Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Materials
2.2. Preparation of the Tea Extracts
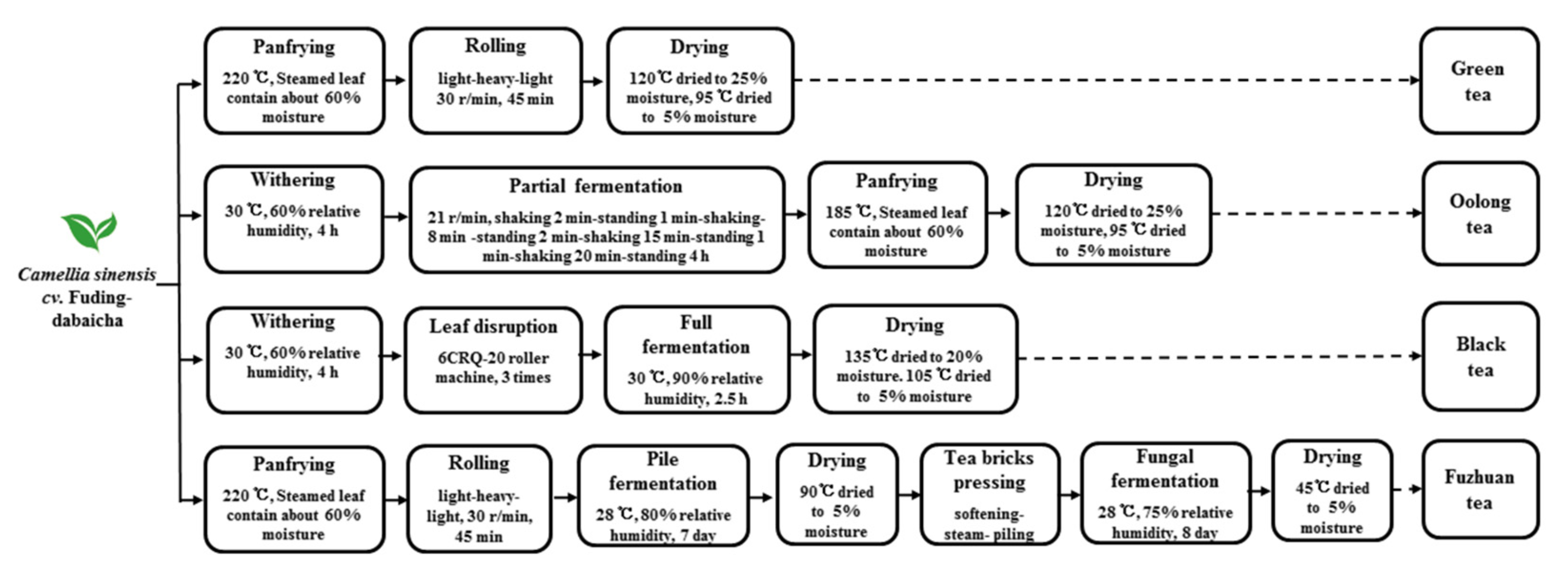
2.3. Extraction of Catechins
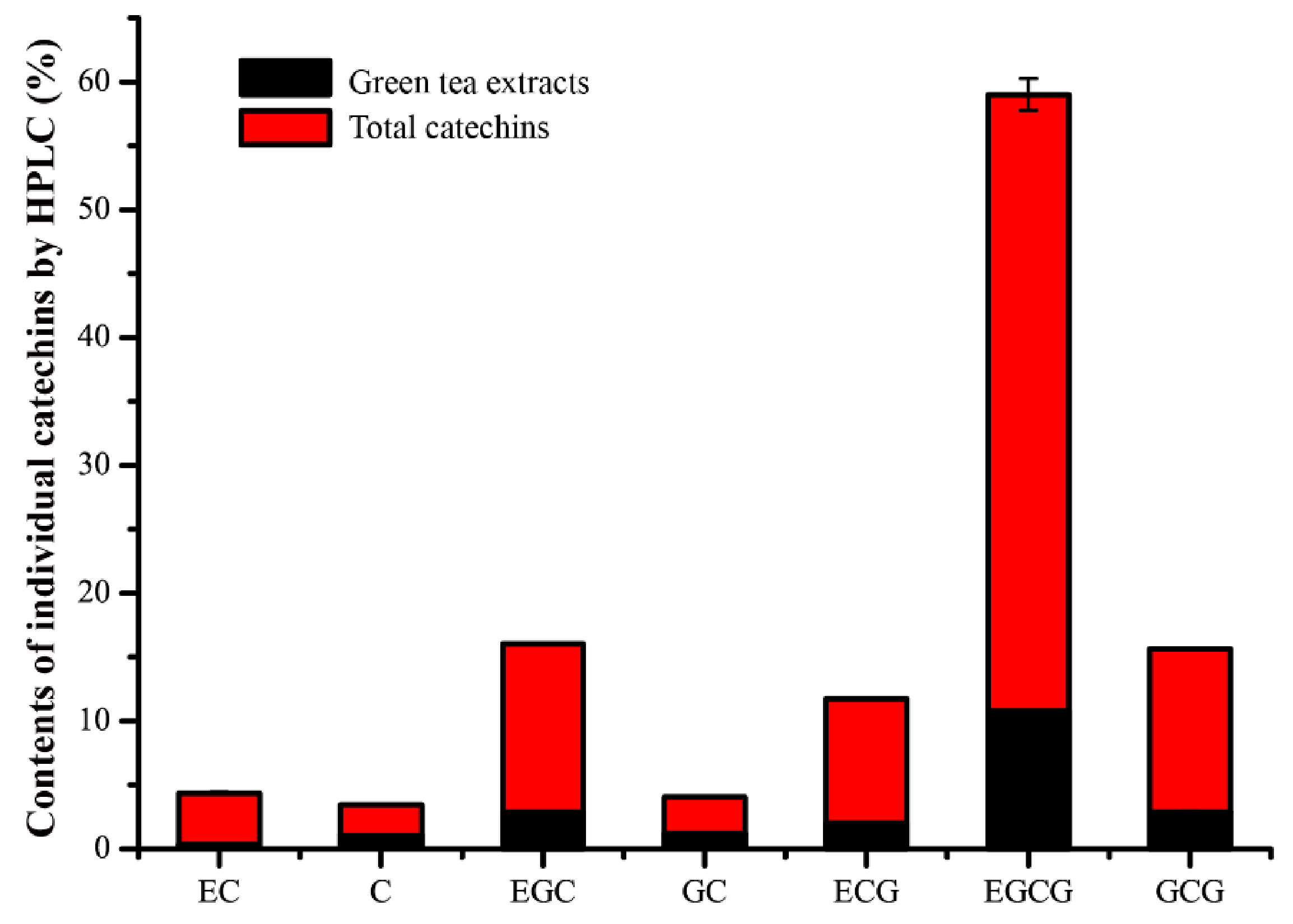
2.4. Chemical Identification of Tea Extracts
2.5. Bacterial Susceptibility Test
2.6. Determination of the Minimum Inhibitory Concentration (MIC)
2.7. Membrane Permeability Assay
2.8. Membrane Integrity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Comparison of the Antibacterial Efficacy of Different Tea Extracts
3.2. Catechins Play the Main Role in Antibacterial Efficacy
3.3. Antibacterial Mechanism of Catechins
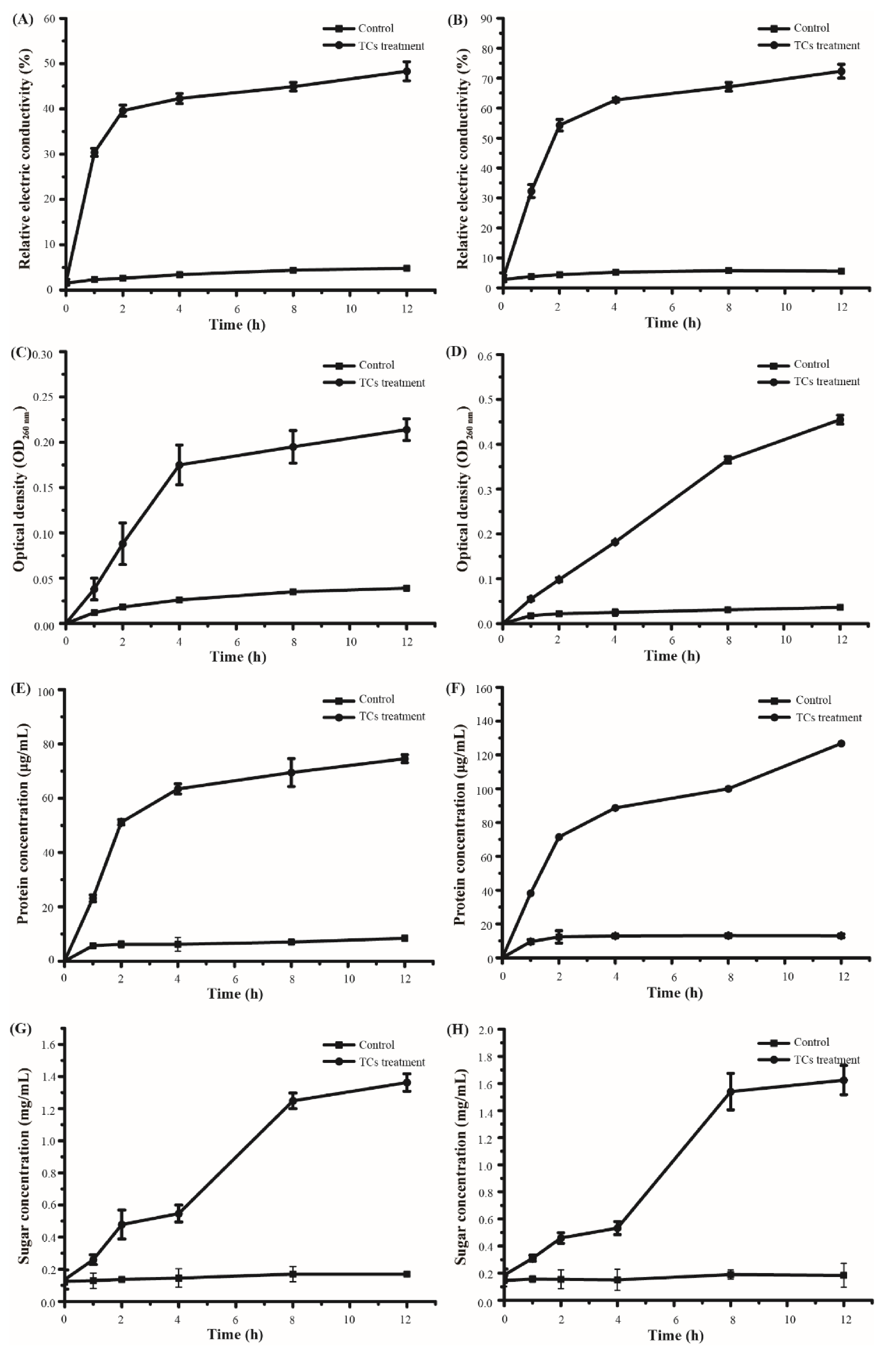
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Esch-erichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Franz, C.M.; Besten, H.M.D.; Böhnlein, C.; Gareis, M.; Zwietering, M.H.; Fusco, V. Microbial food safety in the 21st century: Emerging challenges and foodborne pathogenic bacteria. Trends Food Sci. Technol. 2019, 84, 34–37. [Google Scholar] [CrossRef]
- Fleming-Jones, M.E.; Smith, R.E. Volatile organic compounds in foods: A five year study. J. Agric. Food Chem. 2003, 51, 8120–8127. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.N.S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Qu, F.F.; Liu, S.Y.; He, C.; Zhou, J.T.; Zhang, S.M.; Ai, Z.Y.; Chen, Y.Q.; Yu, Z.; Ni, D.J. Comparison of the effects of green and black tea extracts on Na+/K+-ATPase activity in intestine of type 1 and type 2 diabetic mice. Mol. Nutr. Food Res. 2019, 63, e1801039. [Google Scholar] [CrossRef]
