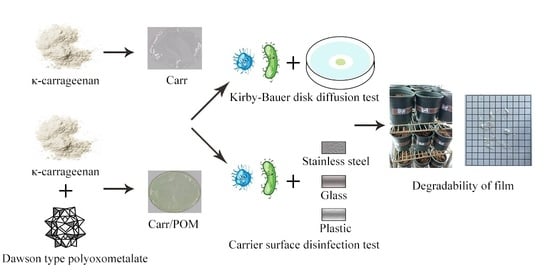
Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Preparation of Carr/POM Film
2.3. Measurement of the Film Thickness
2.4. Measurement of Water Content in the Film
2.5. Measurement of the Tensile Strength of the Film
2.6. Measurement of Color and Transparency of the Film
2.7. Scanning Electron Microscopy (SEM)
2.8. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.9. Thermogravimetric Analysis
2.10. Determination of Bactericidal Activity of Carr/POM Film
2.10.1. Kirby-Bauer Disc Diffusion Method
2.10.2. Carrier Surface Disinfection Test
2.11. Determination of Film Degradability in Soil
2.12. Statistical Analysis
3. Results and Discussion
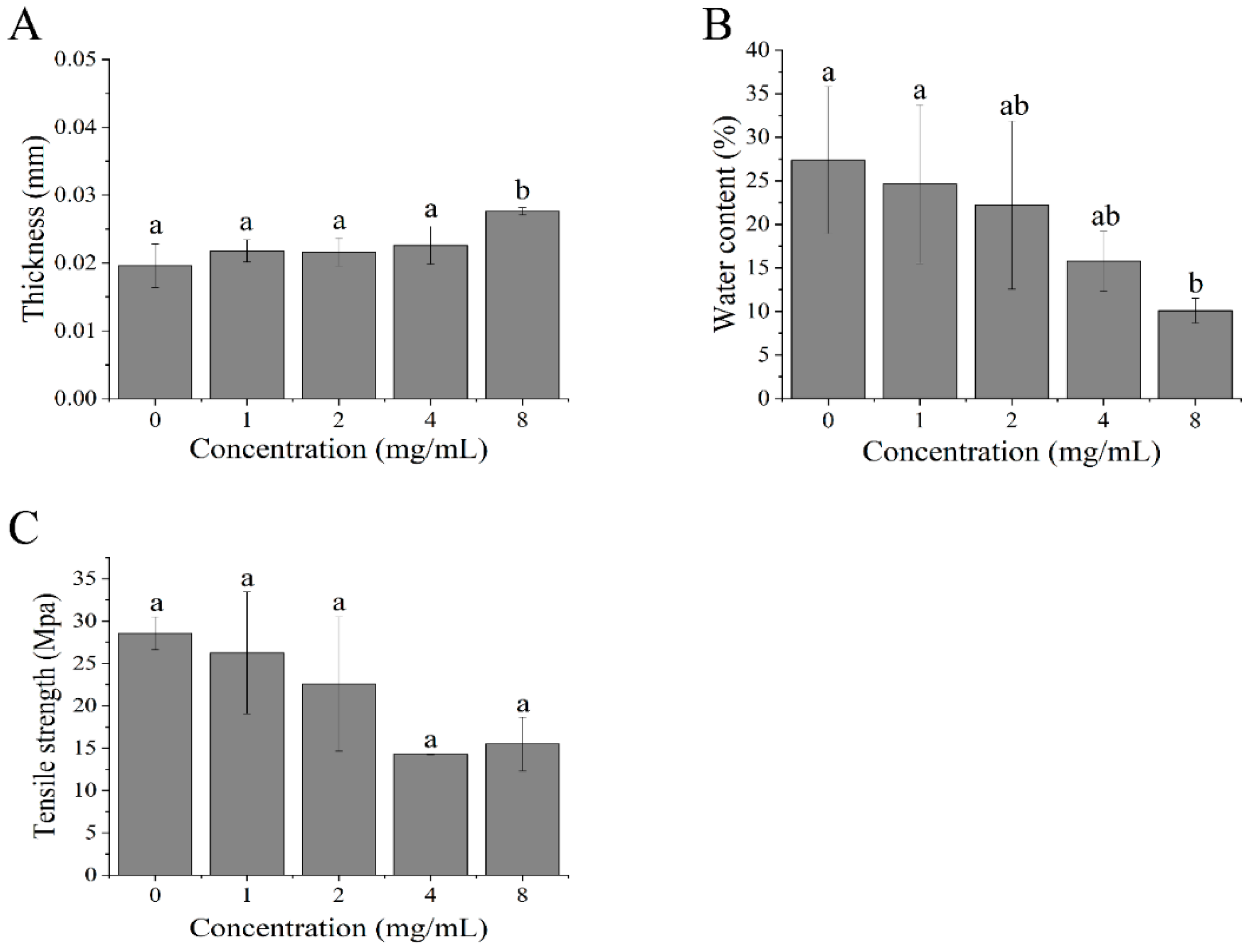
3.1. The Effects of Polyoxometalate Concentration on the Thickness of the Film
3.2. The Effects of Polyoxometalate Concentration on the Water Content of the Film
3.3. The Effects of Polyoxometalate Concentration on the Tensile Strength of the Film
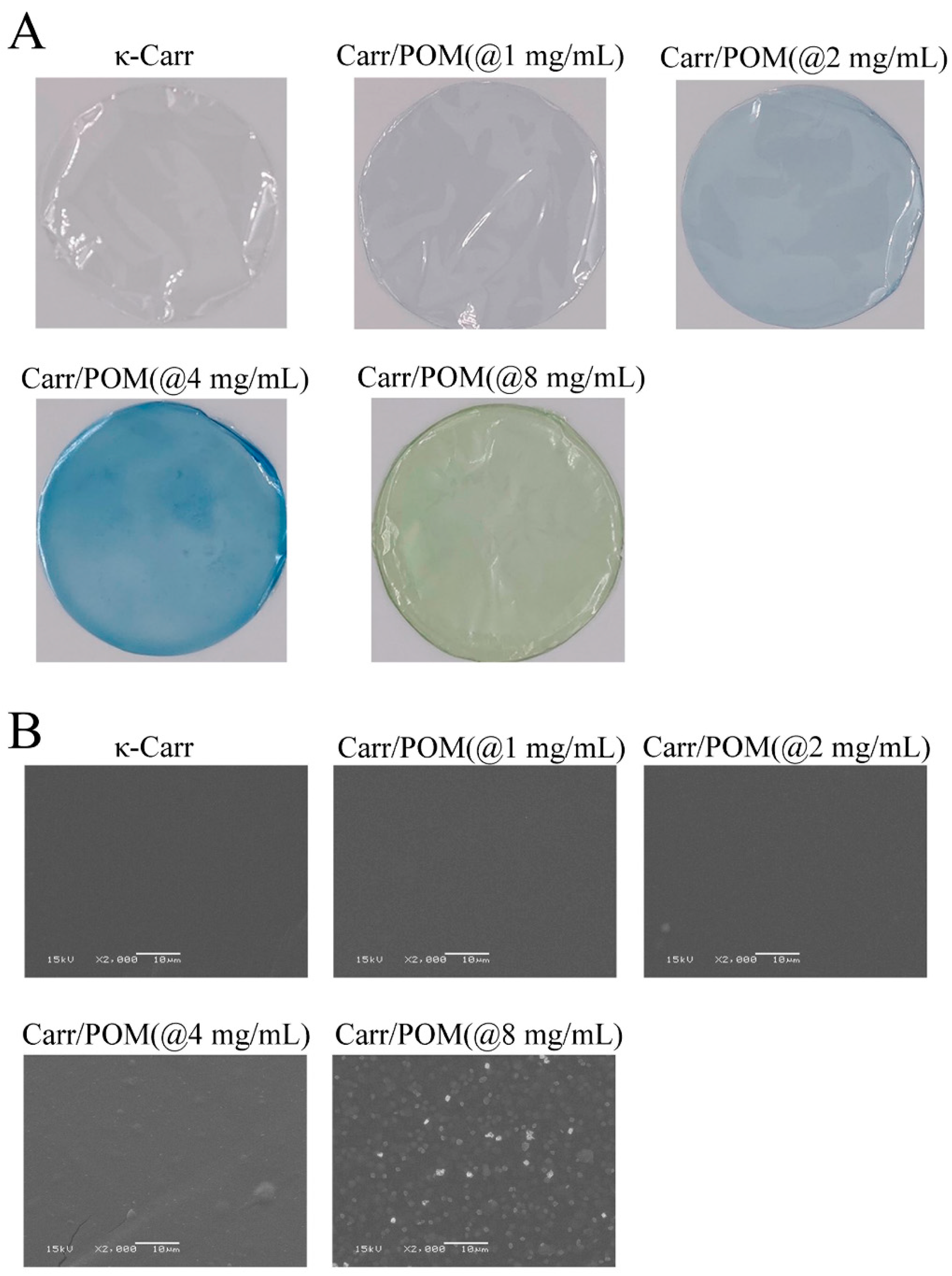
3.4. The Effects of Polyoxometalate Concentration on the Color and Opacity of the Film
3.5. The Effects of Polyoxometalate Concentration on the Microstructures of the Film
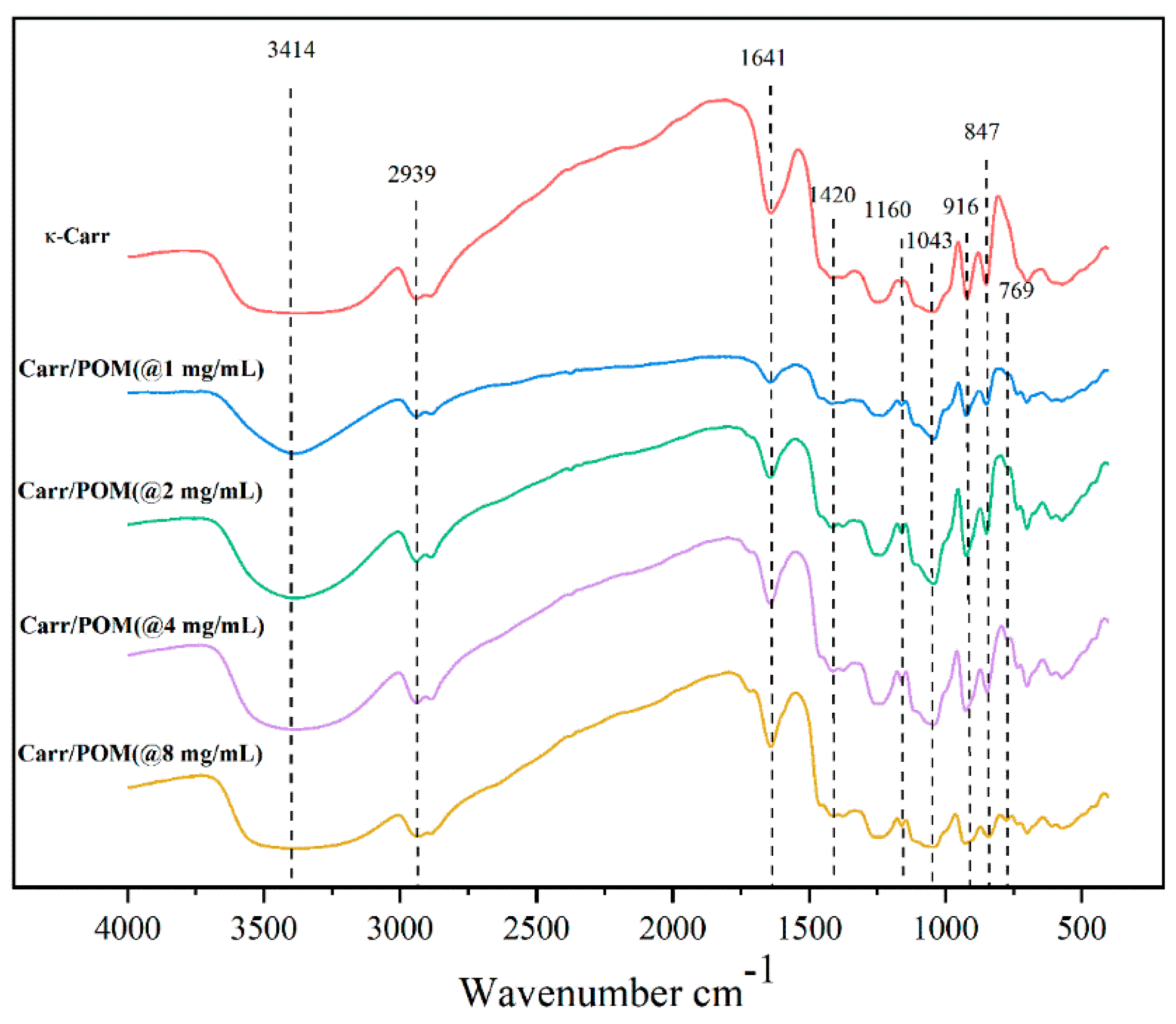
3.6. FTIR Spectroscopic Analysis of Carr/POM Film
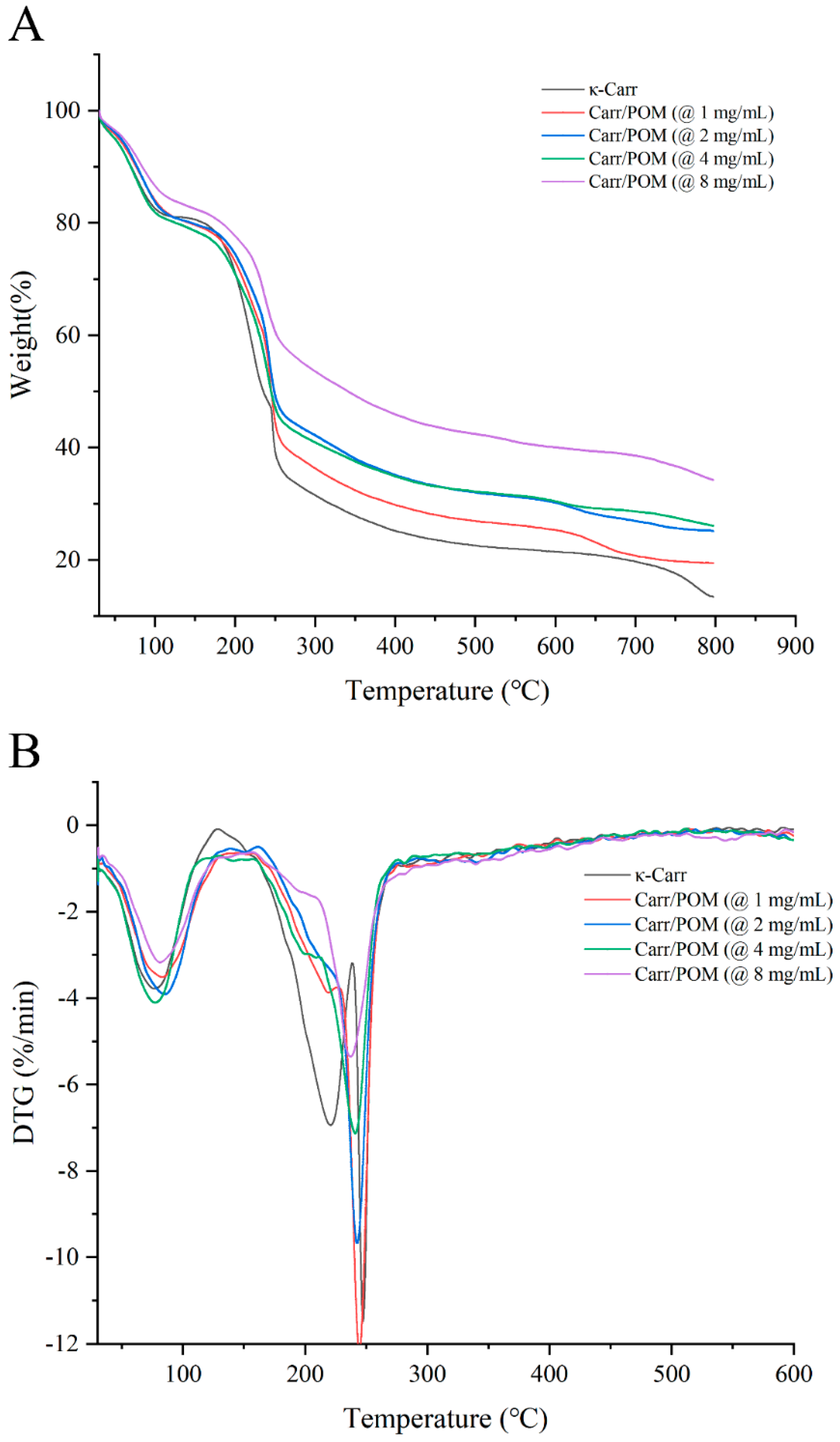
3.7. Thermogravimetric Analysis of Carr/POM Anti-Bacterial Film
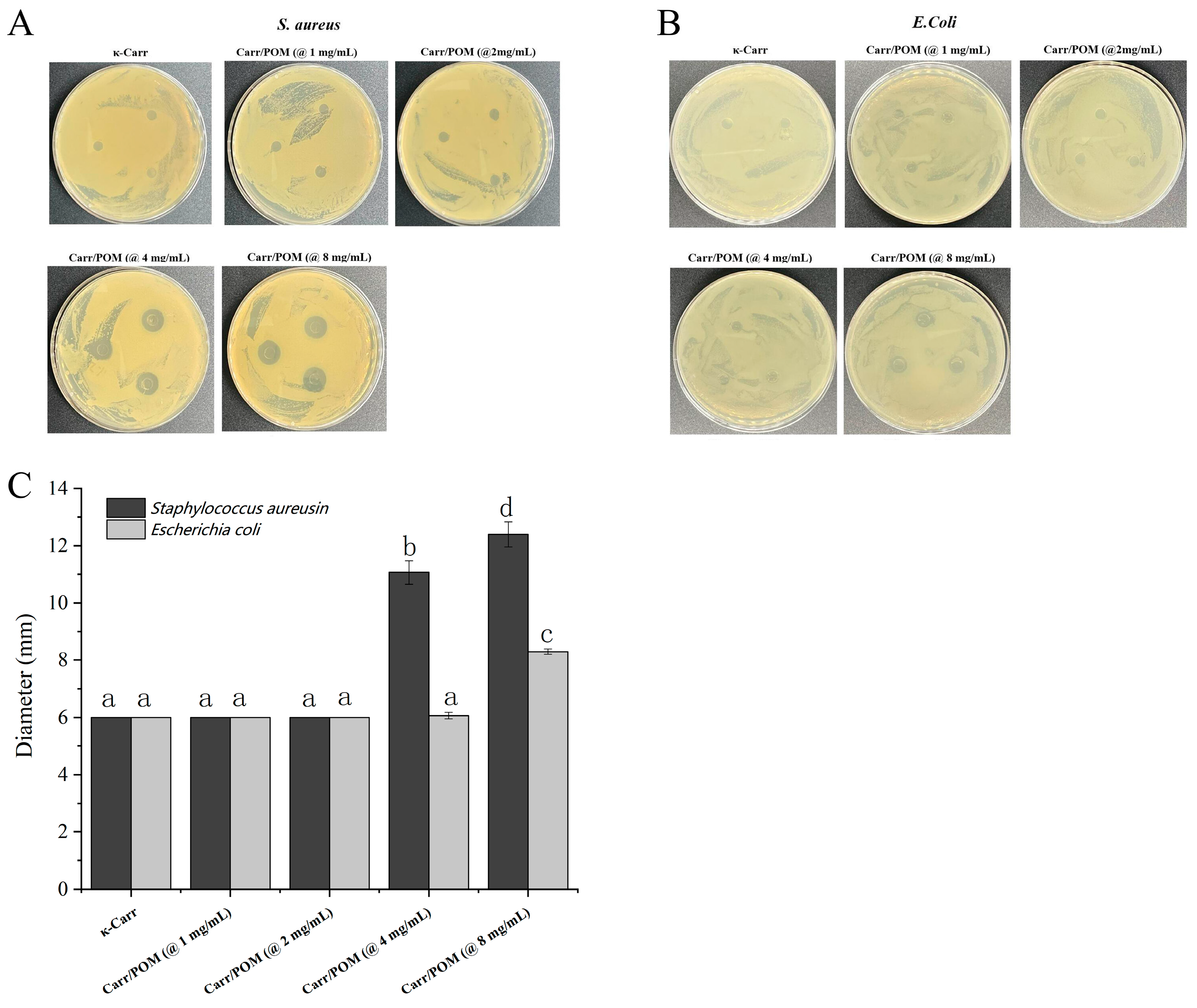
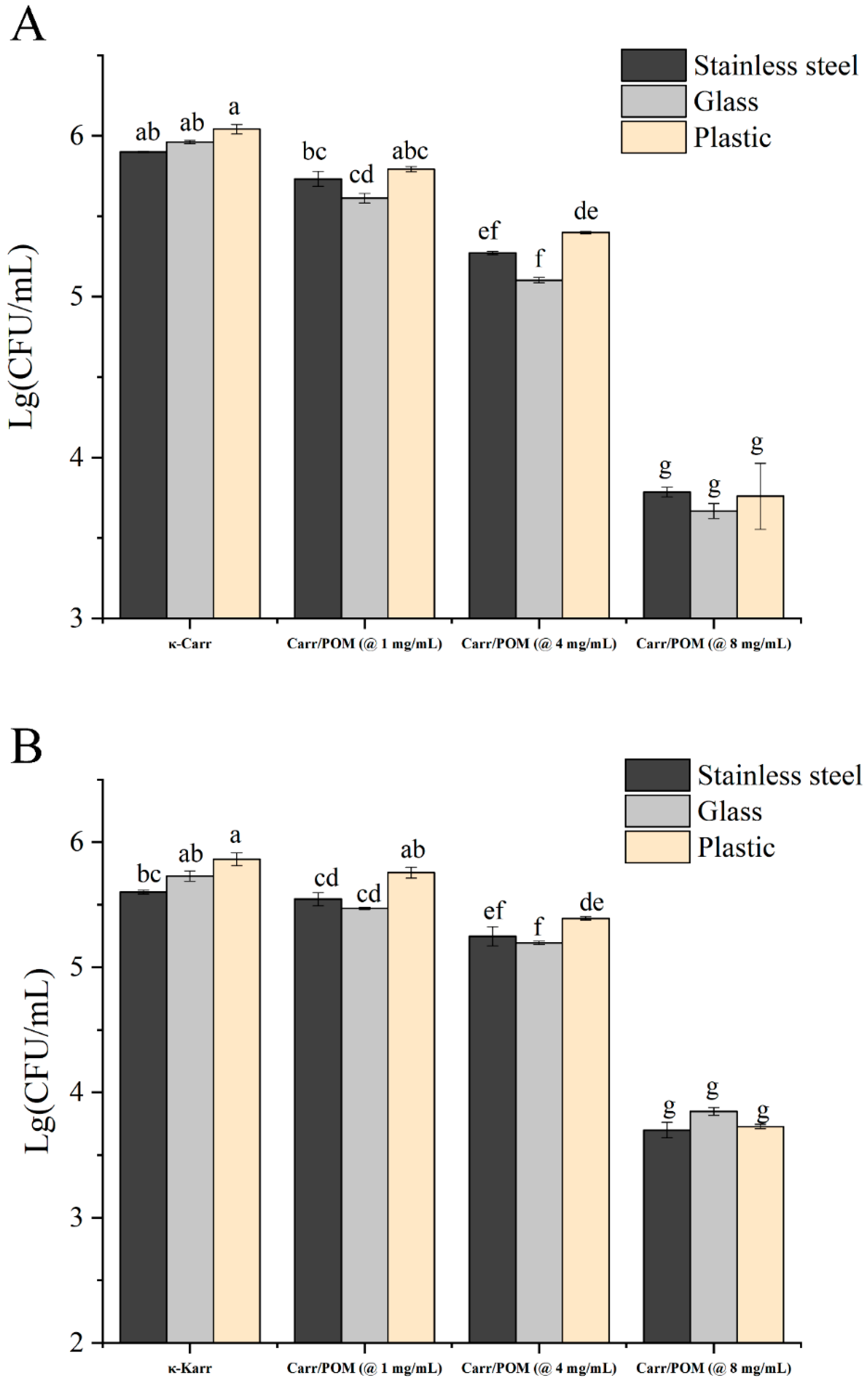
3.8. Bactericidal Activity of Carr/POM Anti-Bacterial Film
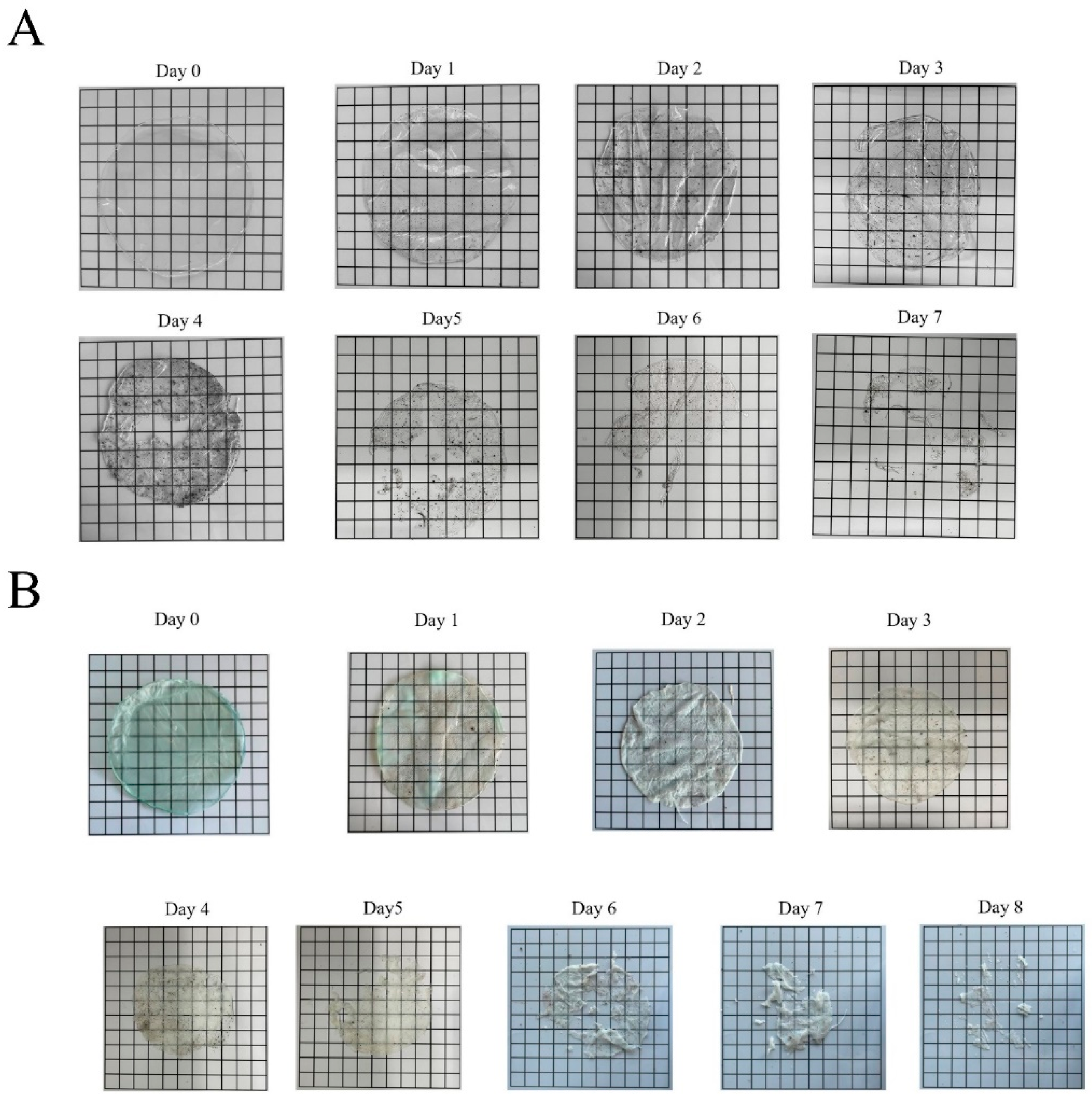
