A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Microorganism and Storage Medium
2.2. Microorganism Growth
2.3. Biosurfactant Production
2.4. Scaled-Up Biosurfactant Production
2.5. Biosurfactant Isolation, Purification, and Characterization
2.6. Phytotoxicity Assays with Seeds
2.7. Toxicity Assay with Artemia salina
2.8. Phytotoxicity Assays in Onions (Allium cepa L.)
2.9. Biosurfactant as a Food Additive
2.10. Microbiological Analyses
3. Results and Discussion
3.1. Biosurfactant Properties
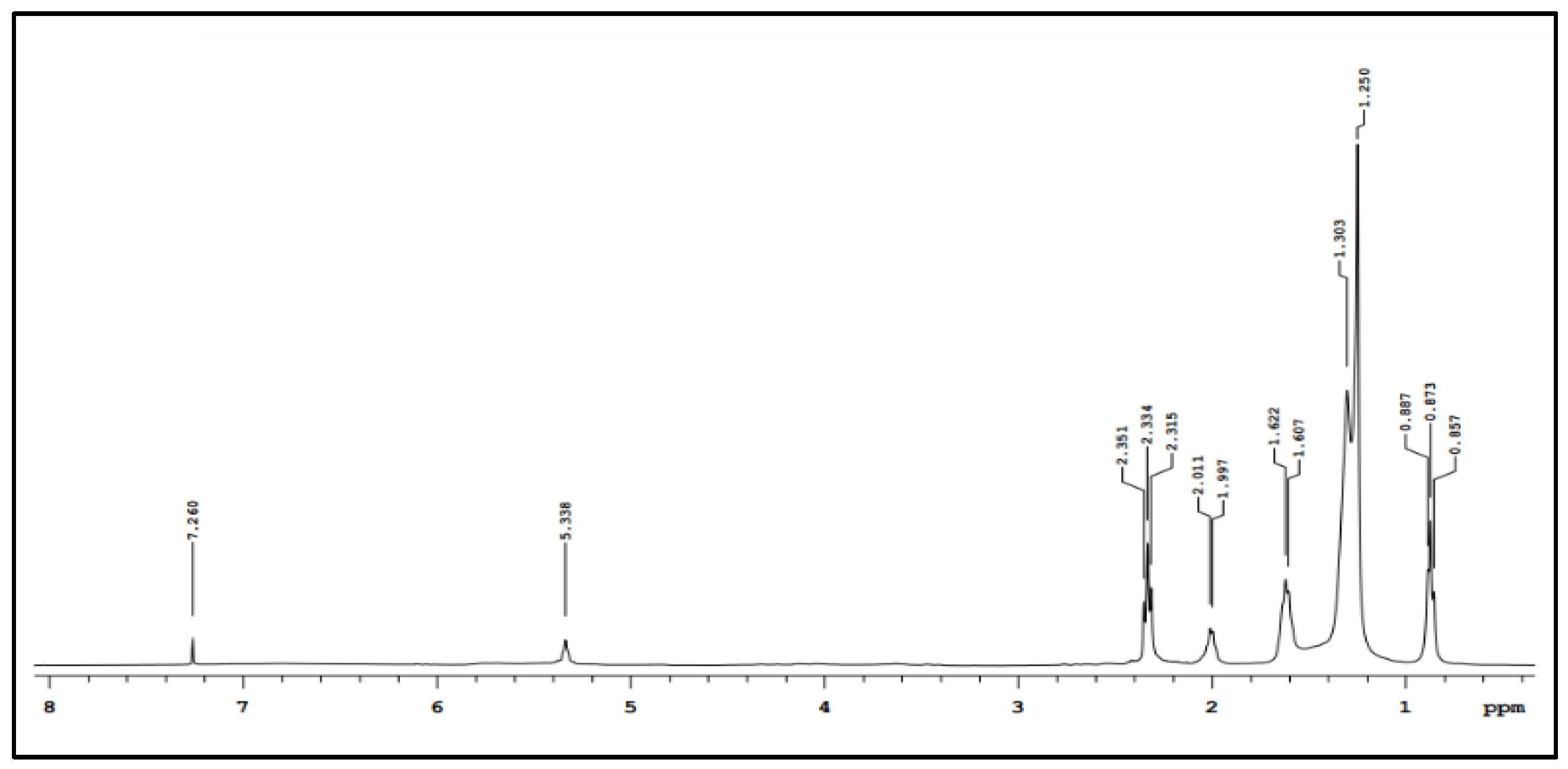
3.2. Biosurfactant Characterization
3.3. Phytotoxicity Assays with Seeds
3.4. Toxicity Assay with Artemia salina
3.5. Phytotoxicity Assays in Onions (Allium cepa L.)
3.6. Biosurfactant as a Food Additive
3.6.1. Selection of the Thickening Agent
3.6.2. Biosurfactant Isolation Influence on Emulsion Stability
3.7. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, V.M.; Jurelevicius, D.; Marques, J.M.; de Souza, P.M.; de Araújo, L.V.; Barros, T.G.; de Souza, R.O.; Freire, D.M.; Seldin, L. Bacillus amyloliquefaciens TSBSO 3.8, a biosurfactant-producing strain with biotechnological potential for microbial enhanced oil recovery. Colloids Surf. B Biointerfaces 2015, 136, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.G.; Soares, S.R.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising Molecules for Petroleum Biotechnology Advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.J.; Lal, S.; Kumar, R.; Verma, C. Bacterial biosurfactants can be an ecofriendly and advanced technology for remediation of heavy metals and co-contaminated soil. Int. J. Environ. Sci. Technol. 2017, 14, 1343–1354. [Google Scholar] [CrossRef]
- Chaprão, M.J.; da Silva, R.C.F.S.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 2018, 27, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, C.; Gutierrez, T. Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.I.; Olaniran, A.O. Production and potential biotechnological applications of microbial surfactants: An overview. Saudi J. Biol. Sci. 2021, 28, 669–679. [Google Scholar] [CrossRef]
- Penha, R.O.; Vandenberghe, L.P.S.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef] [Green Version]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef]
- Anjum, F.; Gautam, G.; Edgard, G.; Negi, S. Biosurfactant production through Bacillus sp. MTCC 5877 and its multifarious applications in food industry. Bioresour. Technol. 2016, 213, 262–269. [Google Scholar] [CrossRef]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of Lipopeptide Biosurfactant by a Marine Nesterenkonia sp. and Its Application in Food Industry. Front. Microbiol. 2017, 28, 1138. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ.-Sci. 2020, 32, 223–227. [Google Scholar] [CrossRef]
- Mohd Isa, M.H.; Shamsudin, N.H.; Al-Shorgani, N.K.; Alsharjabi, F.A.; Kalil, M.S. Evaluation of antibacterial potential of biosurfactant produced by surfactin-producing Bacillus isolated from selected Malaysian fermented foods. Food Biotechnol. 2020, 34, 1–24. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.d.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wróbel, A.; BŁażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Meira, H.M.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochem. 2017, 54, 20–27. [Google Scholar] [CrossRef]
