Vacuum-Packed Steak Tartare: Prevalence of Listeria monocytogenes and Evaluation of Efficacy of ListexTM P100
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prevalence and Characteristics of L. monocytogenes from Steak Tartare Samples
2.2. Model Experiment Using ListexTM P100
3. Results and Discussion
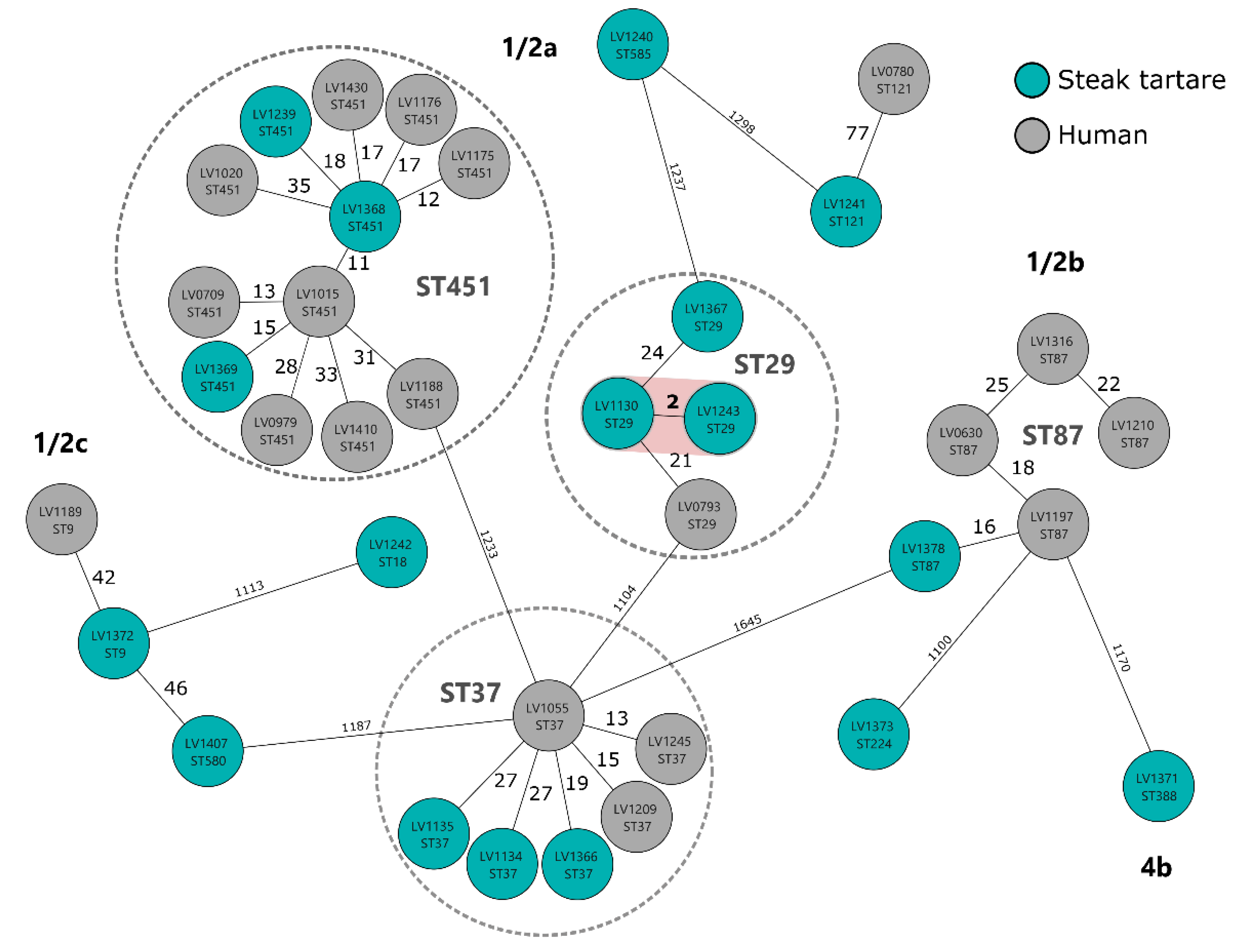
3.1. Prevalence and Characteristics of L. monocytogenes from Steak Tartare Samples
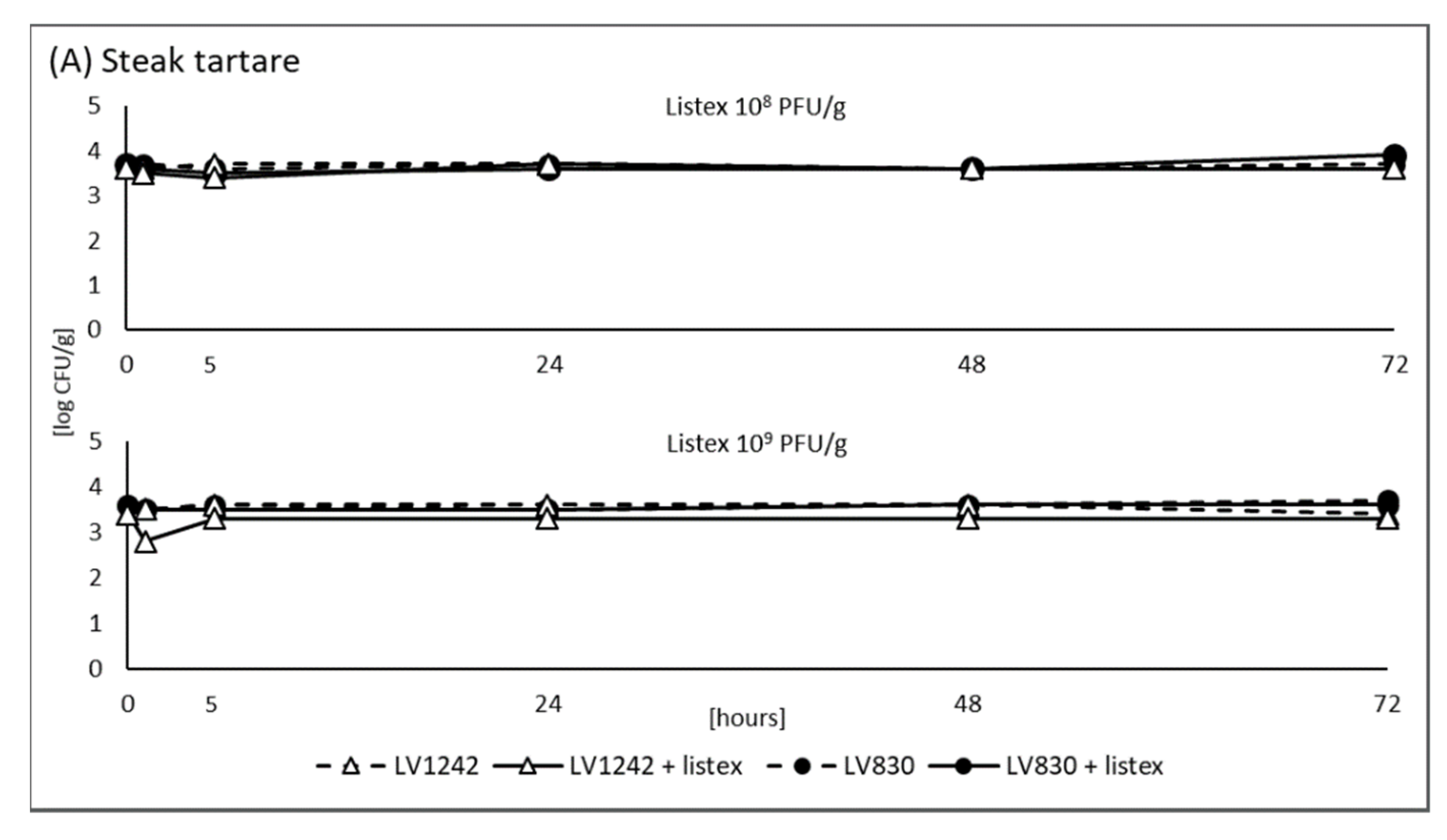
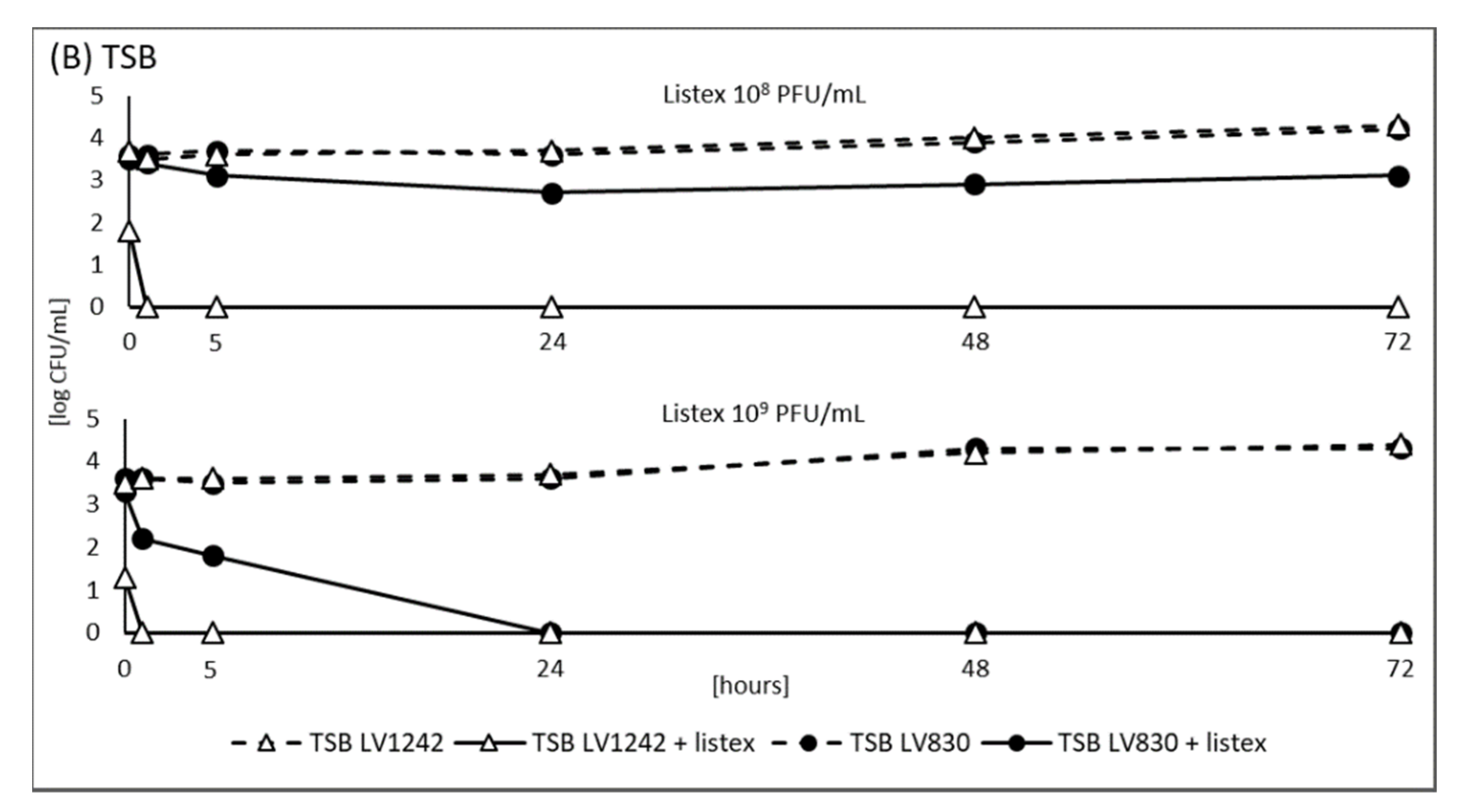
3.2. Model Experiment Using ListexTM P100
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delhalle, L.; Korsak, N.; Taminiau, B.; Nezer, C.; Burteau, S.; Delcenserie, V.; Poullet, J.B.; Daube, G. Exploring the Bacterial Diversity of Belgian Steak Tartare Using Metagenetics and Quantitative Real-Time PCR Analysis. J. Food Prot. 2016, 79, 220–229. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet.-Res. 2020, 87, 20. [Google Scholar] [CrossRef]
- WHO. Listeriosis. Fact Sheet. February 2018. Available online: http://www.who.int/mediacentre/factsheets/listeriosis/en/ (accessed on 14 October 2021).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Melero, B.; Manso, B.; Stessl, B.; Hernández, M.; Wagner, M.; Rovira, J.; Rodríguez-Lázaro, D. Distribution and Persistence of Listeria monocytogenes in a Heavily Contaminated Poultry Processing Facility. J. Food Prot. 2019, 82, 1524–1531. [Google Scholar] [CrossRef]