3.9. Degradability of Carr/POM Anti-Bacterial Film in Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasajpour, A.; Ansari, S.; Rinoldi, C.; Rad, A.S.; Tamayol, A. A Multifunctional Polymeric Periodontal Membrane with Osteogenic and Antibacterial Characteristics. Adv. Funct. Mater. 2018, 28, 1703437. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, J.; Wang, D.; Heise, A.; Lang, M. Chemo-Enzymatic Synthesis of Poly(4-piperidine lactone-b-ω-pentadecalactone) Block Copolymers as Biomaterials with Antibacterial Properties. Biomacromolecules 2018, 19, 2673–2681. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Z.; Wang, X.; Editing, W.R.; Shi, C. A dynamic risk assessment approach based on stochastic hybrid system: Application to microbial hazards in food processing. Microb. Risk Anal. 2021, 19, 100163. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jia, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2021, 375, 131738. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 375, 131738. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, P.; McBain, A.J.; Rickard, A.H. Formation of microbial biofilm in hygienic situations: A problem of control. Int. Biodeterior. Biodegrad. 2003, 51, 245–248. [Google Scholar] [CrossRef]
- Lai, D.; Zhou, F.; Zhou, A.; Hamza, S.S.; Zhang, Y.; Hu, J.; Lin, S. Comprehensive properties of photodynamic antibacterial film based on κ-Carrageenan and curcumin-β-cyclodextrin complex. Carbohydr. Polym. 2022, 282, 119112. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Lajevardi, A.; Rahimi, T.; Tanzifi, M.; Ghorbanpour, M. An investigation of the role of fabrication process in the physicochemical properties of κ-carrageenan-based films incorporated with Zataria multiflora extract and nanoclay. Food Packag. Shelf Life 2020, 23, 100435. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Abdillah, A.A.; Charles, A.L. Characterization of a natural biodegradable edible film obtained from arrowroot starch and iota-carrageenan and application in food packaging. Int. J. Biol. Macromol. 2021, 191, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tao, N.; Li, L.; Xu, C.; Deng, S.; Wang, Y. Non-migrating active antibacterial packaging and its application in grass carp fillets. Food Packag. Shelf Life 2022, 31, 100786. [Google Scholar] [CrossRef]
- Suzuki, K.; Mizuno, N.; Yamaguchi, K. Polyoxometalate Photocatalysis for Liquid-Phase Selective Organic Functional Group Transformations. ACS Catal. 2018, 8, 10809–10825. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2019, 36, 831–858. [Google Scholar] [CrossRef]
- Sabarinathan, C.; Karthikeyan, M.; Harisma, B.R.; Murugappan, R.M.; Arumuganathan, T. One Pot Synthesis of Luminescent Polyoxometalate Supported Transition Metal Complex and biological evaluation as a potential larvicidal and anti-cancer agent. J. Mol. Struct. 2019, 1206, 127486. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Hu, Q.; Yan, H.; Zeng, Y. Synthesis and evaluation of pyridinium polyoxometalates as anti-HIV-1 agents. Bioorg. Med. Chem. Lett. 2017, 27, 2357–2359. [Google Scholar] [CrossRef]
- Qi, Y.-f.; Zhang, H.; Wang, J.; Jiang, Y.; Li, J.; Yuan, Y.; Zhang, S.; Xu, K.; Li, Y.; Li, J.; et al. In vitro anti-hepatitis B and SARS virus activities of a titanium-substituted-heteropolytungstate. Antivir. Res. 2012, 93, 118–125. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, L.; Yuan, X.; Chen, Y.