- Gao, R.; Falkeborg, M.; Xu, X.; Guo, Z. Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Appl. Microbiol. Biotechnol. 2013, 97, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterization, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B. 2013, 102, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Luna, J.M.; Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Guerra, J.M.C.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ 2020, 8, e9064. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.G.; Veras, B.O.; Aguiar, J.S.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactant produced by Candida utilis UFPEDA1009 with potential application in cookie formulation. Electron. J. Biotechnol. 2020, 46, 14–21. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252. [Google Scholar] [CrossRef]
- Campos, J.; Stamford, T.L.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.; Sarubbo, L.A. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicol. Rep. 2015, 2, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.A. Gums: Properties and Uses. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 283–289. [Google Scholar] [CrossRef]
- Hayati, I.N.; Ching, C.W.; Rozaini, M.Z.H. Flow properties of o/w emulsions as affected by xanthan gum, guar gum and carboxymethyl cellulose interactions studied by a mixture regression modelling. Food Hydrocol. 2016, 53, 199–208. [Google Scholar] [CrossRef]
- Ahmed, I.; Thomas, L. Effect of xanthan and guar gum on the pasting, stickiness and extensional properties of brown wheat flour/β-glucan composite doughs. LWT 2018, 87, 443–449. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacilllus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids Production by Candida bombicola ATCC 22214 and its Potential Application in Microbial Enhanced Oil Recovery. Front. Microbiol. 2015, 6, 1324. [Google Scholar] [CrossRef] [Green Version]
- Sobrinho, H.B.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Campos-Takaki, G.M.; Leite, L.F.; Sarubbo, L.A. Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem. 2008, 43, 912–917. [Google Scholar] [CrossRef]
- Santos Santos, M.S.; Lira, I.R.A.S.; Meira, H.M.; Aguiar, J.S.; Rufino, R.D.; Almeida, D.G.; Casazza, A.A.; Converti, A.; Sarubbo, L.A.; Luna, J.M. Enhanced Oil Removal by a Non-Toxic Biosurfactant Formulation. Energies 2021, 14, 467. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tama, N.F.Y.; Hodgkis, I.J. Effects of composting on phytotoxicity of spent ping-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Silva, S.N.; Farias, C.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B Biointerfaces 2010, 79, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Jardim, G.M. Estudos Ecotoxicológicos da Água e do Sedimento do Rio Corumbataí, São Paulo. 2004. Master’s Thesis, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba, Brazil, 2004. [Google Scholar]
- Shepherd, R.; Rockey, J.; Sutherland, I.W. Novel bioemulsifiers from microorganisms. J. Biotechnol. 1995, 40, 207–217. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Shojaosadati, S.A.; Tehrani, H.A. Preparation and Characterisation of Bioemulsifier from Saccharomyces cerevisiae and its Application in Food Products. LWT-Food Sci. Technol. 1996, 29, 734–737. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- BRASIL. Resolução nº 12; Agência Nacional de Vigilância Sanitária—Minostério da Saúde: Brasília, Brazil, 12 January 2001. [Google Scholar]
