Effects of Cathepsins on Gel Strength and Water-Holding Capacity of Myofibrillar Protein Gels from Bighead Carp (Aristichthys nobilis) under a Hydroxyl Radical-Generation Oxidizing System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
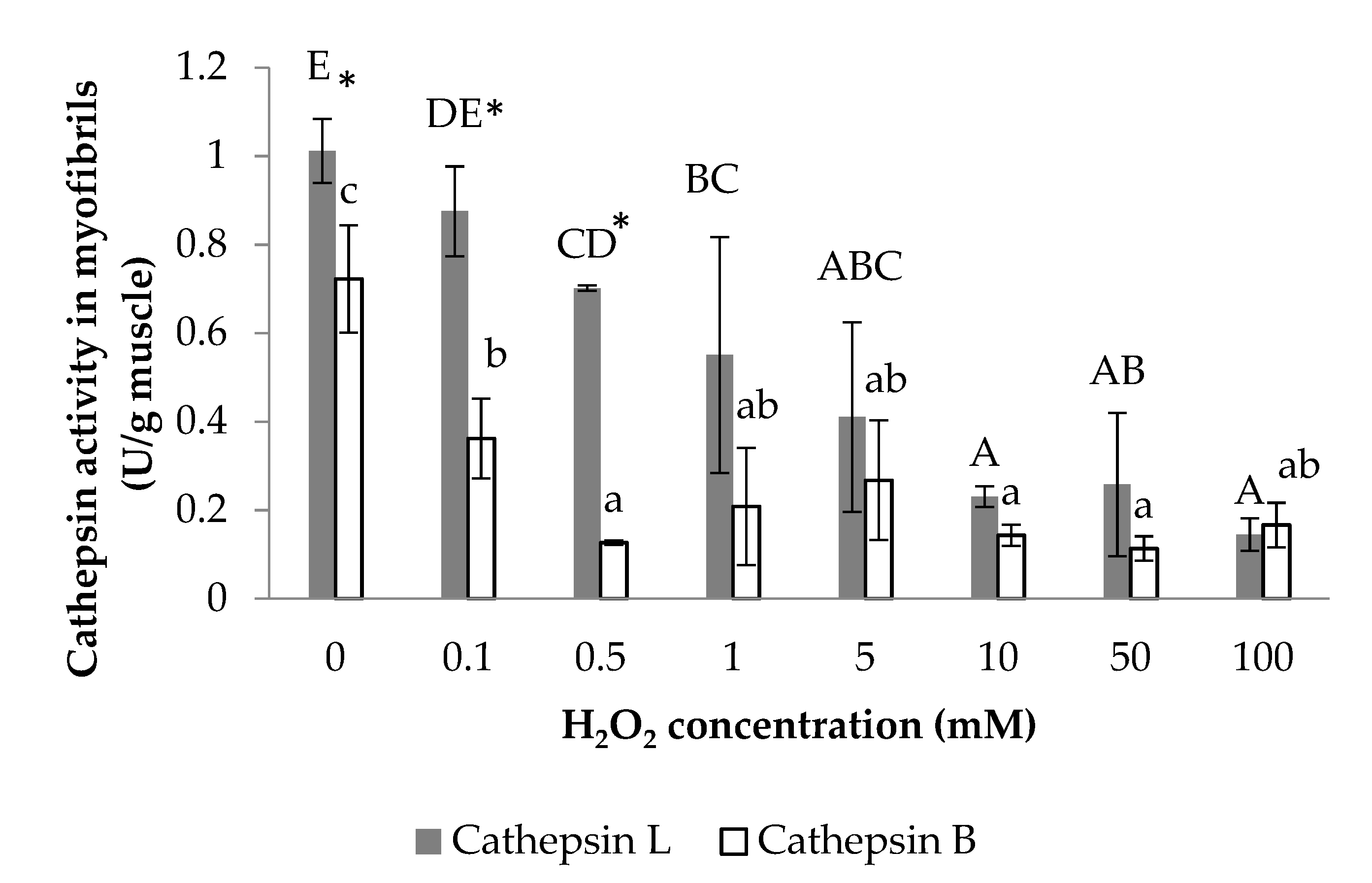