-G.; Gao, X.; Li, D. Synthesis and Antibacterial Activity of Polyoxometalates with Different Structures. Bioinorg. Chem. Appl. 2018, 2018, 9342326. [Google Scholar] [CrossRef] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Suzuki, T.; Fujita, Y.; Oda, M.; Matsumoto, N.; Yamase, T. Enhancement of antibacterial activity of β-lactam antibiotics by [P2W18O62]6−, [SiMo12O40]4−, and [PTi2W10O40]7− against methicillin-resistant and vancomycin-resistant Staphylococcus aureus. J. Inorg. Biochem. 2006, 100, 1225–1233. [Google Scholar] [CrossRef]
- Lampl, R.; Breibeck, J.; Gumerova, N.I.; Galanski, M.S.; Rompel, A. Wells-Dawson phosphotungstates as mushroom tyrosinase inhibitors: A speciation study. Sci. Rep. 2021, 11, 19354. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Chi, G.; Shuai, D.; Wang, L.; Chen, B.; Li, J. Research progress on the inhibition of enzymes by polyoxometalates. Inorg. Chem. Front. 2020, 7, 4320–4332. [Google Scholar] [CrossRef]
- Chen, B.-N.; Xing, R.; Wang, F.; Zheng, A.P.; Wang, L. Inhibitory effects of α-Na8SiW11CoO40 on tyrosinase and its application in controlling browning of fresh-cut apples. Food Chem. 2015, 188, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Zheng, A.; Wang, F.; Wang, L.; Yu, Y.; Jiang, A. Functionality study of Na6PMo11FeO40 as a mushroom tyrosinase inhibitor. Food Chem. 2015, 175, 292–299. [Google Scholar] [CrossRef]
- Hu, J.; Tan, S.K.; Lim, M.G.L.; Chang, S.H.; Cui, G.; Liu, S.; Narasimhan, K.; New, S.Y.; Wang, X.; Chen, C.; et al. Identification of a Wells–Dawson polyoxometalate-based AP-2γ inhibitor with pro-apoptotic activity. Biochem. J. 2018, 475, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, G.; Cao, L.; Wang, L. Accurately intelligent film made from sodium carboxymethyl starch/κ-carrageenan reinforced by mulberry anthocyanins as an indicator—ScienceDirect. Food Hydrocoll. 