- Sarubbo, L.A.; Rocha, R.B., Jr.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.M.; Rufino, R.D.; Jara, A.M.A.T.; Brasileiro, P.P.F.; Sarubbo, L.A. Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Colloids Surf. A 2015, 480, 413–418. [Google Scholar] [CrossRef]
- Rau, U.; Hammen, S.; Heckmann, R.; Wray, V.; Lang, S. Sophorolipids: A source for novel compounds. Ind. Crops Prod. 2001, 13, 85–92. [Google Scholar] [CrossRef]
- Maity, J.P.; Huang, Y.M.; Hsu, C.M.; Wu, C.I.; Chen, C.C.; Li, C.Y.; Jean, J.S.; Chang, Y.F.; Chen, C.Y. Removal of Cu, Pb and Zn by foam fractionation and a soil washing process from contaminated industrial soils using soapberry-derived saponin: A comparative effectiveness assessment. Chemosphere 2013, 92, 1286–1293. [Google Scholar] [CrossRef]
- Zargar, A.N.; Lymperatou, A.; Skiadas, I.; Kumar, M.; Srivastava, P. Structural and functional characterization of a novel biosurfactant from Bacillus sp. IITD106. J. Hazard Mater. 2022, 423, 127201. [Google Scholar] [CrossRef]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Characterization and application of a biosurfactant isolated from Candida utilis in salad dressings. Biodegradation 2019, 30, 313–324. [Google Scholar] [CrossRef]
- Silva, M.D.S.S. Produção de Biossurfactantes por Leveduras Isoladas do Pólen Apícola de Melipona seminigra merrilae da Região do Baixo Amazonas-AM. Master’s Thesis, Dissertação de Mestrado Universidade Federal Rural de Pernambuco, Recife, Brazil, 2013. [Google Scholar]
- Silva, R.D.C.F.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef]
- Santos, A.P.P. Produção de Biossurfactante por Streptomyces sp. Isolado de Liquens da Região Amazônica Para Aplicação Como agente Antifúngico. Master’s Thesis, Dissertação de Mestrado, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2013. [Google Scholar]
- Arambasic, M.B.; Bjelic, S.; Subakov, G. Acute toxicity of heavy metals (copper, lead, zinc), phenol and sodium on Allium cepa L., Lepidium sativum L. and Daphnia magna St.: Comparative investigation and the practical applications. Water Res. 1995, 29, 497–503. [Google Scholar] [CrossRef]
- Fiskesjo, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 12, 99–112. [Google Scholar] [CrossRef]
- Benassi, J.C. O uso de bioindicadores e biomarcadores na avaliação do processo de remediação de efluente de lixiviação de carvão mineral utilizando microesferas de quitosana. Master’s Thesis, Programa de Pós-Graduação em Biotecnologia, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2004. [Google Scholar]
- Grippa, G.A.; Morozesk, M.; Nati, N.; Matsumoto, S.T. Genotoxicity study of the surfactant Tween 80 in Allium cepa. Rev. Bras. Toxicol. 2010, 23, 11–16. [Google Scholar]
- Antonelli, P.J. Avaliação da toxicidade de efluentes de mineração de carvão antes e após a sua remedição, utilizando-se parâmetros físico-químicos e bioindicadores. In II Seminário Integrado de Ensino, Pesquisa e Extensão, V Seminário de Iniciação Científica; UNESC: Criciúma, Brasil, 2005; pp. 1–35. [Google Scholar]
- Chivero, P.; Gohtani, S.; Yoshii, H.; Nakamura, A. Assessment of soy soluble polysaccharide, gum arabic and OSA-Starch as emulsifiers for mayonnaise-like emulsions. LWT-Food Sci. Technol. 2016, 69, 59–66. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Cuadri, A.A.; Berta, M.; Stading, M. Relation between concentration and shear-extensional rheology properties of xanthan and guar gum solutions. Carbohydr. Polym. 2018, 181, 63–70. [Google Scholar] [CrossRef]
