Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
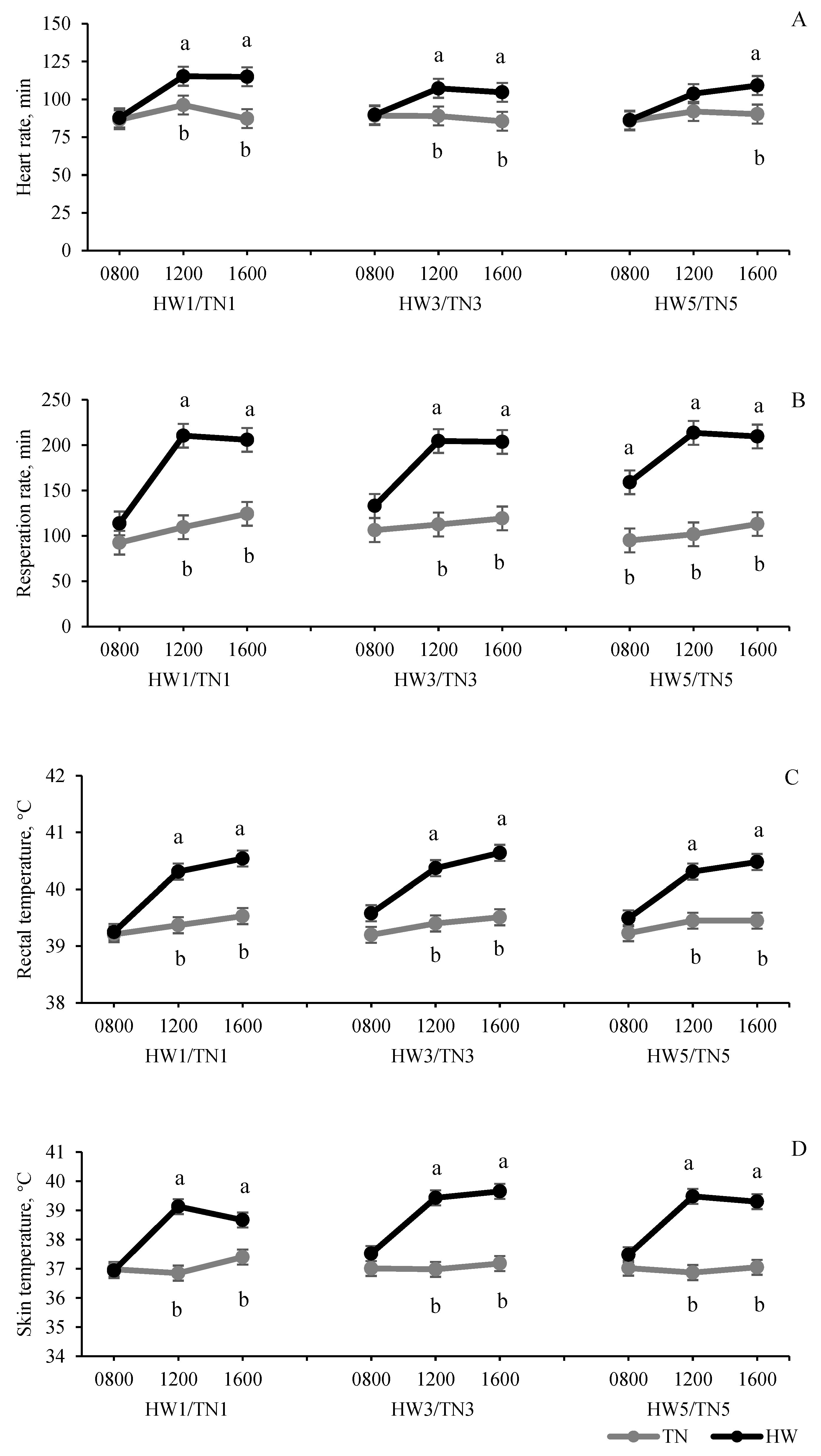
2.2. Physiological Parameters
2.3. Slaughter
Postmortem Muscle Sample Collection
2.4. Meat Quality
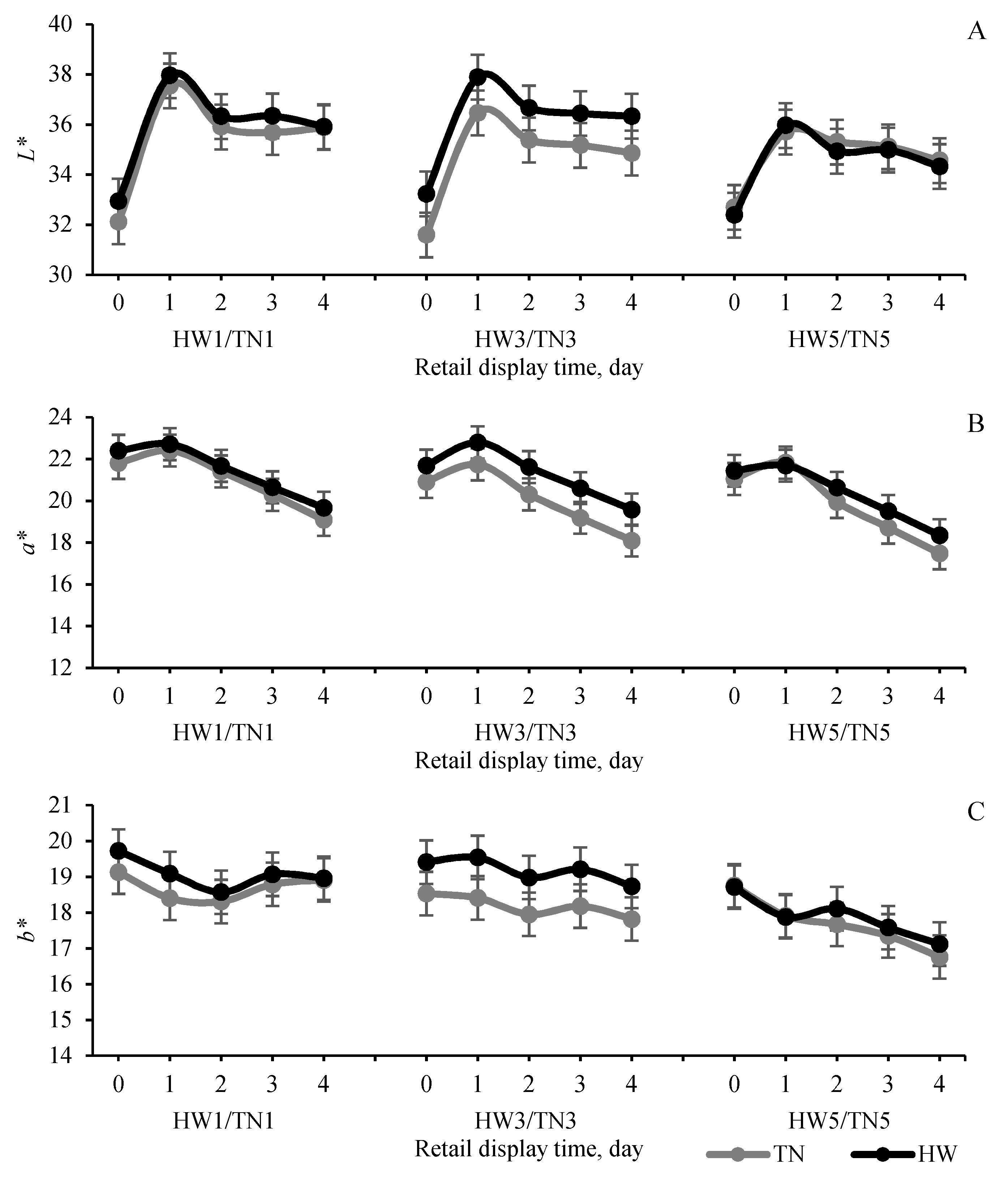
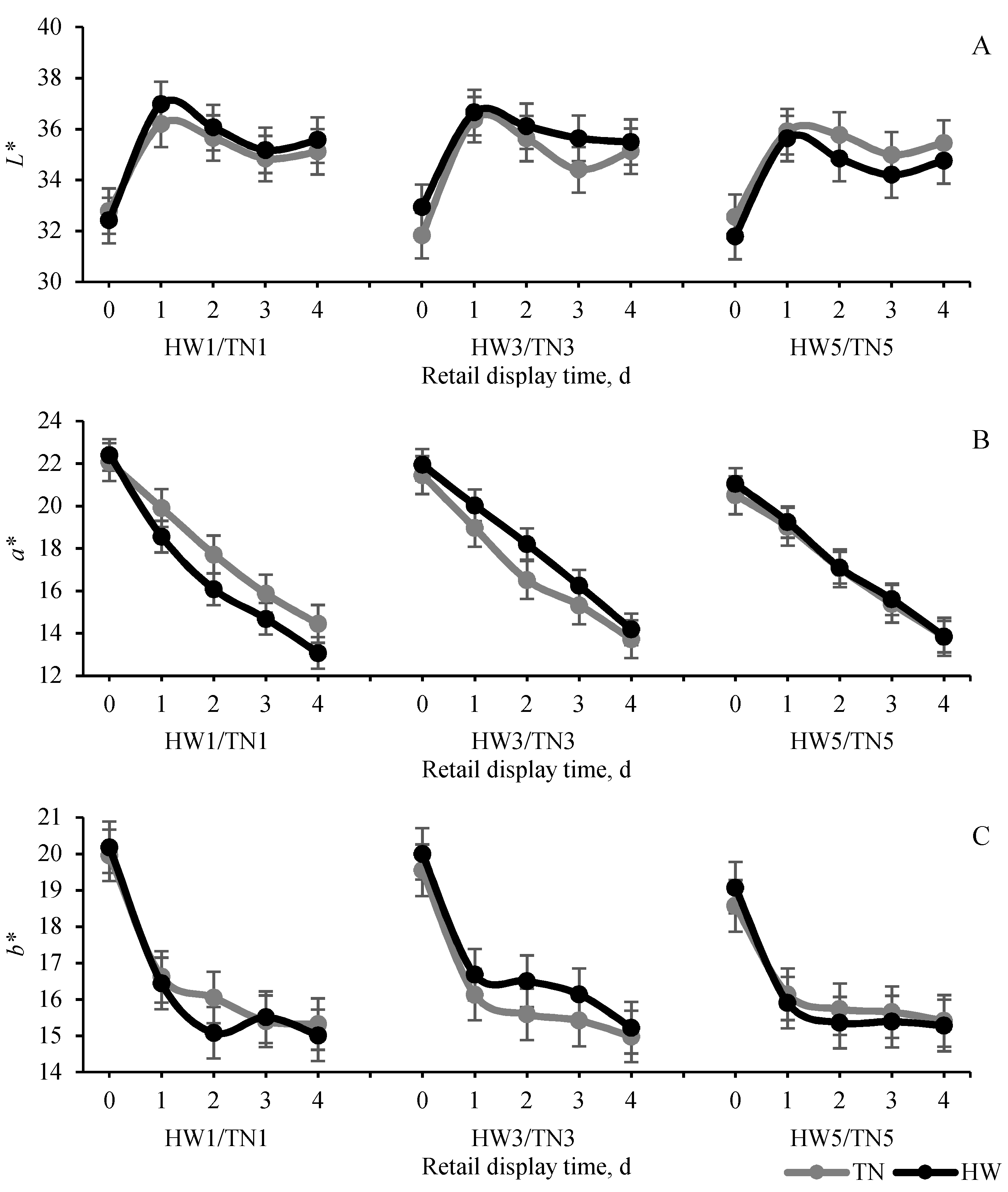
2.4.1. Meat Packaging and Retail Display
2.4.2. Surface Colour
2.4.3. Water Holding Capacity
2.4.4. Warner–Bratzler Shear Force
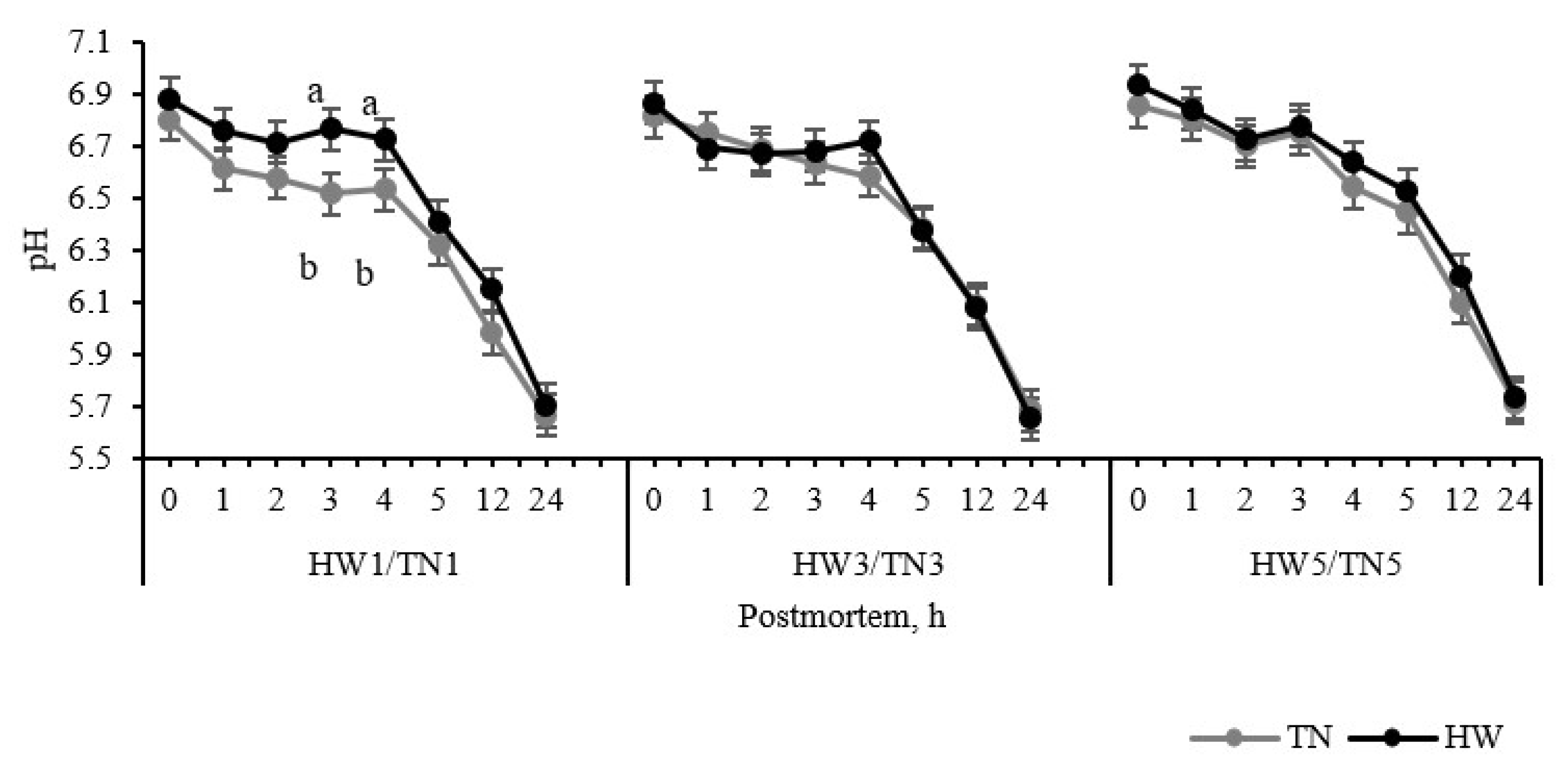
2.5. Muscle pH, Glycogen and Lactates
2.6. Statistical Analysis
3. Results and Discussion
3.1. Temperature–Humidity Index and Physiological Parameters
3.2. Postmortem Muscle Glycolysis and Meat Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, A.; Upadhyay, R. Heat Stress and Reproduction; Springer India: New Delhi, India, 2013; pp. 79–111. [Google Scholar]
- Gregory, N.G. How climatic changes could affect meat quality. Food Res. Int. 2010, 43, 1866–1873. [Google Scholar] [CrossRef]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; DiGiacomo, K.; Osei-Amponsah, R.; Chauhan, S.S. Impacts of heat stress on meat quality and strategies for amelioration: A review. Int. J. Biometeorol. 2020, 64, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Kadim, I.T.; Mahgoub, O.; Al-Marzooqi, W.; Al-Ajmi, D.S.; Al-Maqbali, R.S.; Al-Lawati, S.M. The influence of seasonal temperatures on meat quality characteristics of hot-boned, m. psoas major and minor, from goats and sheep. Meat Sci. 2008, 80, 210–215. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Al-Ajmi, D.S.; Al-Maqbaly, R.S.; Al-Mugheiry, S.M.; Bartolome, D.Y. The influence of season on quality characteristics of hot-boned beef m. longissimus thoracis. Meat Sci. 2004, 66, 831–836. [Google Scholar] [CrossRef]
