meat Sci. 2017, 123, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inguglia, E.S.; Zhang, Z.H.; Tiwaria, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Zanardi, E.; Ghidini, S.; Conter, M.; Lanieri, A. Mineral composition of Italian salami and effect of NaCl partial replacement on compositional, physico-chemical and sensory parameters. Meat Sci. 2010, 86, 742–747. [Google Scholar] [CrossRef]
- Talon, R.; Leroy, S. Diversity and safety hazards of bacteria involved in meat fermentations. Meat Sci. 2011, 89, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Cruxen, C.E.; Funck, G.D.; Haubert, L.; da Silva Dannenberg, G.; de Lima Marques, J.; Chaves, F.C.; da Sliva, W.P. Selection of native bacterial starter culture in the production of fermented meat sausages: Application potential, safety aspects, and emerging technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef]
- Flores, M.; Corral, S.; Cano-García, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Gardini, F.; Suzzi, G.; Lombardi, A.; Galgano, F.; Crudele, M.A.; Andrighetto, C.; Schirone, M.; Tofalo, R. A survey of yeasts in traditional sausages of southern Italy. FEMS Yeast Res. 2001, 1, 161–167. [Google Scholar] [CrossRef]
- Flores, M.; Durá, M.-A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef]
- Copetti, M.V. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.H.; Han, Q.; Liu, Q.; Xu, L. The role of bacterial fermentation in the hydrolysis and oxidation of sarcoplasmic and myofibrillar proteins in Harbin dry sausages. Meat Sci. 2016, 121, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Li, Y.J.; Zhu, J.M.; Kong, B.H.; Liu, Q.; Chen, Q. Improving the taste profile of reduced-salt dry sausage by inoculating different lactic acid bacteria. Food Res. Int. 2021, 145, 110391. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.X.; Sun, F.D.; Li, X.A.; Chen, Q.; Kong, B.H. The potential correlations between the fungal communities and volatile compounds of traditional dry sausages from Northeast China. Food Microbiol. 2021, 98, 103787. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.X.; Yin, X.Y.; Hu, Y.Y.; Chen, Q.; Kong, B.H. Technological properties and flavour formation potential of yeast strains isolated from traditional dry fermented sausages in Northeast China. LWT Food Sci. Technol. 2021, 154, 112853. [Google Scholar] [CrossRef]
- Williams, S. Association of Official Methods of Analysis Methods 925.04, 16th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1995. [Google Scholar]
- Hu, Y.Y.; Wang, H.; Kong, B.H.; Wang, Y.; Chen, Q. The succession and correlation of the bacterial community and flavour characteristics of Harbin dry sausages during fermentation. LWT Food Sci. Technol. 2021, 138, 110689. [Google Scholar] [CrossRef]
- Wen, R.X.; Lv, Y.C.; Li, X.A.; Chen, Q.; Kong, B.H. High-throughput sequencing approach to reveal the bacterial diversity of traditional yak jerky from the Tibetan regions. Meat Sci. 2021, 172, 108348. [Google Scholar] [CrossRef]
