Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sprouts
2.3. Mineralization of Sprout Samples for Multi-Element Analysis
2.4. Determination of Selected Elements by Flame AAS
2.5. Determination of Iodine in Sprouts
2.6. Statistical Approach
3. Results and Discussion
3.1. Concentration of Essential Minerals in Sprouts
3.2. Concentration of Trace Elements in Sprouts
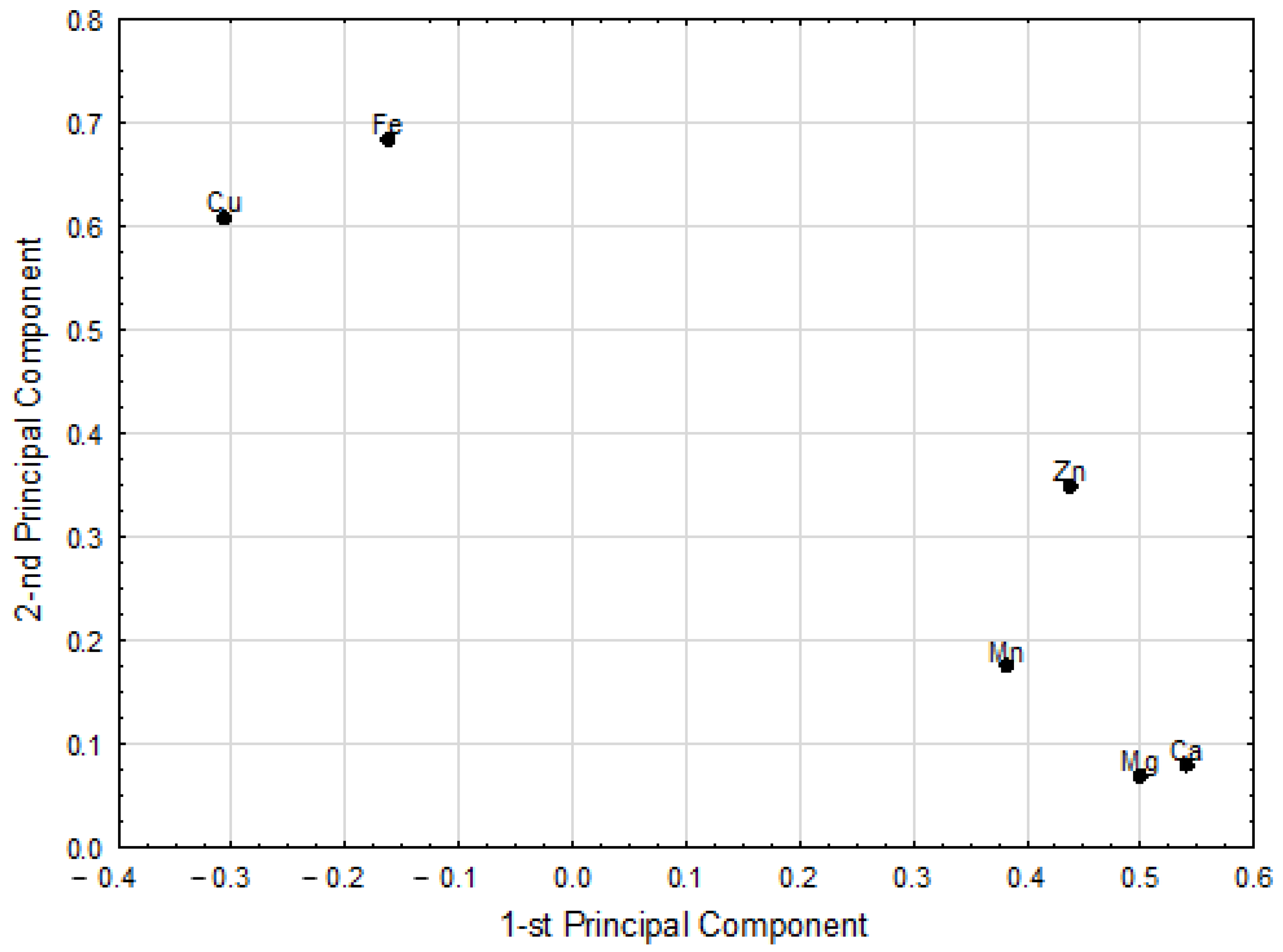
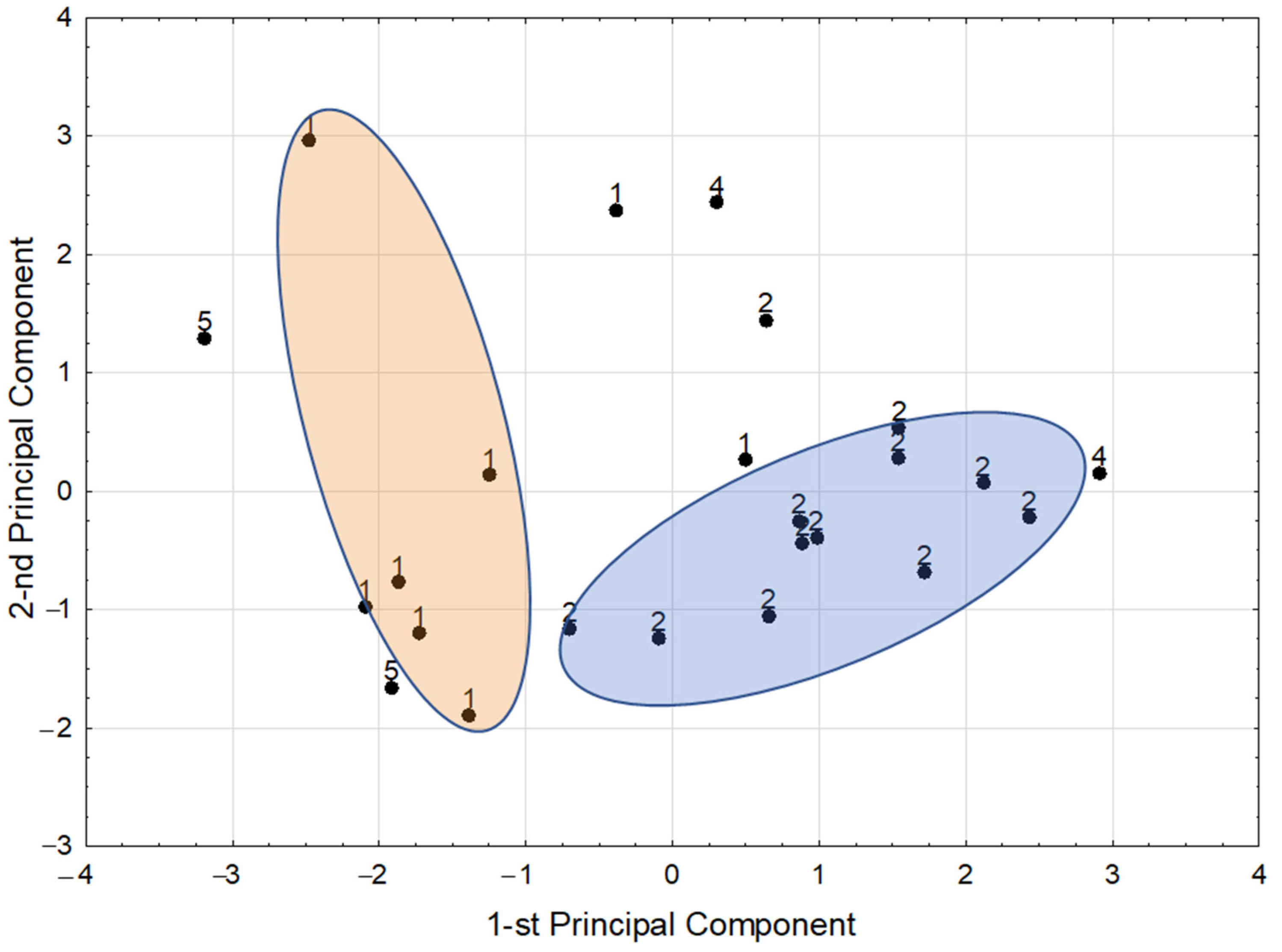
3.3. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Wong, L.S.; Force, L.E.; Irving, D.E. Glucosinolate composition and anti-cancer potential of seed-sprouts from horticultural members of the Brassicaceae. Acta Hortic. 2007, 744, 181–187. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Żmudzki, P.; Zagrodzki, P.; Gdula-Argasińska, J.; Prochownik, E.; Gorinstein, S. Health Promoting vs Anti-nutritive Aspects of Kohlrabi Sprouts, a Promising Candidate for Novel Functional Food. Plant Foods Hum. Nutr. 2021, 76, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Màrton, M.; Màndoki, Z.; Csapó-Kiss, Z.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae 2010, 3, 81–117. [Google Scholar]
- Pająk, P.; Socha, R.; Gałkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MS. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Zielinski, H.; Frias, J.; Piskuła, M.K.; Kozłowska, H.; Vidal-Valverde, C. Vitamin B1 and B2, dietary fiber and minerals content of Cruciferae sprouts. Eur. Food Res. Technol. 2005, 221, 78–83. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Wei, Y.; Yang, X. Changes of folate and other potential health promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef]