- Sharangi, A. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Obanda, M.; Owuor, P.; Mang’Oka, R.; Kavoi, M.M. Changes in thearubigin fractions and theaflavin levels due to variations in processing conditions and their influence on black tea liquor brightness and total colour. Food Chem. 2004, 85, 163–173. [Google Scholar] [CrossRef]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of different teas against starch digestibility by mammalian glycosidases. J. Agric. Food Chem. 2009, 58, 148–154. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.Z.; Zhou, Y.B.; Ling, T.J.; Wan, X.C. Chinese dark teas: Postfermentation, chemistry and biological activ-ities. Food Res. Int. 2013, 53, 600–607. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]
- Wu, C.D.; Wei, G. Tea as a functional food for oral health. Nutrition 2002, 18, 443–444. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef]
- Mbata, T. Preliminary studies of the antibacterial activities of processed Kenyan and Nigerian tea. Afr. J. Biotech. 2007, 6, 278–279. [Google Scholar]
- Almajano, M.; Carbó, R.; Jiménez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Bancirova, M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.C.; Lin, L.L.; Chung, K.T. Antimicrobial activity of tea as affected by the degree of fermentation and manufacturing season. Int. J. Food Microbiol. 1999, 48, 125–130. [Google Scholar] [CrossRef]
- Vieira, C.; Sarmiento-García, A.; García, J.J.; Rubio, B.; Martínez, B. Quality and shelf life of fresh meat from Iberian pigs as affected by a new form of presentation of oleic acid and an organic-acid mix in the diet. Foods 2021, 10, 985. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Bond, A.E.; Yang, L. Essential oil-incorporated carbon nanotubes filters for bacterial removal and inactivation. PLoS ONE 2019, 14, e0227220. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Z.; Qu, F.; Chen, Y.; Ni, D. Effect of steeping temperature on antioxidant and inhibitory activities of green tea extracts against α-amylase, α-glucosidase and intestinal glucose uptake. Food Chem. 2017, 234, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Huang, H.; Kwok, K. Properties of tea-polyphenol-complexed bromelain. Food Res. Int. 1999, 32, 545–551. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Z.; Zhu, H.; Zhang, W.; Chen, Y. In vitroα-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement. Altern. Med. 2016, 16, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L. Physicochemical Analysis on Tea Quality; Shanghai Science and Technology Press: Shanghai, China, 1989. [Google Scholar]
- Goni, P.; Lopez, P.; Sanchez, C.; Gomez-Lus, R.; Becerril, R.; Nerin, C. Antimicrobial activity in the vapour phase of a com-bination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.; Domingues, F. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Chen, X.G.; Liu, C.; Meng, X.H.; Yu, L.J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; Dos Santos D’Almeida, C.T.; Nascimento, T.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Yoda, Y.; Hu, Z.Q.; Shimamura, T.; Zhao, W.H. Different susceptibilities of Staphylococcus and gram-negative rods to epi-gallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef]
- Ignasimuthu, K.; Prakash, R.; Murthy, P.S.; Subban, N. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on gram-negative bacteria. LWT 2019, 105, 103–109. [Google Scholar] [CrossRef]
- Cao, H.; Shi, Y.J.; Chen, X.Q. Advances on the Interaction between tea catechins and plasma proteins: Structure-affinity rela-tionship, influence on antioxidant activity, and molecular docking aspects. Curr. Drug Metab. 2013, 14, 446–450. [Google Scholar] [CrossRef]
- Orak, H.; Yagar, H.; Isbilir, S.; Demirci, A.; Gumus, T. Antioxidant and antimicrobial activities of white, green and black tea extracts. Acta Aliment. 2013, 42, 379–389. [Google Scholar] [CrossRef]