- Bolumar, T.; Sanz, Y.; Flores, M.; Aristoy, M.C.; Toldrá, F.; Flores, J. Sensory improvement of dry-fermented sausages by the addition of cell-free extracts from Debaryomyces hansenii and Lactobacillus sakei. Meat Sci. 2006, 72, 457–466. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Li, Y.J.; Li, X.A.; Zhang, H.; Chen, Q.; Kong, B.H. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT Food Sci. Technol. 2022, 154, 112723. [Google Scholar] [CrossRef]
- Yin, X.Y.; Du, H.Z.; Xu, M.; Chen, Q.; Kong, B.H. Heterocyclic aromatic amine level and quality characteristics of selected Harbin red sausages in the northern Chinese market. Meat Sci. 2021, 172, 108360. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.Y.; Wang, Y.; Kong, B.H.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT Food Sci. Technol. 2021, 140, 110764. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.Y.; Wen, R.X.; Wang, Y.; Qin, L.G.; Kong, B.H. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Hu, Y.Y.; Wen, R.X.; Liu, Q.; Chen, Q.; Kong, B.H. Effect of NaCl substitutes on the physical, microbial and sensory characteristics of Harbin dry sausage. Meat Sci. 2019, 156, 205–213. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; López-Díez, J.J.; Belloch, C.; Flores, M. Counteracting the effect of reducing nitrate/nitrite levels on dry fermented sausage aroma by Debaryomyces hansenii inoculation. Meat Sci. 2020, 164, 108103. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Rendueles, M.; Díaz, M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, H.J.; Lee, J.Y.; Jo, C.; Yoon, Y. Identification of microorganisms associated with the quality improvement of dry-aged beef through microbiome analysis and DNA sequencing, and evaluation of their effects on beef quality. J. Food Sci. 2019, 84, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Marušić, N.; Petrović, M.; Vidaček, S.; Petrak, T.; Medić, H. Characterization of traditional lstrian dry-cured ham by means of physical and chemical analyses and volatile compounds. Meat Sci. 2011, 88, 786–790. [Google Scholar] [CrossRef]
- Murgia, M.A.; Marongiu, A.; Aponteb, M.; Blaiotta, G.; Deiana, P.; Mangia, N.P. Impact of a selected Debaryomyces hansenii strain’s inoculation on the quality of Sardinian fermented sausages. Food Res. Int. 2019, 121, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Moreno, L.; Ruiz-Pérez, F.; Rodríguez-Castro, E.; Ramos, J. Debaryomyces hansenii Is a real tool to improve a diversity of characteristics in sausages and dry-meat products. Microorganisms 2021, 9, 1512. [Google Scholar] [CrossRef]
- Liu, P.X.; Wang, S.W.; Zhang, H.; Wang, H.T.; Kong, B.H. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef]
- Xia, X.F.; Kong, B.H.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Ramos-Moreno, L.; Ruiz-Castilla, F.J.; Bravo, C.; Martínez, E.; Menéndez, M.; Dios-Palomares, R.; Ramos, J. Inoculation with a terroir selected Debaryomyces hansenii strain changes physico-chemical characteristics of Iberian cured pork loin. Meat Sci. 2019, 157, 107875. [Google Scholar] [CrossRef]