- Gan, R.Y.; Wang, M.; Lui, W.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C.; Frias, J. Role of elicitation on the health-promoting properties of kidney bean sprouts. LWT Food Sci. Technol. 2014, 56, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Sulkowska-Ziaja, K.; Muszynska, B.; Zagrodzki, P. Serotonin, melatonin, and certain indole derivatives profiles in rutabaga and kohlrabi seeds, sprouts, bulbs, and roots. LWT-Food Sci. Technol. 2014, 59, 740–745. [Google Scholar] [CrossRef]
- Yang, Y.A.O.; Cheng, X.Z.; Ren, G.X. Contents of D-chiro-Inositol, vitexin, and isovitexin in various varieties of mung bean and its products. Agric. Sci. China 2011, 10, 1710–1715. [Google Scholar] [CrossRef]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Boil. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Miyahira, R.F.; de Oliveira Lopes, J.; Antunes, A.E.C. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional quality of fresh and stored legumes sprouts—Effect of Lactobacillus plantarum 299v enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Using combined optimization, GC–MS and analytical technique to analyze the germination effect on phenolics, dietary fibers, minerals and GABA contents of Kodo millet (Paspalum scrobiculatum). Food Chem. 2017, 233, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res. Int. 2021, 142, 110193. [Google Scholar] [CrossRef] [PubMed]
- Suma, P.F.; Urooj, A. Influence of germination on bioaccessible iron and calcium in pearl millet (Pennisetum typhoideum). J. Food Sci. Technol. 2014, 51, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Lintschinger, J.; Fuchs, N.; Moser, H.; Jager, R.; Hlebeina, T.; Markolin, G.; Gossler, W. Uptake of various trace elements during germination of wheat, buckwheat and quinoa. Plant Foods Hum. Nutr. 1997, 50, 223–237. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, X.; Xia, Z. Effects of sodium metavanadate and germination on the sprouting of chickpeas and its content of vanadium, formononetin and biochanin a in the sprouts. J. Diet. Suppl. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Wietecha-Posłuszny, R.; Rubió, P.S.; Bartoń, H.; Prochownik, E.; Muszyńska, B.; Sułkowska-Ziaja, K.; et al. Does selenium fortification of kale and kohlrabi sprouts change significantly their biochemical and cytotoxic properties? J. Trace Elem. Med. Biol. 2020, 59, 126466. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.F.; Zhao, J. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Ebert, A.W.; Chang, C.H.; Yan, M.R.; Yang, R.Y. Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. Food Chem. 2017, 237, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Jerše, A.; Kacjan-Maršić, N.; Šircelj, H.; Germ, M.; Kroflič, A.; Stibilj, V. Seed soaking in I and Se solutions increases concentrations of both elements and changes morphological and some physiological parameters of pea sprouts. Plant Physiol. Biochem. 2017, 118, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bibak, A.; Stümp, S.; Knudsen, L.; Gundersen, V. Concentrations of 63 elements in cabbage and sprouts in Denmark. Commun. Soil Sci. Plant Anal. 1999, 30, 2409–2418. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Zheng, X. Bioactive ingredients in adzuki bean sprouts. J. Med. Plants Res. 2011, 5, 5894–5898. [Google Scholar] [CrossRef]
- Wietecha-Posłuszny, R.; Dobrowolska, J.; Kościelniak, P. Method for determination of selenium and arsenic in human urine by atomic fluorescence spectrometry. Anal. Lett. 2006, 39, 2787–2796. [Google Scholar] [CrossRef]
- Paśko, P.; Okoń, K.; Krośniak, M.; Prochownik, E.; Żmudzki, P.; Kryczyk-Kozioł, J.; Zagrodzki, P. Interaction between iodine and glucosinolates in rutabaga sprouts and selected biomarkers of thyroid function in male rats. J. Trace Elem. Med. Boil. 2018, 46, 110–116. [Google Scholar] [CrossRef]