2020, 108, 106012. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Red pomelo peel pectin based edible composite films: Effect of pectin incorporation on mechanical, structural, morphological and thermal properties of composite films. Food Hydrocoll. 2022, 123, 107135. [Google Scholar] [CrossRef]
- Yu, X.; Chen, C.; Peng, J.; Shi, Z.; Shen, Y.; Mei, J.; Ren, Z. Antibacterial-active multilayer films composed of polyoxometalate and Methyl Violet: Fabrication, characterization and properties. Thin Solid Films 2014, 571, 69–74. [Google Scholar] [CrossRef]
- MSc, H.H.; Niemeyer, B. Aerosol disinfection of bacterial spores by peracetic acid on antibacterial surfaces and other technical materials. Am. J. Infect. Control 2020, 48, 1200–1203. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.d.S.; Silva, B.E.B.e.; Bittencourt, P.R.S.; Koschevic, M.T.; Silveira, T.F.S.d.; Martines, M.A.U.; Caon, T.; MariaMartelli, S. Biodegradation of polyhydroxybutyrate films incorporated with eugenol in different soil types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Valenzuela, L.M.; Giordano, A.; Cabrera-Barjas, G.; Martin-Belloso, O. k-carrageenan edible films for beef: Honey and bee pollen phenolic compounds improve their antioxidant capacity. Food Hydrocoll. 2021, 124, 107250. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a green film with pH-sensitivity and antioxidant activity based on K-carrageenan and hydroxypropyl methylcellulose incorporating Primus maackii juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Azizullah; Nisar-ur-Rehman; Haider, A.; Kortz, U.; Afridi, S.; Sohail, M.; Joshi, S.A.; Iqbal, J. Novel pH responsive supramolecular hydrogels of chitosan hydrochloride and polyoxometalate: In-vitro, in-vivo and preliminary safety evaluation. Int. J. Pharm. 