- Alim, A.; Song, H.L.; Liu, Y.; Zou, T.T.; Zhang, Y.; Zhang, S.P. Flavor-active compounds in thermally treated yeast extracts. J. Sci. Food Agric. 2018, 98, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.F.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT Food Sci. Technol. 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhao, B.; Zhang, S.L.; Wu, Q.R.; Zhu, N.; Li, S.; Pan, X.; Wang, S.W.; Qiao, X.L. Development of volatiles and odor-active compounds in Chinese dry sausage at different stages of process and storage. Food Sci. Hum. Well. 2021, 10, 316–326. [Google Scholar] [CrossRef]
- Gómez, M.; Lorenzo, J.M. Effect of fat level on physicochemical, volatile compounds and sensory characteristics of dry-ripened “chorizo” from Celta pig breed. Meat Sci. 2013, 95, 658–666. [Google Scholar] [CrossRef]
- Azarbad, M.H.; Jeleń, H. Determination of hexanal-an indicator of lipid oxidation by static headspace gas chromatography (SHS-GC) in fat-rich food matrices. Food Anal. Methods 2014, 8, 1727–1733. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.Q.; Wang, H.B.; Xi, B.; He, X.N.; Yang, X.L.; Li, W.H. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography—Ion mobility spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K.; Faithong, N. Changes in volatile compounds, ATP-related compounds and antioxidative properties of Kapi, produced from Acetes vulgaris, during processing and fermentation. Food Biosci. 2017, 19, 49–56. [Google Scholar] [CrossRef]
- De Lima Alves, L.; Donadel, J.Z.; Athayde, D.R.; da Silva, M.S.; Klein, B.; Fagundes, M.B.; de Menezes, C.R.; Barin, J.S.; Campagnol, P.C.B.; Wagner, R.; et al. Effect of ultrasound on proteolysis and the formation of volatile compounds in dry fermented sausages. Ultrason. Sonochem. 2020, 67, 105161. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of curing salts and probiotic cultures on the evolution of flavor compounds in dry-fermented sausages during ripening. Food Chem. 2016, 15, 334–338. [Google Scholar] [CrossRef]
- Zhong, A.; Chen, W.; Duan, Y.; Li, K.; Tang, X.; Tian, X.; Wu, Z.; Li, Z.; Wang, Y.; Wang, C. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT Food Sci. Technol. 2021, 149, 111873. [Google Scholar] [CrossRef]
- Andrade, M.J.; Córdoba, J.J.; Casado, E.M.; Córdoba, M.G.; Rodríguez, M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichón”. Meat Sci. 2010, 85, 256–264. [Google Scholar] [CrossRef]
- Olesen, P.T.; Stahnke, L.H. The influence of Debaryomyces hansenii and Candida utilis on the aroma formation in garlic spiced fermented sausages and model minces. Meat Sci. 2000, 56, 357–368. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B.H. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Landaud, S.; Sandra, H.; Pascal, B. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biot. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Durá, M.A.; Flores, M.; Toldrá, F. Purification and characterisation of a glutaminase from Debaryomyces spp. Int. J. Food Microbiol. 2002, 76, 117–126. [Google Scholar] [CrossRef]


| CT | CR | PK | TD | DH | |
|---|---|---|---|---|---|
| Starter cultures | - | - | P. kudriavzevii | T. delbrueckii | D. hansenii |
| Lean pork (g) | 5400 | 5400 | 5400 | 5400 | 5400 |
| Pork back fat (g) | 600 | 600 | 600 | 600 | 600 |
| Salt (g) | 150 | 105 | 105 | 105 | 105 |
| Sodium nitrite (g) | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 |
| Monosodium glutamate (g) | 18 | 18 | 18 | 18 | 18 |