- Pino, S.; Fang, S.L.; Braverman, L.E. Ammonium persulfate: A safe alternative oxidizing reagent for measuring urinary iodine. Clin. Chem. 1996, 42, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Pasko, P.; Gdula-Argasinska, J.; Podporska-Carroll, J.; Quilty, B.; Wietecha-Posluszny, R.; Tyszka-Czochara, M.; Zagrodzki, P. Influence of selenium supplementation on fatty acids profile and biological activity of four edible amaranth sprouts as new kind of functional food. J. Food Sci. Technol. 2015, 52, 4724–4736. [Google Scholar] [CrossRef] [Green Version]
- Zagrodzki, P.; Paśko, P.; Domínguez-Álvarez, E.; Salardón-Jiménez, N.; Sevilla-Hernández, C.; Sanmartín, C.; Gorinstein, S. Synthesis of novel organic selenium compounds and speciation of their metabolites in biofortified kale sprouts. Microchem. J. 2022, 172, 106962. [Google Scholar] [CrossRef]
- Jarosz, M. (Ed.) Human Nutrition Recommendations for Polish Population; IZZ: Warsaw, Poland, 2020. (In Polish) [Google Scholar]
- Santos, C.S.; Silva, B.; Valente, L.M.P.; Gruber, S.; Vasconcelos, M.W. The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile. Foods 2020, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Nunes, J.V.D.; Nóbrega, L.H.P.; da Cruz-Silva, C.T.A.; Pacheco, F.P. Comparison among beans species for food sprouts yield. Biosci. J. 2015, 31, 1682–1691. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.L.D.L.; Barcelos, M.D.F.P.; Teixeira, A.H.R.; Nogueira, D.A. Avaliação de componentes químicos em brotos de Fabaceae para o consumo humano. Cienc. Agrotecnologia 2009, 33, 1071–1078. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; Eel-Bedawey, A.A.; El-Beltagy, A.E. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods Hum. Nutr. 2003, 58, 1–13. [Google Scholar] [CrossRef]