2017, 533, 125–137. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. Incorporating κ-carrageenan colorimetric film with a wide pH–sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Cui, B.; Liu, Y. Influence of cations on texture, compressive elastic modulus, sol-gel transition and freeze-thaw properties of kappa-carrageenan gel. Carbohydr. Polym. 2018, 202, 530–535. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag. Shelf Life 2020, 24, 100476. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Bio-based films prepared with soybean by-products and pine (Pinus densiflora) bark extract. J. Clean. Prod. 2018, 187, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Li, J.; Wang, L. A green strategy for maintaining intelligent response and improving antioxidant properties of κ-carrageenan-based film via cork bark extractive addition. Food Hydrocoll. 2020, 113, 106470. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, X.; Wang, S.; Yin, C.; Gao, X.; Xiong, G.; Xu, X.; Qi, J.; Mei, L. Emulsified blend film based on konjac glucomannan/carrageenan/camellia oil: Physical, structural, and water barrier properties. Carbohydr. Polym. 2021, 251, 117100. [Google Scholar] [CrossRef]
- Zhao, M.; Fang, Y.; Ma, L.; Zhu, X.; Jiang, L.; Li, M.; Han, Q. Synthesis, characterization and in vitro antibacterial mechanism study of two Keggin-type polyoxometalates. J. Inorg. Biochem. 2020, 210, 111131. [Google Scholar] [CrossRef]