- Parvez, M.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.A.; Islam, M.; Shariare, M.H. Antibac-terial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Perez-Alvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity rela-tionship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Zhou, W.; Li, P.; Liu, G.; Zhang, J.; Dai, Y. Mode of action of pentocin 31-1: An antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control 2008, 19, 817–822. [Google Scholar] [CrossRef]
| Test Solution | DIZ (mm) | ||||
|---|---|---|---|---|---|
| E. coli | S. typhimurium | E. faecalis | S. aureus | ||
| Green tea | 20 mg/mL | 6.60 ± 0.19 c | 8.80 ± 0.15 c | 8.50 ± 0.16 a | 21.16 ± 0.08 c |
| 40 mg/mL | 7.20 ± 0.09 b | 14.25 ± 0.19 b | 19.80 ± 0.22 b | 24.50 ± 0.16 b | |
| 80 mg/mL | 12.40 ± 0.17 a | 22.00 ± 0.13 a | 25.90 ± 0.13 a | 31.33 ± 0.12 a | |
| Oolong tea | 20 mg/mL | 6.10 ± 0.04 c | 7.20 ± 0.10 c | 7.50 ± 0.06 c | 18.15 ± 0.12 c |
| 40 mg/mL | 6.70 ± 0.11 b | 12.65 ± 0.07 b | 16.90 ± 0.11 b | 22.60 ± 0.13 b | |
| 80 mg/mL | 11.40 ± 0.17 a | 20.00 ± 0.11 a | 21.90 ± 0.09 a | 27.33 ± 0.17 a | |
| Black tea | 20 mg/mL | 6.10 ± 0.08 b | 6.10 ± 0.07 c | 6.40 ± 0.05 c | 12.02 ± 0.09 c |
| 40 mg/mL | 6.30 ± 0.13 b | 9.90 ± 0.03 b | 14.80 ± 0.08 b | 18.33 ± 0.16 b | |
| 80 mg/mL | 10.10 ± 0.04 a | 15.30 ± 0.11 a | 20.00 ± 0.13 a | 25.50 ± 0.13 a | |
| Fuzhuan tea | 20 mg/mL | 6.10 ± 0.18 b | 6.30 ± 0.13 c | 7.10 ± 0.13 c | 14.33 ± 0.11 c |
| 40 mg/mL | 6.40 ± 0.21 b | 10.75 ± 0.12 b | 16.50 ± 0.16 b | 20.83 ± 0.08 b | |
| 80 mg/mL | 11.93 ± 0.10 a | 17.10 ± 0.14 a | 20.80 ± 0.08 a | 25.17 ± 0.17 a | |
| Test Solution | MIC (mg/mL) | |||
|---|---|---|---|---|
| E. coli | S. typhimurium | E. faecalis | S. aureus | |
| Green tea | 35 | 16 | 20 | 10 |
| Oolong tea | 45 | 20 | 32 | 12 |
| Black tea | 65 | 36 | 40 | 16 |
| Fuzhuan tea | 45 | 38 | 32 | 18 |