- Mun, J.H.; Kim, I.D.; Dhungana, S.K.; Park, Y.S.; Kim, J.H.; Shin, D.H. Yield and quality characteristics of Korean red bean sprouts produced with different time of seed soaking. Food Sci. Biotechnol. 2020, 29, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bączek-Kwinta, R.; Baran, A.; Simlat, M.; Lang, J.; Bieniek, M.; Florek, B. Enrichment of Different Plant Seeds with Zinc and Assessment of Health Risk of Zn-Fortified Sprouts Consumption. Agronomy 2020, 10, 937. [Google Scholar] [CrossRef]
- Zou, T.; Xu, N.; Hu, G.; Pang, J.; Xu, H. Biofortification of soybean sprouts with zinc and bioaccessibility of zinc in the sprouts. J. Sci. Food Agric. 2014, 94, 3053–3060. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative Study of the Bioactive Properties and Elemental Composition of Red Clover (Trifolium pratense) and Alfalfa (Medicago sativa) Sprouts during Germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Fazaeli, H.; Golmohammadi, H.A.; Tabatabayee, S.N.; Asghari-Tabrizi, M. Productivity and nutritive value of barley green fodder yield in hydroponic system. World Appl. Sci. J. 2012, 16, 531–539. [Google Scholar]
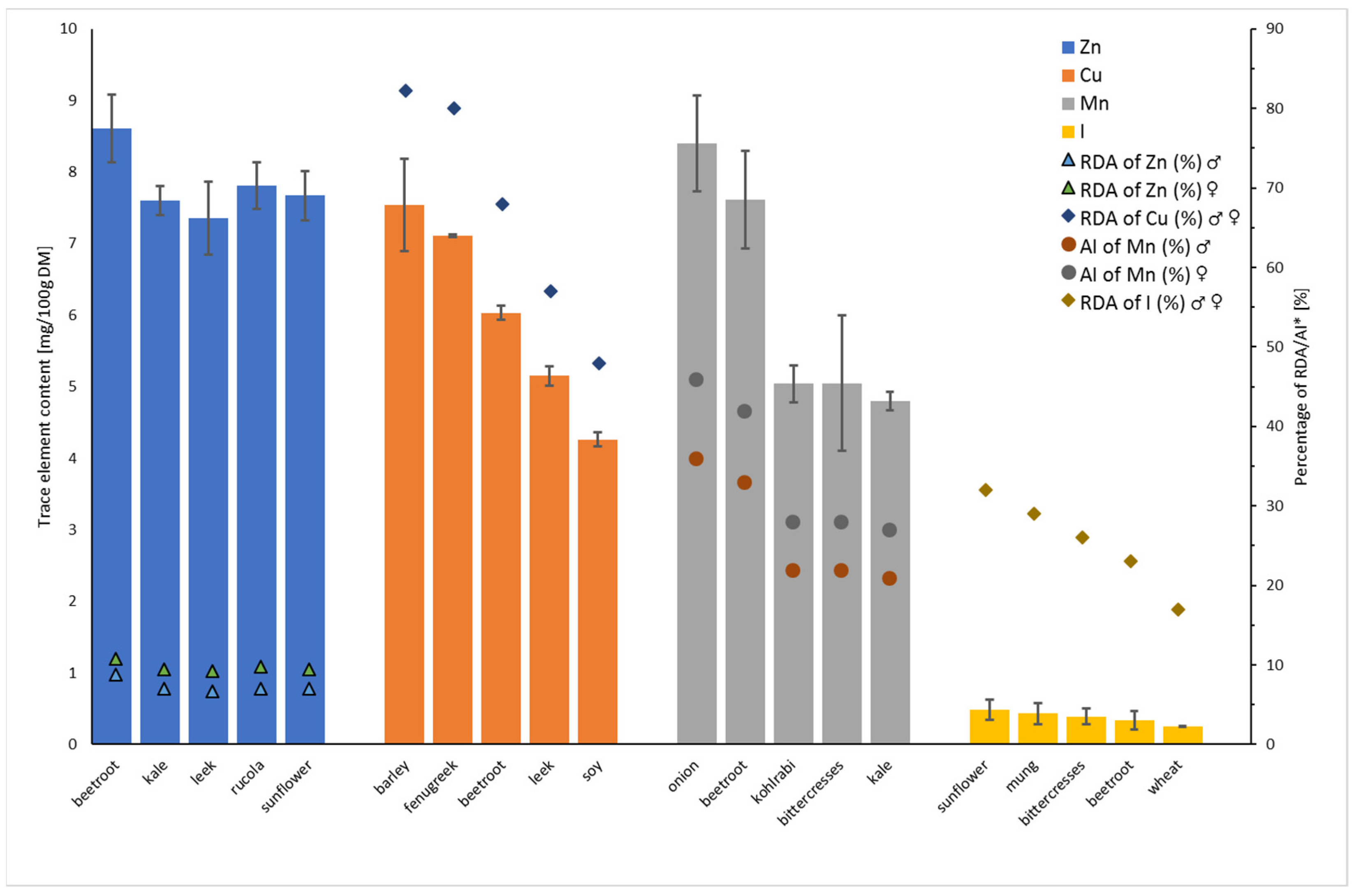

| Ca | Fe | Mg | Cu | I | Mn | Zn | |
|---|---|---|---|---|---|---|---|
| Sprouts | Fabaceae | ||||||
| Mung | 115 ± 3 | 5.2 ± 0.1 | 119 ± 6 | 1.24 ± 0.05 | 0.43 ± 0.15 | 1.86 ± 0.12 | 3.69 ± 0.02 |
| Lentil | 60 ± 5 | 12.0 ± 0.6 | 82 ± 4 | 1.55 ± 0.56 | 0.07 ± 0.02 | 1.75 ± 0.37 | 3.58 ± 0.35 |
| Lucerne | 351 ± 10 | 14.3 ± 1.0 | 190 ± 3 | 1.46 ± 0.01 | 0.24 ± 0.03 | 3.18 ± 0.14 | 6.25 ± 0.49 |
| Pea | 65 ± 6 | 17.7 ± 5.6 | 127 ± 12 | 3.12 ± 1.03 | 0.03 ± 0.01 | 3.31 ± 1.03 | 5.27 ± 0.26 |
| Soy | 202 ± 6 | 24.1 ± 0.9 | 233 ± 11 | 4.26 ± 0.10 | 0.12 ± 0.06 | 4.24 ± 0.67 | 5.81 ± 0.13 |
| Adzuki beans | 65 ± 2 | 12.8 ± 0.4 | 140 ± 6 | 1.87 ± 0.04 | 0.04 ± 0.02 | 1.82 ± 0.25 | 3.43 ± 0.14 |