- Shen, M.; Dong, W.; Qian, J.; Zou, L. Antimicrobial activity and membrane interaction mechanism of the antimicrobial peptides derived from Rana chensinensis with short sequences. Biologia 2017, 72, 1089–1097. [Google Scholar] [CrossRef]
- Klobucar, K.; Brown, E.D. New potentiators of ineffective antibiotics: Targeting the Gram-negative outer membrane to overcome intrinsic resistance. Curr. Opin. Chem. Biol. 2022, 66, 102099. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Morgan, J.A.W.; Whipps, J.M.; Saunders, J.R. The role of motility in the in vitro attachment of Pseudomonas putida PaW8 to wheat roots. FEMS Microbiol. Ecol. 2001, 35, 57–65. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E.; Ista, L.K.; Lopez, G.; Chaudhury, M.K. The influence of surface energy on the wetting behaviour of the spore adhesive of the marine alga Ulva linza (synonym Enteromorpha linza). J. R. Soc. Interface 2005, 2, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Kusumaningrum, H.D.; Riboldi, G.; Hazeleger, W.C.; Beumer, R.R. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 2003, 85, 227–236. [Google Scholar] [CrossRef]
- Alves, V.D.; Costa, N.; Coelhoso, I.M. Barrier properties of biodegradable composite films based on kappa-carrageenan/pectin blends and mica flakes. Carbohydr. Polym. 2010, 79, 269–276. [Google Scholar] [CrossRef]
- Rajeswari, A.; Christy, E.J.S.; Pius, E.S.A. Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ. Chem. Ecotoxicol. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Xing, R.; Wang, F.; Dong, L.; Zheng, A.-P.; Wang, L.; Su, W.-J.; Lin, T. Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem. 2016, 197, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Segawa, K.; Matsunag, S.; Matsumoto, N.; Oda, M.; Yamase, T. Antibacterial activity of highly negative charged polyoxotungstates, K27[KAs4W40O140] and K18[KSb9W21O86], and Keggin-structural polyoxotungstates against Helicobacter pylori. J. Inorg. Biochem. 2005, 99, 1023–1031. [Google Scholar] [CrossRef]
| Concentration (mg/mL) | Opacity | L | a | b | ∆E |
|---|---|---|---|---|---|
| 0 | 22.704 ± 5.4115 a | 20.960 ± 2.588 a | 114.353 ± 5.542 a | −41.59 ± 2.326 a | 123.509 ± 5.455 a |
| 1 | 23.028 ± 0.260 b | 20.857 ± 2.051 a | 115.267 ± 5.832 a | −41.817 ± 2.353 a | 124.405 ± 5.827 a |
| 2 | 36.968 ± 2.980 b | 23.423 ± 2.319 a | 119.523 ± 9.984 a | −43.720 ± 4.720 a | 129.412 ± 11.189 a |
| 4 | 90.031 ± 4.783 c | 34.433 ± 4.702 b | 90.787 ± 7.040 b | −33.263 ± 3.178 b | 102.779 ± 6.176 b |
| 8 | 82.047 ± 2.024 c | 40.653 ± 1.620 c | 54.393 ± 1.747 c | −18.927 ± 1.234 c | 70.526 ± 0.816 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Wang, D.; Zhang, J.; Li, J.; Lai, D.; Lin, S.; Hu, J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods 2022, 11, 586. https://doi.org/10.3390/foods11040586
Zhou F, Wang D, Zhang J, Li J, Lai D, Lin S, Hu J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods. 2022; 11(4):586. https://doi.org/10.3390/foods11040586
Chicago/Turabian StyleZhou, Feng, Dehua Wang, Jiawen Zhang, Jing Li, Danning Lai, Shaoling Lin, and Jiamiao Hu. 2022. "Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate" Foods 11, no. 4: 586. https://doi.org/10.3390/foods11040586
APA StyleZhou, F., Wang, D., Zhang, J., Li, J., Lai, D., Lin, S., & Hu, J. (2022). Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods, 11(4), 586. https://doi.org/10.3390/foods11040586