| Tea Extracts | Polyphenol | Catechin | Gallic acid | Caffeine | Carbohydrate | Amino Acid | Theaflavin | Thearubigin | Theabrownin |
|---|---|---|---|---|---|---|---|---|---|
| Green tea | 27.10 ± 0. 05 a | 21.30 ± 0. 05 a | 0.13 ± 0.01 c | 3.40 ± 0.02 c | 10.41 ± 0.10 b | 4.88 ± 0.16 a | 0.09 ± 0.01 d | 2.71 ± 0.04 d | 1.70 ± 0.02 d |
| Oolong tea | 21.60 ± 0.04 b | 11.16 ± 0.03 b | 0.55 ± 0.02 b | 4.12 ± 0.01 b | 10.24 ± 0.07 b | 4.64 ± 0.24 a | 0.87 ± 0.01 b | 3.13 ± 0.01 c | 3.19 ± 0.06 c |
| Black tea | 17.26 ± 0.03 c | 1.67 ± 0.01 d | 0.78 ± 0.04 a | 5.23 ± 0.02 a | 13.24 ± 0.01 a | 4.73 ± 0.11 a | 1.80 ± 0.01 a | 16.13 ± 0.01 a | 17.80 ± 0.06 b |
| Fuzhuan tea | 19.60 ± 0.08 d | 2.22 ± 0.05 c | 0.75 ± 0.004 a | 5.48 ± 0.05 a | 8.91 ± 0.27 c | 4.43 ± 0.37 a | 0.24 ± 0.02 c | 6.76 ± 0.06 b | 18.77 ± 0.15 a |
| Microorganisms | DIZ (mm) | MIC (mg/mL) | Correlation | ||
|---|---|---|---|---|---|
| 1.0 mg/mL | 5.0 mg/mL | 10.0 mg/mL | |||
| E. coli | 6.30 ± 0.04 b | 11.50 ± 0.16 d | 12.20 ± 0.29 d | 2.5 | 0.9866 |
| S. typhimurium | 6.30 ± 0.05 b | 14.30 ± 0.19 c | 24.60 ± 0.18 c | 2.0 | 0.9946 |
| E. faecalis | 7.30 ± 0.05 b | 17.90 ± 0.08 b | 27.20 ± 0.09 b | 2.0 | 0.9791 |
| S. aureus | 9.90 ± 0.22 a | 22.20 ± 0.10 a | 32.20 ± 0.32 a | 1.5 | 0.9997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. https://doi.org/10.3390/foods11040620
Liu S, Zhang Q, Li H, Qiu Z, Yu Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods. 2022; 11(4):620. https://doi.org/10.3390/foods11040620
Chicago/Turabian StyleLiu, Shuyuan, Qiqi Zhang, Hang Li, Zheyu Qiu, and Youben Yu. 2022. "Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts" Foods 11, no. 4: 620. https://doi.org/10.3390/foods11040620
APA StyleLiu, S., Zhang, Q., Li, H., Qiu, Z., & Yu, Y. (2022). Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods, 11(4), 620. https://doi.org/10.3390/foods11040620