| Kidney beans (red) | 68 ± 2 | 10.1 ± 0.5 | 133 ± 3 | 1.67 ± 0.08 | 0.03 ± 0.00 | 2.38 ± 0.45 | 3.13 ± 0.03 |
| Fenugreek | 132 ± 4 | 48.2 ± 23.1 | 161 ± 11 | 7.11 ± 0.02 | 0.18 ± 0.06 | 2.30 ± 0.82 | 4.09 ± 0.20 |
| Brassicaceae | |||||||
| Rutabaga | 432 ± 18 | 9.3 ± 0.7 | 312 ± 1 | 0.37 ± 0.01 | 0.10 ± 0.02 | 4.11 ± 0.12 | 5.36 ± 0.04 |
| Radish | 196 ± 6 | 9.3 ± 0.1 | 280 ± 12 | 0.47 ± 0.02 | 0.14 ± 0.02 | 1.71 ± 0.09 | 4.58 ± 0.06 |
| Red kale | 415 ± 11 | 9.5 ± 0.6 | 263 ± 4 | 0.62 ± 0.09 | 0.23 ± 0.03 | 2.47 ± 0.07 | 4.25 ± 0.40 |
| Broccoli | 413 ± 8 | 14.3 ± 0.5 | 295 ± 13 | 0.49 ± 0.05 | 0.17 ± 0.05 | 2.02 ± 0.23 | 5.32 ± 0.02 |
| Red cabbage | 392 ± 6 | 11.8 ± 0.4 | 210 ± 5 | 0.58 ± 0.01 | 0.17 ± 0.03 | 4.27 ± 0.24 | 5.02 ± 0.25 |
| Rucola | 332 ± 16 | 7.1 ± 0.3 | 399 ± 12 | 2.31 ± 0.06 | 0.21 ± 0.08 | 3.51 ± 0.29 | 7.81 ± 0.33 |
| China rose | 127 ± 8 | 10.7 ± 0.3 | 270 ± 17 | 0.92 ± 0.03 | 0.14 ± 0.03 | 2.09 ± 0.51 | 3.47 ± 0.22 |
| White mustard | 383 ± 15 | 15.0 ± 0.2 | 334 ± 14 | 1.22 ± 0.18 | 0.08 ± 0.01 | 3.22 ± 0.35 | 6.88 ± 0.07 |
| Kale | 424 ± 11 | 11.9 ± 0.2 | 163 ± 6 | 0.92 ± 0.13 | 0.24 ± 0.06 | 4.80 ± 0.13 | 7.60 ± 0.02 |
| Kohlrabi | 404 ± 17 | 11.9 ± 0.1 | 261 ± 3 | 1.10 ± 0.10 | 0.22 ± 0.05 | 5.04 ± 0.26 | 3.84 ± 0.16 |
| Bittercress | 228 ± 11 | 15.9 ± 0.5 | 314 ± 13 | 4.17 ± 0.10 | 0.39 ± 0.11 | 5.05 ± 0.95 | 5.96 ± 0.66 |
| Amaryllidaceae | |||||||
| Leek | 335 ± 16 | 20.1 ± 0.7 | 222 ± 6 | 5.15 ± 0.14 | 0.12 ± 0.04 | 3.70 ± 0.72 | 7.36 ± 0.51 |
| Onion | 444 ± 22 | 10.4 ± 0.5 | 329 ± 10 | 0.77 ± 0.03 | 0.10 ± 0.03 | 8.40 ± 0.67 | 5.73 ± 0.23 |
| Poaceae | |||||||
| Barley | 25 ± 1 | 15.9 ± 0.5 | 94 ± 3 | 7.54 ± 0.64 | 0.20 ± 0.07 | 1.53 ± 0.18 | 3.71 ± 0.25 |
| Wheat | 18 ± 1 | 8.5 ± 0.1 | 102 ± 3 | 1.22 ± 0.10 | 0.25 ± 0.01 | 3.61 ± 0.62 | 2.12 ± 0.07 |
| Amaranthaceae | |||||||
| Beetroot | 370 ± 10 | 68.0 ± 3.3 | 836 ± 29 | 6.03 ± 0.07 | 0.34 ± 0.13 | 7.61 ± 0.68 | 8.61 ± 0.47 |