- Lowe, T.E.; Gregory, N.G.; Fisher, A.D.; Payne, S.R. The effects of temperature elevation and water deprivation on lamb physiology, welfare, and meat quality. Aust. J. Agric. Res. 2002, 53, 707–714. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Dunshea, F.R.; Plozza, T.E.; Hopkins, D.L.; Ponnampalam, E.N. The impact of antioxidant supplementation and heat stress on carcass characteristics, muscle nutritional profile and functionality of lamb meat. Animals 2020, 10, 1286. [Google Scholar] [CrossRef]
- Zhang, M.; Warner, R.D.; Dunshea, F.R.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Osei-Amponsah, R.; Hopkins, D.L.; Ha, M.; Chauhan, S.S. Impact of heat stress on the growth performance and retail meat quality of 2nd cross (Poll Dorset × (Border Leicester × Merino)) and Dorper lambs. Meat Sci. 2021, 181, 108581. [Google Scholar] [CrossRef]
- Cowan, T.; Purich, A.; Perkins, S.; Pezza, A.; Boschat, G.; Sadler, K. More Frequent, Longer, and Hotter Heat Waves for Australia in the Twenty-First Century. J. Clim. 2014, 27, 5851–5871. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.H.; Li, W.J. Observed trends in various aspects of compound heat waves across china from 1961 to 2015. J. Meteorol. Res. 2017, 31, 455–467. [Google Scholar] [CrossRef]
- Rastogi, D.; Lehner, F.; Ashfaq, M. Revisiting recent U.S. Heat waves in a warmer and more humid climate. Geophys. Res. Lett. 2020, 47, e2019GL086736. [Google Scholar]
- Marai, I.F.M.; El Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep—A review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Colorimetry, Central Bureau of the CIE; The International Commission on Illumination: Vienna, Austria, 1986; pp. 64–65.