| Dextrose (g) | 20 | 20 | 20 | 20 | 20 |
| Wine (g) | 60 | 60 | 60 | 60 | 60 |
| Ginger powder (g) | 30 | 30 | 30 | 30 | 30 |
| Mixed spices (g) | 48 | 48 | 48 | 48 | 48 |
| Sensor Name | Representative Substance Type | Description |
|---|---|---|
| W1C | Aromatic | Sensitive to aromatic compounds |
| W5S | Broad range | Sensitive to nitrogen oxides |
| W3C | Aromatic | Sensitive to aroma, aromatic compounds |
| W6S | Hydrogen | Sensitive to hydrides |
| W5C | Arom-aliph | Sensitive to short-chain alkane aromatic compounds |
| W1S | Broad-methane | Sensitive to methyl |
| W1W | Sulphur-organic | Sensitive to sulphides |
| W2S | Broad-alcohol | Sensitive to alcohol, aldehydes and ketones |
| W2W | Sulph-chlor | Sensitive to organic sulphides |
| W3S | Methane-aliph | Sensitive to long-chain alkanes |
| Fermentation Time (Day) | CT | CR | PK | TD | DH | |
|---|---|---|---|---|---|---|
| Moisture content (%) | 0 | 70.60 ± 2.25 Aa | 69.63 ± 3.12 Aa | 71.28 ± 3.72 Aa | 70.65 ± 1.13 Aa | 71.47 ± 0.52 Aa |
| 4 | 37.27 ± 1.24 Bc | 40.79 ± 0.98 Ba | 40.54 ± 0.37 Bab | 42.27 ± 0.54 Ba | 38.58 ± 0.60 Bbc | |
| 8 | 22.89 ± 0.28 Cc | 25.81 ± 0.16 Cb | 25.77 ± 0.70 Cb | 25.63 ± 0.25 Cb | 27.46 ± 0.19 Ca | |
| 12 | 18.35 ± 0.04 Dc | 19.36 ± 0.32 Db | 19.79 ± 0.27 Db | 19.85 ± 0.19 Db | 21.56 ± 0.44 Da | |
| pH | 0 | 6.10 ± 0.03 Aa | 6.09 ± 0.04 Aa | 6.08 ± 0.03 Aa | 6.09 ± 0.03 Aa | 6.09 ± 0.04 Aa |
| 4 | 5.69 ± 0.02 Ba | 5.55 ± 0.02 Bb | 5.56 ± 0.03 Bb | 5.28 ± 0.04 Bc | 5.56 ± 0.02 Bb | |
| 8 | 5.59 ± 0.01 Ca | 5.40 ± 0.02 Cb | 5.16 ± 0.02 Cd | 5.07 ± 0.02 Ce | 5.31 ± 0.01 Cc | |
| 12 | 5.65 ± 0.04 BCa | 5.45 ± 0.02 Cb | 5.20 ± 0.01 Cd | 5.12 ± 0.02 Ce | 5.34 ± 0.01 Cc | |
| Yeast count (log CFU/g) | 0 | 4.80 ± 0.02 Db | 4.80 ± 0.01 Cb | 6.12 ± 0.04 Ca | 6.08 ± 0.04 Da | 6.07 ± 0.04 Da |
| 4 | 5.35 ± 0.03 Ce | 6.04 ± 0.03 Bd | 7.42 ± 0.05 Bc | 7.75 ± 0.02 Aa | 7.52 ± 0.04 Ab | |
| 8 | 5.68 ± 0.03 Ad | 6.30 ± 0.06 Ac | 7.56 ± 0.03 Aa | 7.39 ± 0.03 Bb | 7.36 ± 0.03 Bb | |
| 12 | 5.54 ± 0.02 Bc | 6.23 ± 0.02 Ab | 7.28 ± 0.04 Ba | 7.22 ± 0.01 Ca | 7.21 ± 0.03 Ca | |
| LAB count (log CFU/g) | 0 | 5.14 ± 0.02 Ca | 5.14 ± 0.01 Da | 5.15 ± 0.01 Da | 5.14 ± 0.01 Ca | 5.15 ± 0.01 Da |
| 4 | 6.86 ± 0.01 Bd | 6.93 ± 0.01 Cc | 7.25 ± 0.02 Cb | 7.42 ± 0.02 Aa | 7.24 ± 0.02 Cb | |
| 8 | 7.09 ± 0.06 Ae | 7.45 ± 0.03 Ac | 7.81 ± 0.02 Aa | 7.35 ± 0.03 Ad | 7.65 ± 0.02 Ab | |
| 12 | 7.04 ± 0.04 Ad | 7.33 ± 0.03 Bb | 7.56 ± 0.03 Ba | 7.20 ± 0.05 Bc | 7.56 ± 0.02 Ba | |
| Shear force (N) | 0 | 3.03 ± 0.18 Da | 2.83 ± 0.28 Da | 2.82 ± 0.12 Da | 2.75 ± 0.41 Da | 3.33 ± 0.15 Da |
| 4 | 7.62 ± 0.30 Ca | 7.62 ± 0.18 Ca | 7.60 ± 0.16 Ca | 7.55 ± 0.13 Ca | 7.54 ± 0.42 Ca | |
| 8 | 19.56 ± 0.34 Ba | 16.61 ± 0.21 Bb | 14.54 ± 0.17 Be | 15.90 ± 0.14 Bc | 15.17 ± 0.13 Bd | |
| 12 | 26.05 ± 0.79 Aa | 20.57 ± 0.26 Ab | 16.56 ± 0.34 Ad | 18.58 ± 0.21 Ac | 16.59 ± 0.18 Ad | |
| L*-value | 0 | 44.54 ± 0.78 Aa | 44.17 ± 0.39 Aa | 44.53 ± 0.18 Aa | 44.16 ± 0.27 Aa | 44.40 ± 0.69 Aa |
| 4 | 40.52 ± 0.20 Ba | 40.46 ± 0.54 Ba | 40.21 ± 0.27 Ba | 40.39 ± 0.28 Ba | 40.61 ± 0.27 Ba | |
| 8 | 38.34 ± 0.12 Cc | 38.52 ± 0.25 Cc | 38.72 ± 0.38 Cbc | 39.60 ± 0.43 Ca | 39.47 ± 0.26 Cab | |