- Bak, J.H.; Yoo, B. Intrinsic viscosity of binary gum mixtures with xanthan gum and guar gum: Effect of NaCl, sucrose, and pH. Int. J. Biol. Macromol. 2018, 111, 77–81. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Mück, D.; Grossmann, L.; Greiner, L.; Klausmann, P.; Henkel, M.; Lilge, L.; Weiss, J.; Hausmann, R. Surfactin from Bacillus subtilis displays promising characteristics as O/W-emulsifier for food formulations. Colloids Surf. B Biointerfaces 2021, 203, 111749. [Google Scholar] [CrossRef]
- Chen, X.-W.; Yin, W.-J.; Yang, D.-X.; Wan, Z.-L.; Ma, C.-G.; Yang, X.-Q. One-pot ultrasonic cavitational emulsification of phytosterols oleogel-based flavor emulsions and oil powder stabilized by natural saponin. Food Res. Int. 2021, 150, 110757. [Google Scholar] [CrossRef]

| Method | ST a (mN·m−1) | Emulsification Index (%) | Yield (g·L−1) b | |||
|---|---|---|---|---|---|---|
| Canola Oil | Corn Oil | Soya Oil | LE Isolation | AP Isolation | ||
| Flask | 29.0 ± 1.0 | 49.0 ± 1.2 | 47.0 ± 0.9 | 45.0 ± 1.1 | 12.5 ± 1.4 | 5.9 ± 1.3 |
| 1.2-L bioreactor | 31.0 ± 1.1 | 29.0 ± 1.4 | 32.0 ± 0.9 | 29.0 ± 1.1 | 19.8 ± 0.9 | 2.8 ± 1.0 |
| 3.0-L bioreactor | 33.0 ± 1.3 | 10.0 ± 1.2 | 10.0 ± 1.1 | 12.0 ± 1.6 | 61.0 ± 0.8 | 1.1 ± 1.1 |
| 50-L bioreactor | 30.0 ± 1.3 | 59.0 ± 0.9 | 58.0 ± 1.1 | 57.0 ± 1.2 | 221.9 ± 1.1 | 2.4 ± 1.3 |
| Germination Index of Isolated Biosurfactants (%) | ||||||
|---|---|---|---|---|---|---|
| ½ CMC | CMC | 2 CMC | ||||
| Seeds | LE a | AP b | LE | AP | LE | AP |
| Cucumis anguria (gherkin) | 100% ± 0.1 | 41.0%± 0.1 | 100% ± 0.1 | 28.0% ± 0.1 | 80.0% ± 0.2 | 0% ± 0.1 |
| Solanum lycopersicum (tomato) | 85.0% ± 0.1 | 64.0% ± 0.1 | 38.0% ± 0.1 | 45.0% ± 0.2 | 22.0% ± 0.2 | 0% ± 0.1 |
| Thickening Agent (% p/p) | pH | |
|---|---|---|
| Xanthan gum | 0.2 | 3.70 |
| 0.5 | 3.70 | |
| 0.8 | 3.71 | |
| Guar gum | 0.2 | 3.56 |
| 0.5 | 3.63 | |
| 0.8 | 3.70 | |
| Carboxymethylcellulose | 0.2 | 3.78 |
| 0.5 | 3.76 | |
| 0.8 | 3.66 | |
| Arabic Gum | 0.2 | 3.71 |
| 0.5 | 3.71 | |
| 0.8 | 3.71 | |
| Biosurfactant Concentration (% v/v) | Guar Gum | Carboxymethylcellulose | ||
|---|---|---|---|---|
| LE Isolation | AP Isolation | LE Isolation | AP Isolation | |
| 0.2 | + | − | − | + |
| 0.3 | + | + | + | − |
| 0.4 | + | + | − | + |
| 0.5 | − | − | − | − |
| 0.6 | − | − | − | + |
| 0.7 | − | + | + | − |
| 0.8 | + | + | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, M.I.S.; Campos Guerra, J.M.; Meira, H.M.; Sarubbo, L.A.; de Luna, J.M. A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions. Foods 2022, 11, 561. https://doi.org/10.3390/foods11040561
Pinto MIS, Campos Guerra JM, Meira HM, Sarubbo LA, de Luna JM. A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions. Foods. 2022; 11(4):561. https://doi.org/10.3390/foods11040561
Chicago/Turabian StylePinto, Maria Isabel Silveira, Jenyffer Medeiros Campos Guerra, Hugo Morais Meira, Leonie Asfora Sarubbo, and Juliana Moura de Luna. 2022. "A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions" Foods 11, no. 4: 561. https://doi.org/10.3390/foods11040561
APA StylePinto, M. I. S., Campos Guerra, J. M., Meira, H. M., Sarubbo, L. A., & de Luna, J. M. (2022). A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions. Foods, 11(4), 561. https://doi.org/10.3390/foods11040561