- Abhijith, A. Effect of slaughter age and post-mortem days on meat quality of longissimus and semimembranosus muscles of Boer goats. Meat Sci. 2021, 175, 108466. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D. Genotype and age at slaughter influence the retail shelf-life of the loin and knuckle from sheep carcasses. Aust. J. Exp. Agric. 2007, 47, 1190. [Google Scholar] [CrossRef]
- Otto, G.; Roehe, R.; Looft, H.; Thoelking, L.; Kalm, E. Comparison of different methods for determination of drip loss and their relationships to meat quality and carcass characteristics in pigs. Meat Sci. 2004, 68, 401–409. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Ponnampalam, E.N.; van de Ven, R.J.; Warner, R.D. The effect of pH decline rate on the meat and eating quality of beef carcasses. Anim. Prod. Sci. 2014, 54, 407–413. [Google Scholar] [CrossRef]
- Ha, M.; Dunshea, F.; Warner, R. Investigation of Tenderness and water Holding Capacity of aged Pork Loins in Two Packaging Systems; Final Report; Co-Operative Research Center for High Integrity Australian Pork, University of Melbourne: Parkville, VIC, Australia, 2017. [Google Scholar]
- Bendall, J.R. Variability in rates of pH fall and lactate production in the muscles of cooling beef carcasses. Meat Sci. 1978, 2, 91–104. [Google Scholar] [CrossRef]
- Chauhan, S.S.; LeMaster, M.N.; Clark, D.L.; Foster, M.K.; Miller, C.E.; England, E.M. Glycolysis and pH decline terminate prematurely in oxidative muscles despite the presence of excess glycogen. Meat Muscle Biol. 2019, 3. [Google Scholar] [CrossRef]
- Margolis, L. Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metabolism 2019, 97, 1–8. [Google Scholar] [CrossRef]