| 12 | 38.13 ± 0.09 Cb | 38.39 ± 0.09 Cb | 38.21 ± 0.23 Cb | 38.14 ± 0.13 Db | 39.12 ± 0.22 Ca | |
| a*-value | 0 | 11.48 ± 0.33 Da | 11.63 ± 0.48 Ca | 11.60 ± 0.09 Da | 11.67 ± 0.07 Da | 11.56 ± 0.35 Ca |
| 4 | 13.23 ± 0.19 Ca | 13.42 ± 0.18 Ba | 13.42 ± 0.17 Ca | 13.34 ± 0.37 Ca | 13.33 ± 0.22 Ba | |
| 8 | 14.20 ± 0.05 Bb | 14.15 ± 0.20 Bb | 14.82 ± 0.21 Ba | 14.28 ± 0.27 Bb | 14.23 ± 0.19 Ab | |
| 12 | 14.89 ± 0.14 Aab | 15.00 ± 0.18 Aab | 15.41 ± 0.02 Aa | 15.01 ± 0.29 Aab | 14.82 ± 0.24 Ab | |
| b*-value | 0 | 15.37 ± 0.17 Ca | 15.33 ± 0.12 Ca | 15.31 ± 0.21 Ca | 15.39 ± 0.13 Ca | 15.34 ± 0.11 Ca |
| 4 | 17.46 ± 0.51 Ba | 17.38 ± 0.34 Ba | 17.22 ± 0.23 Ba | 17.36 ± 0.39 Ba | 17.42 ± 0.28 Ba | |
| 8 | 17.62 ± 0.10 Aa | 17.78 ± 0.14 Ba | 17.53 ± 0.23 Ba | 17.48 ± 0.18 Ba | 17.75 ± 0.16 Ba | |
| 12 | 18.32 ± 0.14 Aa | 18.39 ± 0.14 Aa | 18.37 ± 0.17 Aa | 18.44 ± 0.17 Aa | 18.46 ± 0.29 Aa |
| Sensor Name | CT-0d | CT-12d | CR-12d | PK-12d | TD-12d | DH-12d |
|---|---|---|---|---|---|---|
| W1C | 0.14 ± 0.04 b | 0.33 ± 0.07 a | 0.30 ± 0.06 a | 0.31 ± 0.02 a | 0.39 ± 0.05 a | 0.33 ± 0.02 a |
| W5S | 2.11 ± 0.13 a | 1.69 ± 0.13 b | 1.88 ± 0.18 ab | 1.76 ± 0.08 ab | 1.73 ± 0.20 ab | 1.95 ± 0.11 ab |
| W3C | 0.18 ± 0.02 c | 0.41 ± 0.03 ab | 0.38 ± 0.02 ab | 0.35 ± 0.02 b | 0.43 ± 0.04 a | 0.38 ± 0.01 ab |
| W6S | 8.67 ± 2.65 a | 3.67 ± 0.68 b | 4.22 ± 0.72 b | 3.90 ± 0.24 b | 3.47 ± 0.47 b | 4.20 ± 0.27 b |
| W5C | 0.24 ± 0.03 c | 0.58 ± 0.03 a | 0.53 ± 0.03 ab | 0.47 ± 0.03 b | 0.58 ± 0.04 a | 0.53 ± 0.01 ab |
| W1S | 79.95 ± 0.12 a | 20.70 ± 0.64 e | 22.53 ± 0.19 d | 27.03 ± 0.13 b | 19.56 ± 0.29 f | 25.50 ± 0.16 c |
| W1W | 2.42 ± 0.25 ab | 1.72 ± 0.11 c | 1.84 ± 0.12 c | 1.78 ± 0.07 c | 1.77 ± 0.10 c | 1.93 ± 0.06 bc |
| W2S | 13.18 ± 4.06 a | 4.56 ± 0.73 b | 5.13 ± 0.71 b | 5.26 ± 0.48 b | 4.09 ± 0.5 b | 4.87 ± 0.24 b |
| W2W | 0.76 ± 0.02 a | 0.73 ± 0.02 abc | 0.70 ± 0.01 bc | 0.75 ± 0.02 ab | 0.72 ± 0.01 abc | 0.69 ± 0.01 c |
| W3S | 3.40 ± 0.31 a | 1.62 ± 0.07 b | 1.80 ± 0.10 b | 1.88 ± 0.08 b | 1.68 ± 0.10 b | 1.82 ± 0.06 b |
| Volatile Compound | CAS | LRI | CT-0d | CT-12d | CR-12d | PK-12d | TD-12d | DH-12d | |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| V1 | Hexanal | 66-25-1 | 1084 | n.d. | 8.58 ± 0.30 c | 12.03 ± 1.61 c | 35.79 ± 2.19 a | 11.93 ± 0.38 c | 25.05 ± 0.85 b |
| V2 | Nonanal | 124-19-6 | 1385 | 1.43 ± 0.08 e | 10.50 ± 0.54 c | 16.25 ± 1.40 b | 20.83 ± 0.55 a | 11.31 ± 0.45 c | 7.74 ± 0.55 d |
| V3 | Tridecanal | 10486-19-8 | 1767 | 1.39 ± 0.11 e | 2.04 ± 0.06 d | 2.07 ± 0.10 d | 3.77 ± 0.16 b | 3.02 ± 0.18 c | 4.89 ± 0.20 a |
| V4 | Cinnamaldehyde | 104-55-2 | 2170 | 9.48 ± 0.51 a | 3.52 ± 0.10 d | 2.08 ± 0.06 e | 4.41 ± 0.13 c | 2.57 ± 0.06 e | 6.41 ± 0.14 b |
| Total | 12.30 ± 0.18 f | 24.64 ± 0.16 e | 32.43 ± 0.33 c | 64.80 ± 0.74 a | 28.83 ± 0.34 d | 44.09 ± 0.52 b | |||
| Ketones | |||||||||
| V5 | 3-Hydroxy-2-butanone | 513-86-0 | 1299 | 3.86 ± 0.10 b | 24.29 ± 1.81 a | 23.96 ± 0.78 a | 23.19± 0.33 a | 24.75 ± 0.30 a | 24.41 ± 0.85 a |
| V6 | 2-Nonanone | 821-55-6 | 1396 | 5.78 ± 0.30 a | 4.19 ± 0.23 c | 3.30 ± 0.18 d | 4.11 ± 0.27 c | 5.02 ± 0.33 b | 4.16 ± 0.21 c |