- Thomas, J.; Govender, N.; McCarthy, K.M.; Erasmus, L.K.; Doyle, T.J.; Allam, M.; Ismail, A.; Ramalwa, N.; Sekwadi, P.; Ntshoe, G.; et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. N. Engl. J. Med. 2020, 382, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Gelbíčová, T.; Zobaníková, M.; Tomáštíková, Z.; Van Walle, I.; Ruppitsch, W.; Karpíšková, R. An outbreak of listeriosis linked to turkey meat products in the Czech Republic, 2012–2016. Epidemiol. Infect. 2018, 146, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wu, Q.; Zhang, J.; Chen, M.; Yan, Z.; Hu, H. Listeria monocytogenes Prevalence and Characteristics in Retail Raw Foods in China. PLoS ONE 2015, 10, e0136682. [Google Scholar] [CrossRef]
- Kramarenko, T.; Roasto, M.; Keto-Timonen, R.; Mäesaar, M.; Meremäe, K.; Kuningas, M.; Hörman, A.; Korkeala, H. Listeria monocytogenes in ready-to-eat vacuum and modified atmosphere packaged meat and fish products of Estonian origin at retail level. Food Control 2016, 67, 48–52. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601&from=DA (accessed on 20 October 2021).
- D’Arrigo, M.; Mateo-Vivaracho, L.; Guillamón, E.; Fernández-León, M.F.; Bravo, D.; Peirotén, Á.; Medina, M.; García-Lafuente, A. Characterization of persistent Listeria monocytogenes strains from ten dry-cured ham processing facilities. Food Microbiol. 2020, 92, 103581. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Desai, M.; Oladunjoye, A.; Skrobot, F.; Nannapaneni, R. Reduction of Listeria monocytogenes in queso fresco cheese by a combination of listericidal and listeriostatic GRAS antimicrobials. Int. J. Food Microbiol. 2012, 155, 82–88. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, 4565. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.; Part 1: Detection method, ISO 11290-1; Part 2: Enumeration method, ISO 11290-2; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the Major Listeria monocytogenes Serovars by Multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppitsch, W.; Pietzka, A.T.; Prior, K.; Bletz, S.; Fernandez, H.L.; Allerberger, F.; Harmsen, D.; Mellmann, A. Defining and Evaluating a Core Genome Multilocus Sequence Typing Scheme for Whole-Genome Sequence-Based Typing of Listeria monocytogenes. J. Clin. Microbiol. 2015, 53, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Khen, B.K.; Lynch, O.; Carroll, J.; McDowell, D.; Duffy, G. Occurrence, Antibiotic Resistance and Molecular Characterization ofListeria monocytogenesin the Beef Chain in the Republic of Ireland. Zoonoses Public Health 2014, 62, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bohaychuk, V.M.; Gensler, G.E.; King, R.K.; Manninen, K.I.; Sorensen, O.; Wu, J.T.; Stiles, M.E.; McMullen, L.M. Occurrence of Pathogens in Raw and Ready-to-Eat Meat and Poultry Products Collected from the Retail Marketplace in Edmonton, Alberta, Canada. J. Food Prot. 2006, 69, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Kalender, H. Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2013, 26, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Pouillot, R.; Klontz, K.C.; Chen, Y.; Al, R.P.E.; Macarisin, D.; Doyle, M.; Bally, K.M.; Strain, E.; Datta, A.R.; Hammack, T.S.; et al. Infectious Dose of Listeria monocytogenesin Outbreak Linked to Ice Cream, United States, 2015. Emerg. Infect. Dis. 2016, 22, 2113–2119. [Google Scholar] [CrossRef] [Green Version]