- St Pierre, N.R. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52. [Google Scholar] [CrossRef] [Green Version]
- Srikandakumar, A.; Johnson, E.H.; Mahgoub, O. Effect of heat stress on respiratory rate, rectal temperature and blood chemistry in Omani and Australian Merino sheep. Small Rumin. Res. 2003, 49, 193–198. [Google Scholar] [CrossRef]
- Wojtas, K.; Cwynar, P.; Kolacz, R. Effect of thermal stress on physiological and blood parameters in merino sheep. Bull. Vet. Inst. Pulawy 2014, 58, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Joy, A.; Dunshea, F.; Leury, B.; DiGiacomo, K.; Clarke, I.; Zhang, M.; Abhijith, A.; Osei Amponsah, R.; Chauhan, S.S. Comparative assessment of thermotolerance in dorper and second-cross (poll dorset/merino × border leicester) lambs. Animals 2020, 10, 2441. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Hales, J.R.; Brown, G.D. Net energetic and thermoregulatory efficiency during panting in the sheep. Comp. Biochem. Physiol. A Comp. Physiol. 1974, 49, 413–422. [Google Scholar] [CrossRef]
- Franzmann, A.W.; Hebert, D.M. Variation of Rectal Temperature in Bighorn Sheep. J. Wildl. Manag. 1971, 35, 488–494. [Google Scholar] [CrossRef]
- Aleksiev, J. Thermoregulation in sheep. IV. Effect of heat stress on heart rate dynamics in shorn and inshorn ewes from three breeds. J. Anim. Sci. 2004, 41, 16–21. [Google Scholar]
- Al Haidary, A.A. Physiological responses of Naimey sheep to heat stress challenge under semi-arid environments. Int. J. Agric. Biol. 2004, 2, 307–309. [Google Scholar]
- Cheng, H.; Song, S.; Kim, G.D. Frozen/thawed meat quality associated with muscle fiber characteristics of porcine longissimus thoracis et lumborum, psoas major, semimembranosus, and semitendinosus muscles. Sci. Rep. 2021, 11, 13354. [Google Scholar] [CrossRef]