| V7 | 2,3-Pentanedione | 600-14-6 | 1120 | n.d. | n.d. | n.d. | 1.79 ± 0.09 a | n.d. | n.d. |
| V8 | 1-Octen-3-one | 4312-99-6 | 1283 | n.d. | n.d. | n.d. | 1.43 ± 0.04 b | 1.57 ± 0.06 b | 2.09 ± 0.07 a |
| V9 | 6-Methyl-5-hepten-2-one | 110-93-0 | 1544 | 17.20 ± 0.66 a | 9.47 ± 0.45 b | 5.59 ± 0.14 c | 3.82 ± 0.16 d | n.d. | n.d. |
| Total | 26.84 ± 0.71 c | 37.95 ± 2.21 a | 32.85 ± 1.19 b | 34.34 ± 0.45 ab | 31.34 ± 0.79 bc | 30.66 ± 1.17 bc | |||
| Alcohols | |||||||||
| V10 | Ethanol | 64-17-5 | 928 | 109.89 ± 4.30 b | 67.16 ± 0.95 e | 69.72 ± 1.15 e | 80.14 ± 1.23 d | 88.62 ± 2.43 c | 143.23 ± 3.45 a |
| V11 | 2,3-Butanediol | 513-85-9 | 1590 | n.d. | 10.96 ± 0.38 c | 24.50 ±1.16 a | 25.29 ± 1.61 a | 17.83 ± 1.53 b | 26.96 ± 1.92 a |
| V12 | 1-Octen-3-ol | 3391-86-4 | 1451 | 0.77 ± 0.06 e | 3.10 ± 0.16 d | 3.15 ± 0.16 d | 7.84 ± 0.38 a | 4.66 ± 0.20 c | 6.82 ± 0.41 b |
| V13 | 2-Heptanol | 543-49-7 | 1285 | 2.30 ± 0.16 bc | 2.07 ± 0.23 cd | 1.59 ± 0.08 e | 2.52 ± 0.07 b | 1.87 ± 0.04 de | 3.05 ± 0.14 a |
| V14 | 3-Phenyl-1-propanol | 122-97-4 | 1715 | 6.00 ± 0.21 a | n.d. | n.d. | n.d. | n.d. | n.d. |
| V15 | Benzyl alcohol | 100-51-6 | 1618 | n.d. | 5.58 ± 0.24 a | 3.18 ± 0.13 c | 3.78 ± 0.11 b | 5.46 ± 0.20 a | 3.51 ± 0.08 bc |
| V16 | Decyl alcohol | 112-30-1 | 1300 | n.d. | n.d. | n.d. | n.d. | n.d. | 14.39 ± 1.37 a |
| V17 | Cineole | 470-82-6 | 1224 | 120.64 ± 4.65 a | 82.64 ± 2.28 c | 66.79 ± 1.92 d | 92.12 ± 3.86 b | 83.14 ± 2.94 bc | 75.24 ± 3.69 cd |
| V18 | Linalool | 78-70-6 | 1552 | 117.24 ± 3.41 bc | 119.31 ± 3.82 b | 101.63 ± 4.92 d | 126.70 ± 3.28 b | 107.17 ± 3.35 cd | 168.38 ± 3.78 a |
| V19 | Geraniol | 106-24-1 | 1849 | 5.12 ± 0.16 bc | 8.99 ± 0.83 a | 5.25 ± 0.69 bc | 4.07 ± 0.31 c | 4.00 ± 0.25 c | 6.44 ± 0.51 b |
| V20 | (-)-α-Terpineol | 10482-56-1 | 1517 | 22.59 ± 1.32 a | 12.92 ± 0.69 c | 9.91 ± 0.44 d | 12.48 ± 0.75 c | 11.60 ± 0.57 cd | 16.21 ± 1.16 b |
| V21 | (-)-Terpinen-4-ol | 20126-76-5 | 1466 | 44.14 ± 2.09 b | 37.81 ± 1.71 cd | 30.07 ± 0.98 e | 40.04 ± 1.19 bc | 35.83 ± 1.34 d | 51.78 ± 1.43 a |
| Total | 428.69 ± 4.78 b | 350.54 ± 6.29 d | 315.79 ± 5.89 e | 394.98 ± 6.75 c | 360.18 ± 4.21 d | 516.01 ± 7.81 a | |||
| Acids | |||||||||
| V22 | Acetic acid | 64-19-7 | 1450 | 5.49 ± 0.48 e | 90.27 ± 1.81 d | 183.34 ± 4.85 bc | 191.23 ± 5.63 b | 171.00 ± 5.32 c | 227.66 ± 5.94 a |
| V23 | Isovaleric acid | 503-74-2 | 1665 | 2.48 ± 0.18 c | 5.15 ± 0.41 b | 5.39 ± 0.35 b | 6.37 ± 0.81 b | 6.41 ± 0.76 b | 9.23 ± 0.88 a |
| V24 | Heptanoic acid | 111-14-8 | 2168 | n.d. | 2.22 ± 0.33 c | 1.24 ± 0.16 c | 4.82 ± 0.57 b | 6.26 ± 0.64 b | 15.06 ± 0.92 a |
| V25 | Nonanoic acid | 112-05-0 | 2202 | 0.88 ± 0.10 c | 1.28 ± 0.18 c | 1.30 ± 0.13 c | 2.94 ± 0.20 b | 0.92 ± 0.07 c | 3.73 ± 0.23 a |
| V26 | Butanoic acid | 107-92-6 | 1477 | 3.62 ± 0.18 e | 4.92 ± 0.42 de | 6.80 ± 0.25 bc | 8.53 ± 0.61 b | 6.41 ± 0.82 cd | 12.18 ± 1.17 a |
| V27 | Octanoic acid | 124-07-2 | 2083 | n.d. | 8.57 ± 0.45 b | 8.18 ± 0.71 b | 8.61 ± 0.76 b | 10.11 ± 0.86 b | 18.75 ±1.19 a |
| Total | 12.47 ± 0.37 e | 112.41 ± 4.30 d | 206.25 ± 6.01 bc | 222.50 ± 6.82 b | 201.11 ± 3.63 c | 286.61 ± 7.45 a | |||
| Esters | |||||||||