| Asteraceae | |||||||
| Sunflower | 376 ± 61 | 11.4 ± 3.3 | 416 ± 47 | 0.82 ± 0.17 | 0.48 ± 0.14 | 3.25 ± 0.47 | 7.67 ± 0.34 |
| Fabaceae vs. Brassicaceae (Mean ± SD, significance level) | 132.3 ± 101.0 340.5 ± 106.7 p = 0.000 | 18.1 ± 13.4 11.5 ± 2.7 NS | 148.1 ± 46.4 281.9 ± 62.3 p = 0.000 | 2.79 ± 2.03 1.20 ± 1.13 p = 0.043 | 0.14 ± 0.14 0.19 ± 0.08 NS | 2.61 ± 0.89 3.48 ± 1.26 NS | 4.41 ± 1.19 5.46 ± 1.46 NS |
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| Cu | Fe | 0.404 |
| Ca | Mg | 0.270 |
| Zn | Ca | 0.205 |
| Mn | Ca | 0.198 |
| Zn | Cu | 0.192 |
| Zn | Mg | 0.188 |
| Zn | Fe | 0.187 |
| Mn | Mg | 0.183 |
| Zn | Mn | 0.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; Saraiva de Carvalho, I.; Paśko, P. Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis. Foods 2022, 11, 371. https://doi.org/10.3390/foods11030371
Dobrowolska-Iwanek J, Zagrodzki P, Galanty A, Fołta M, Kryczyk-Kozioł J, Szlósarczyk M, Rubio PS, Saraiva de Carvalho I, Paśko P. Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis. Foods. 2022; 11(3):371. https://doi.org/10.3390/foods11030371
Chicago/Turabian StyleDobrowolska-Iwanek, Justyna, Paweł Zagrodzki, Agnieszka Galanty, Maria Fołta, Jadwiga Kryczyk-Kozioł, Marek Szlósarczyk, Pol Salvans Rubio, Isabel Saraiva de Carvalho, and Paweł Paśko. 2022. "Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis" Foods 11, no. 3: 371. https://doi.org/10.3390/foods11030371
APA StyleDobrowolska-Iwanek, J., Zagrodzki, P., Galanty, A., Fołta, M., Kryczyk-Kozioł, J., Szlósarczyk, M., Rubio, P. S., Saraiva de Carvalho, I., & Paśko, P. (2022). Determination of Essential Minerals and Trace Elements in Edible Sprouts from Different Botanical Families—Application of Chemometric Analysis. Foods, 11(3), 371. https://doi.org/10.3390/foods11030371