- Tarrant, P.V. The Effects of Handling, Transport, Slaughter and Chilling on Meat Quality and Yield in Pigs—A Review. Irish J. Food Sci. Technol. 1989, 13, 79–107. [Google Scholar]
- Greenwood, P.L.; Gardner, G.E.; Hegarty, R.S. Lamb myofibre characteristics are influenced by sire estimated breeding values and pastoral nutritional system. Aust. J. Agric. Res. 2006, 57, 627–639. [Google Scholar] [CrossRef]
- Oishi, Y.; Taniguchi, K.; Matsumoto, H.; Ishihara, A.; Ohira, Y.; Roy, R.R. Differential responses of HSPs to heat stress in slow and fast regions of rat gastrocnemius muscle. Muscle Nerve 2003, 28, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Macías Cruz, U. Feedlot growth, carcass characteristics and meat quality of hair breed male lambs exposed to seasonal heat stress (winter vs. summer) in an arid climate. Meat Sci. 2020, 169, 108202. [Google Scholar] [CrossRef] [PubMed]
- Archana, P.R.; Sejian, V.; Ruban, W.; Bagath, M.; Krishnan, G.; Aleena, J.; Manjunathareddy, G.B.; Beena, V.; Bhatta, R. Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Sci. 2018, 141, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Celi, P.; Leury, B.J.; Dunshea, F.R. High dietary selenium and vitamin E supplementation ameliorates the impacts of heat load on oxidative status and acid-base balance in sheep. J. Anim. Sci. 2015, 93, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, M.J. Effects of farm management practices and transport duration on stress response and meat quality traits of suckling goat kids. Animal: Int. J. Anim. Biosci. 