| V28 | Ethyl acetate | 141-78-6 | 907 | 1.21 ± 0.13 d | 4.07 ± 0.25 d | 18.45 ± 0.93 b | 16.34 ± 0.99 b | 23.12 ±1.12 a | 11.87 ± 0.66 c |
| V29 | Ethyl lactate | 97-64-3 | 1358 | n.d. | n.d. | 5.13 ± 0.44 c | 20.27 ± 0.76 b | 21.42 ± 0.85 b | 32.52 ± 0.61 a |
| V30 | Methyl butyrate | 623-42-7 | 963 | n.d. | n.d. | n.d. | 7.84 ± 0.52 b | 1.05 ± 0.08 c | 9.31 ± 0.68 a |
| V31 | Ethyl butyrate | 105-54-4 | 1028 | n.d. | 2.92 ± 0.35 c | 3.94 ± 0.44 bc | 4.53 ± 0.28 b | 4.26 ± 0.40 bc | 12.14 ± 0.88 a |
| V32 | Methyl hexanoate | 106-70-7 | 1188 | 13.66 ± 0.59 d | 25.62 ± 0.99 c | 39.92 ± 1.34 b | 43.47 ±1.63 b | 28.98 ± 1.46 c | 64.29 ± 2.26 a |
| V33 | Ethyl hexanoate | 123-66-0 | 1120 | 18.71 ± 0.72 e | 51.85 ± 2.29 c | 42.34 ± 1.34 d | 74.67 ± 1.43 b | 72.45 ± 1.67 b | 159.18 ± 3.78 a |
| V34 | Ethyl heptanoate | 106-30-9 | 1328 | 1.08 ± 0.17 b | 3.30 ± 0.37 a | 2.71 ± 0.38 a | n.d. | 3.01 ± 0.42 a | n.d. |
| V35 | Ethyl caprylate | 106-32-1 | 1437 | 1.43 ± 0.23 d | 6.59 ± 0.54 c | 7.59 ± 0.61 c | 10.08 ± 0.82 b | 11.36 ± 0.59 b | 20.04 ± 1.00 a |
| V36 | Bornyl acetate | 76-49-3 | 1288 | 10.92 ± 0.41 c | 12.30 ± 0.48 abc | 9.09 ± 0.59 d | 11.57 ± 0.64 bc | 13.53 ± 0.57 a | 13.19 ± 0.93 ab |
| V37 | Ethyl caprate | 110-38-3 | 1634 | n.d. | 5.18 ± 0.47 c | 5.73 ± 0.55 c | 6.16 ± 0.50 c | 8.40 ± 0.96 b | 11.22 ± 0.96 a |
| V38 | Methyl lactate | 547-64-8 | 1293 | n.d. | 0.96 ± 0.08 c | 4.14 ± 0.44 b | n.d. | 5.19 ± 0.31 b | 6.68 ± 0.72 a |
| Total | 47.01 ± 1.74 e | 112.79 ± 3.49 d | 139.04 ± 0.98 c | 194.93 ± 3.56 b | 192.77 ± 2.96 b | 340.44 ± 5.98 a | |||
| Terpenes | |||||||||
| V39 | Sabinene | 3387-41-5 | 1160 | 2.84 ± 0.52 c | 11.25 ± 0.92 b | 8.43 ± 0.95 b | 10.71 ± 1.13 b | 11.02 ± 0.86 b | 15.31 ± 0.93 a |
| V40 | Myrcene | 123-35-3 | 1145 | 8.38 ± 0.75 b | 12.34 ± 0.93 a | 7.84 ± 0.98 b | 9.31 ± 1.17 b | 12.09 ± 1.09 a | 12.17 ± 0.95 a |
| V41 | (+)-Dipentene | 5989-27-5 | 1203 | 76.70 ± 3.11 e | 174.82 ± 6.01 ab | 126.56 ± 5.16 d | 152.97 ± 4.27 c | 181.89 ± 4.51 a | 162.42 ± 4.45 bc |
| V42 | γ-Terpinene | 99-85-4 | 1178 | 15.05 ± 0.54 f | 35.65 ± 0.62 b | 22.08 ± 0.27 e | 25.18 ± 0.33 d | 34.10 ± 0.44 c | 43.34 ± 0.54 a |
| V43 | α-Caryophyllene | 6753-98-6 | 2209 | 8.66 ± 0.51 bc | 3.12 ± 0.31 e | 7.04 ± 0.59 d | 7.73 ± 0.55 cd | 10.36 ± 0.79 a | 10.04 ± 0.30 ab |
| V44 | α-Curcumene | 644-30-4 | 1773 | 80.18 ± 3.47 a | 63.90 ± 1.53 b | 40.78 ± 0.76 c | 45.22 ± 1.10 c | 60.45 ± 1.98 b | 64.78 ± 1.46 b |
| V45 | α-Farnesene | 502-61-4 | 1543 | 18.50 ± 0.79 a | 14.98 ± 0.50 b | 9.44 ± 0.81 c | 9.43 ± 0.61 c | 14.38 ±0.83 b | 14.33 ± 0.88 b |
| V46 | α-Terpinene | 99-86-5 | 1282 | 4.21 ± 0.44 b | 6.12 ± 0.55 a | 4.50 ± 0.68 b | 4.13 ± 0.25 b | 5.24 ± 0.72 ab | 4.51 ± 0.68 b |
| V47 | β-Bisabolene | 495-61-4 | 1536 | 23.70 ± 1.95 a | 17.96 ± 0.81 b | 11.52 ± 0.60 c | 11.99 ± 0.45 c | 17.52 ± 0.91 b | 17.33 ± 0.83 b |
| V48 | β-Caryophyllene | 87-44-5 | 1594 | 57.39 ± 1.61 c | 82.40 ± 2.28 a | 54.82 ± 1.54 c | 65.7 ± 1.70 b | 79.08 ± 1.34 a | 83.45 ± 1.60 a |
| V49 | β-Famesene | 18794-84-8 | 1661 | 2.29 ± 0.20 d | 2.51 ± 0.51 d | 3.19 ± 0.14 cd | 4.58 ± 0.45 b | 3.78 ± 0.35 bc | 6.62± 0.55 a |
| V50 | (1 S)-(1)-β-Pinene | 18172-67-3 | 1152 | 1.83 ± 0.18 d | 5.20 ± 0.17 b | 3.76 ± 0.47 c | 5.10 ± 0.25 b | 5.22 ± 0.23 b | 6.36 ± 0.34 a |