- Awaisheh, S.S. Incidence and Contamination Level of Listeria monocytogenes and Other Listeria spp. in Ready-to-Eat Meat Products in Jordan. J. Food Prot. 2010, 73, 535–540. [Google Scholar] [CrossRef]
- Bērziņš, A.; Hörman, A.; Lundén, J.; Korkeala, H. Factors associated with Listeria monocytogenes contamination of cold-smoked pork products produced in Latvia and Lithuania. Int. J. Food Microbiol. 2007, 115, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Kramarenko, T.; Roasto, M.; Meremäe, K.; Kuningas, M.; Põltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Hlucháňová, L.; Gelbíčová, T.; Karpíšková, R. Genetic diversity of human Listeria monocytogenes strains from the Czech Republic in 2016–2020. Epidemiol. Mikrobiol. Imunol. 2022; in press. [Google Scholar]
- Šteingolde, Ž.; Meistere, I.; Avsejenko, J.; Ķibilds, J.; Bergšpica, I.; Streikiša, M.; Gradovska, S.; Alksne, L.; Roussel, S.; Terentjeva, M.; et al. Characterization and Genetic Diversity of Listeria monocytogenes Isolated from Cattle Abortions in Latvia, 2013–2018. Veter-Sci. 2021, 8, 195. [Google Scholar] [CrossRef]
- Kim, S.W.; Haendiges, J.; Keller, E.N.; Myers, R.; Kim, A.; Lombard, J.E.; Karns, J.S.; Van Kessel, J.A.S.; Haley, B.J. Genetic diversity and virulence profiles of Listeria monocytogenes recovered from bulk tank milk, milk filters, and milking equipment from dairies in the United States (2002 to 2014). PLoS ONE 2018, 13, e0197053. [Google Scholar] [CrossRef] [PubMed]