2017, 11, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Warriss, P.D.; Kestin, S.C.; Brown, S.N.; Knowles, T.G.; Wilkins, L.J.; Edwards, J.E.; Austin, S.D.; Nicol, C.J. The depletion of glycogen stores and indices of dehydration in transported broilers. Br. Vet. J. 1993, 149, 391–398. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Alkindi, A.Y.; Al-Marzooqi, W.; Al-Saqri, N.M.; Almaney, M.; Mahmoud, I.Y. Effect of transportation at high ambient temperatures on physiological responses, carcass and meat quality characteristics in two age groups of Omani sheep. Asian-Aust. J. Anim. Sci. 2007, 20, 424–431. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Khalaf, S. Effects of the transportation during hot season and electrical stimulation on meat quality characteristics of goat Longissimus dorsi muscle. Small Rumin. Res. 2014, 121, 120–124. [Google Scholar] [CrossRef]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef]

| 1 Day | 3 Days | 5 Days | SED 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TN | HW | TN | HW | TN | HW | Temp. 2 | Day | T × D 3 | ||
| LTL | ||||||||||
| Drip loss | 2.02 a | 1.65 ab | 2.10 a | 1.74 ab | 1.95 a | 1.33 b | 0.287 | 0.01 | 0.36 | 0.77 |
| Purge loss/% | 2.47 | 2.34 | 2.54 | 2.44 | 2.32 | 2.45 | 0.194 | 0.24 | 0.17 | 0.97 |
| Cooking loss 0 day/% | 19.9 | 19.8 | 20.6 | 20.9 | 20.5 | 18.9 | 1.22 | 0.50 | 0.41 | 0.50 |
| Cooking loss 4 days/% | 22.8 | 23.8 | 23.6 | 23.2 | 22.7 | 23.3 | 0.97 | 0.46 | 0.73 | 0.62 |
| SM | ||||||||||
| Drip loss | 1.55 a | 1.23 b | 1.42 a | 1.36 ab | 1.22 b | 1.07 b | 0.157 | 0.04 | 0.07 | 0.52 |
| Purge loss/% | 3.43 a | 2.62 b | 2.96 ab | 2.83 ab | 2.76 b | 2.36 b | 0.332 | 0.02 | 0.13 | 0.36 |
| Cooking loss 0 day/% | 18.8 ab | 18.9 ab | 20.1 a | 18.4 ab | 17.9 ab | 16.5 b | 1.41 | 0.24 | 0.11 | 0.63 |