| V51 | 3-Carene | 13466-78-9 | 1146 | 1.32 ± 0.18 c | 3.09 ± 0.27 ab | 2.51 ± 0.25 b | 3.12 ± 0.40 ab | 3.61 ± 0.21 a | 3.09 ± 0.28 ab |
| V52 | Camphene | 79-92-5 | 1705 | 9.35 ± 0.65 a | 4.00 ± 0.51 b | 3.20 ± 0.27 b | 3.59 ± 0.21 b | 4.10 ± 0.42 b | 9.99 ± 1.12 a |
| V53 | Cyclosativene | 22469-52-9 | 1373 | 3.14 ± 0.23 b | 3.75 ± 0.34 b | 3.05 ± 0.52 b | 3.93 ± 0.40 b | 4.08 ± 0.45 b | 5.42 ± 0.62 a |
| Total | 325.40 ± 5.15 c | 453.54 ± 7.54 a | 316.05 ± 2.63 c | 407.91 ± 5.97 b | 457.38 ± 6.22 a | 470.86 ± 7.16 a | |||
| Others | |||||||||
| V54 | D-Camphor | 464-49-3 | 1427 | 12.34 ± 0.24 f | 17.03 ± 0.20 d | 15.33 ± 0.41 e | 25.08 ± 0.61 b | 18.97 ± 0.54 c | 27.64 ±0.34 a |
| V55 | 4-Allylanisole | 140-67-0 | 1655 | 164.56 ± 4.45 b | 161.06 ± 4.14 b | 108.28 ± 2.45 d | 129.21 ± 2.56 d | 146.72 ± 2.23 c | 188.07 ± 4.21 a |
| V56 | Anethole | 104-46-1 | 1595 | 315.01 ± 11.00 b | 246.66 ± 8.66 d | 232.42 ± 7.37 d | 275.28 ± 5.57 c | 224.05 ± 6.32 e | 344.05 ± 5.87 a |
| V57 | Safrole | 94-59-7 | 1628 | 12.59 ± 0.51 bc | 14.59± 0.45 ab | 8.91 ± 0.68 d | 10.48 ± 0.75 cd | 13.99 ± 0.65 ab | 15.97 ± 1.34 a |
| V58 | Eugenol | 97-53-0 | 2141 | 240.48 ± 6.94 a | 187.47 ± 5.33 b | 136.75 ± 5.46 c | 140.72 ± 4.47 c | 148.83 ± 5.12 c | 231.42 ± 6.27 a |
| V59 | Methyl eugenol | 93-15-2 | 1755 | 5.89 ± 0.24 c | 8.00 ± 0.37 b | 2.49 ± 0.08 d | 3.38 ± 0.13 d | 6.78 ± 0.34 c | 14.27 ± 0.55 a |
| Total | 750.87 ± 10.83 b | 634.81 ± 19.57 c | 504.18 ± 6.86 e | 584.15 ± 5.57 d | 559.34 ± 7.57 d | 821.42 ± 12.30 a |
| Attribute | CT | CR | PK | TD | DH |
|---|---|---|---|---|---|
| Colour | 5.52 ± 0.33 a | 5.15 ± 0.26 a | 5.32 ± 0.43 a | 5.18 ± 0.33 a | 5.48 ± 0.38 a |
| Hardness | 6.23 ± 0.42 a | 5.53 ± 0.35 ab | 4.33 ± 0.45 c | 5.47 ± 0.31 ab | 4.83 ± 0.15 bc |
| Amora | 4.83 ± 0.25 b | 4.80 ± 0.46 b | 5.73 ± 0.31 a | 5.97 ± 0.25 a | 6.30 ± 0.20 a |
| Sourness | 4.37 ± 0.40 a | 4.60 ± 0.26 a | 4.43 ± 0.25 a | 4.40 ± 0.30 a | 4.47 ± 0.21 a |
| Saltiness | 5.60 ±0.30 a | 3.27 ± 0.31 c | 4.20 ± 0.26 b | 3.33 ± 0.32 c | 4.40 ± 0.26 b |
| Umami | 5.07 ± 0.35 a | 4.60 ± 0.26 ab | 4.97 ± 0.31 a | 4.33 ± 0.25 ab | 4.07 ± 0.25 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-A.; Kong, B.; Wen, R.; Wang, H.; Li, M.; Chen, Q. Flavour Compensation Role of Yeast Strains in Reduced-Salt Dry Sausages: Taste and Odour Profiles. Foods 2022, 11, 650. https://doi.org/10.3390/foods11050650
Li X-A, Kong B, Wen R, Wang H, Li M, Chen Q. Flavour Compensation Role of Yeast Strains in Reduced-Salt Dry Sausages: Taste and Odour Profiles. Foods. 2022; 11(5):650. https://doi.org/10.3390/foods11050650
Chicago/Turabian StyleLi, Xiang-Ao, Baohua Kong, Rongxin Wen, Huiping Wang, Mengtong Li, and Qian Chen. 2022. "Flavour Compensation Role of Yeast Strains in Reduced-Salt Dry Sausages: Taste and Odour Profiles" Foods 11, no. 5: 650. https://doi.org/10.3390/foods11050650
APA StyleLi, X.-A., Kong, B., Wen, R., Wang, H., Li, M., & Chen, Q. (2022). Flavour Compensation Role of Yeast Strains in Reduced-Salt Dry Sausages: Taste and Odour Profiles. Foods, 11(5), 650. https://doi.org/10.3390/foods11050650