- Gelbíčová, T.; Florianová, M.; Tomáštíková, Z.; Pospíšilová, L.; Koláčková, I.; Karpíšková, R. Prediction of Persistence of Listeria monocytogenes ST451 in a Rabbit Meat Processing Plant in the Czech Republic. J. Food Prot. 2019, 82, 1350–1356. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Müller, A.; Stessl, B.; Wagner, M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front. Microbiol. 2015, 6, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, K.A.; Nannapaneni, R. Bacteriophage Significantly Reduces Listeria monocytogenes on Raw Salmon Fillet Tissue†. J. Food Prot. 2010, 73, 32–38. [Google Scholar] [CrossRef]
- Carlton, R.M.; Noordman, W.H.; Biswas, B.; de Meester, E.D.; Loessner, M.J. Bacteriophage P100 for control of Listeria monocytogenes in foods: Genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 2005, 43, 301–312. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hluchanova, L.; Korena, K.; Juricova, H. Vacuum-Packed Steak Tartare: Prevalence of Listeria monocytogenes and Evaluation of Efficacy of ListexTM P100. Foods 2022, 11, 533. https://doi.org/10.3390/foods11040533
Hluchanova L, Korena K, Juricova H. Vacuum-Packed Steak Tartare: Prevalence of Listeria monocytogenes and Evaluation of Efficacy of ListexTM P100. Foods. 2022; 11(4):533. https://doi.org/10.3390/foods11040533
Chicago/Turabian StyleHluchanova, Lucie, Kristyna Korena, and Helena Juricova. 2022. "Vacuum-Packed Steak Tartare: Prevalence of Listeria monocytogenes and Evaluation of Efficacy of ListexTM P100" Foods 11, no. 4: 533. https://doi.org/10.3390/foods11040533
APA StyleHluchanova, L., Korena, K., & Juricova, H. (2022). Vacuum-Packed Steak Tartare: Prevalence of Listeria monocytogenes and Evaluation of Efficacy of ListexTM P100. Foods, 11(4), 533. https://doi.org/10.3390/foods11040533