| Cooking loss 4 days/% | 22.8 | 21.9 | 21.9 | 22.2 | 21.1 | 22.3 | 1.13 | 0.73 | 0.73 | 0.42 |
| 1 Day | 3 Day | 5 Day | SED 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TN | HW | TN | HW | TN | HW | Temp. 2 | Day | T × D 3 | ||
| LTL | ||||||||||
| WBSF 0 day | 27.9 | 31.3 | 35.0 | 32.1 | 30.6 | 27.2 | 36.6 | 0.13 | 0.16 | 0.99 |
| WBSF 4 days | 23.9 | 25.9 | 30.0 | 28.6 | 29.9 | 26.4 | 35.3 | 0.64 | 0.21 | 0.55 |
| SM | ||||||||||
| WBSF 0 day | 40.9 a | 36.9 ab | 33.1 b | 39.6 a | 33.5 b | 36.9 ab | 29.1 | 0.32 | 0.19 | 0.04 |
| WBSF 4 days | 26.6 | 26.0 | 23.6 | 25.3 | 24.8 | 26.2 | 20.0 | 0.45 | 0.43 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Warner, R.D.; Dunshea, F.R.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Prathap, P.; Ma, T.; Chauhan, S.S. Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs. Foods 2022, 11, 414. https://doi.org/10.3390/foods11030414
Zhang M, Warner RD, Dunshea FR, DiGiacomo K, Joy A, Abhijith A, Prathap P, Ma T, Chauhan SS. Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs. Foods. 2022; 11(3):414. https://doi.org/10.3390/foods11030414
Chicago/Turabian StyleZhang, Minghao, Robyn D. Warner, Frank R. Dunshea, Kristy DiGiacomo, Aleena Joy, Archana Abhijith, Pragna Prathap, Ting Ma, and Surinder S. Chauhan. 2022. "Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs" Foods 11, no. 3: 414. https://doi.org/10.3390/foods11030414
APA StyleZhang, M., Warner, R. D., Dunshea, F. R., DiGiacomo, K., Joy, A., Abhijith, A., Prathap, P., Ma, T., & Chauhan, S. S. (2022). Impact of Heatwaves on the Physiology and Retail Meat Quality of Lambs. Foods, 11(3), 414. https://doi.org/10.3390/foods11030414