2.2. Measurement of Cathepsin B and Cathepsin L Activities in Myofibrils under Hydroxyl Radical-Generation Oxidizing System
2.3. Preparation of Myofibrillar Protein from Bighead Carp Fillets
2.4. Incubation of Myofibrillar Protein in Hydroxyl Radical-Generation Oxidizing System
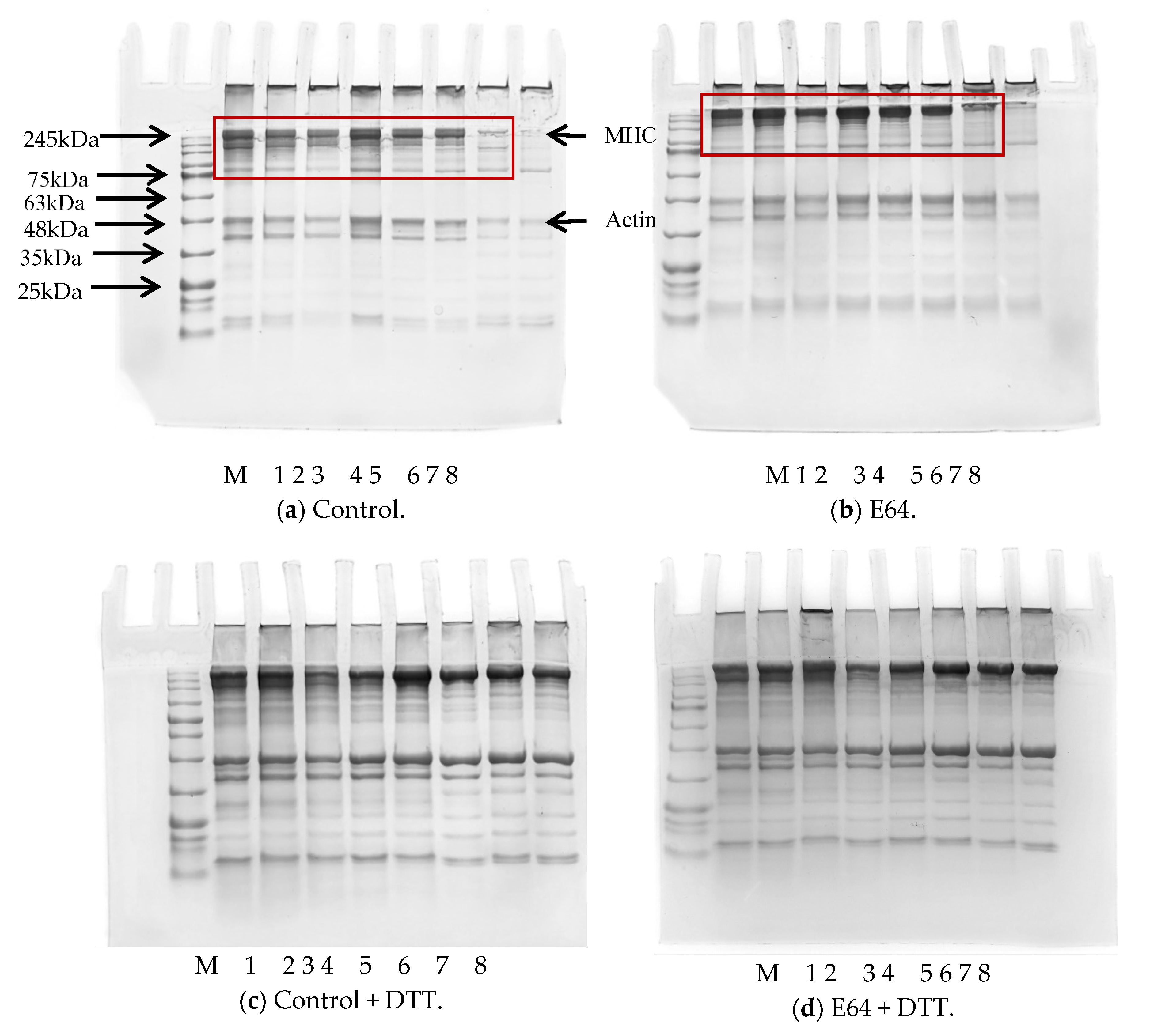
2.5. SDS-PAGE
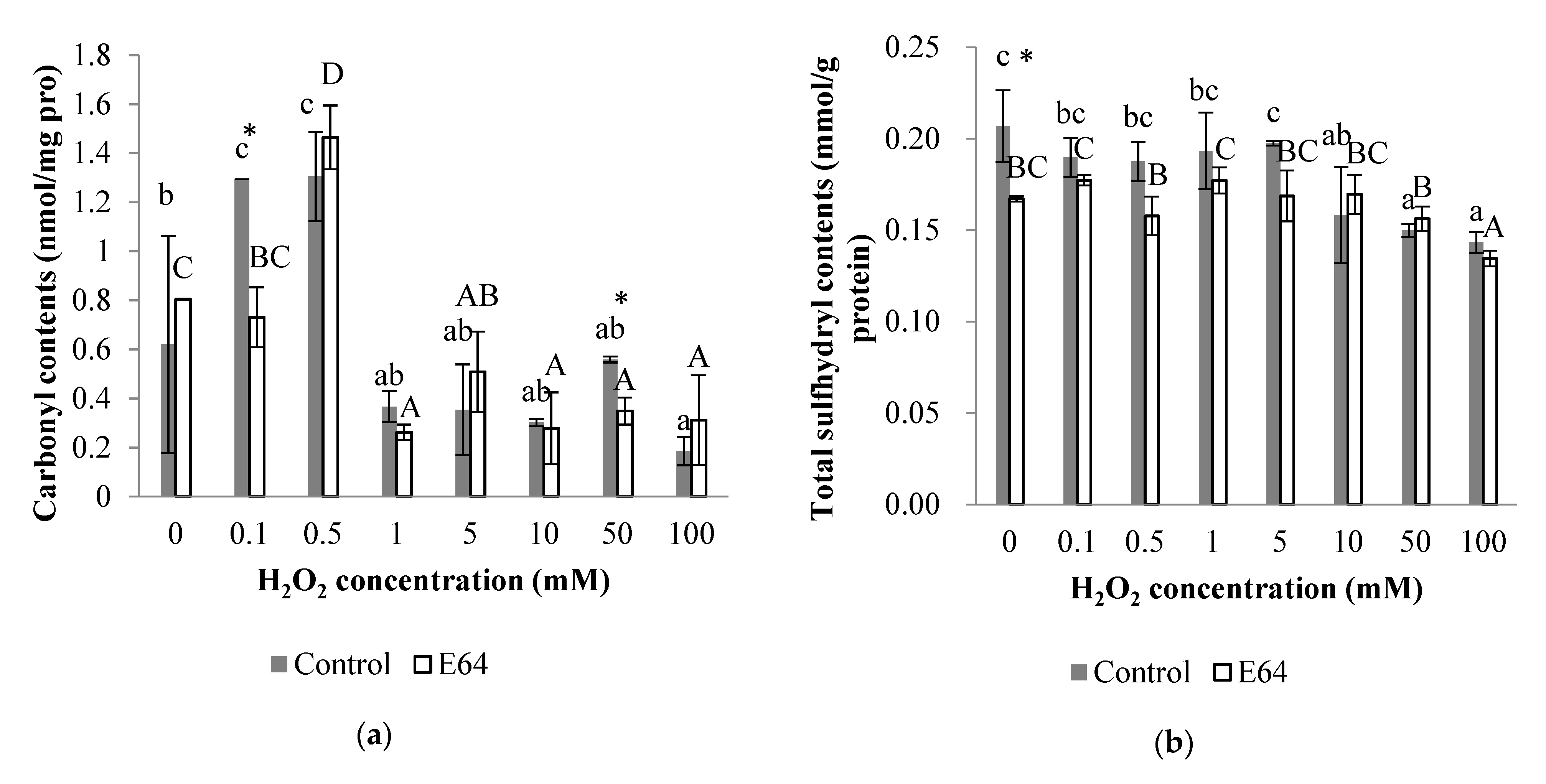
2.6. Determination of Protein Oxidation
2.6.1. Carbonyl Content
2.6.2. Total Sulfhydryl Contents
2.7. Preparation of Gels and Gel Properties
2.7.1. Preparation of Heat-Induced Gel
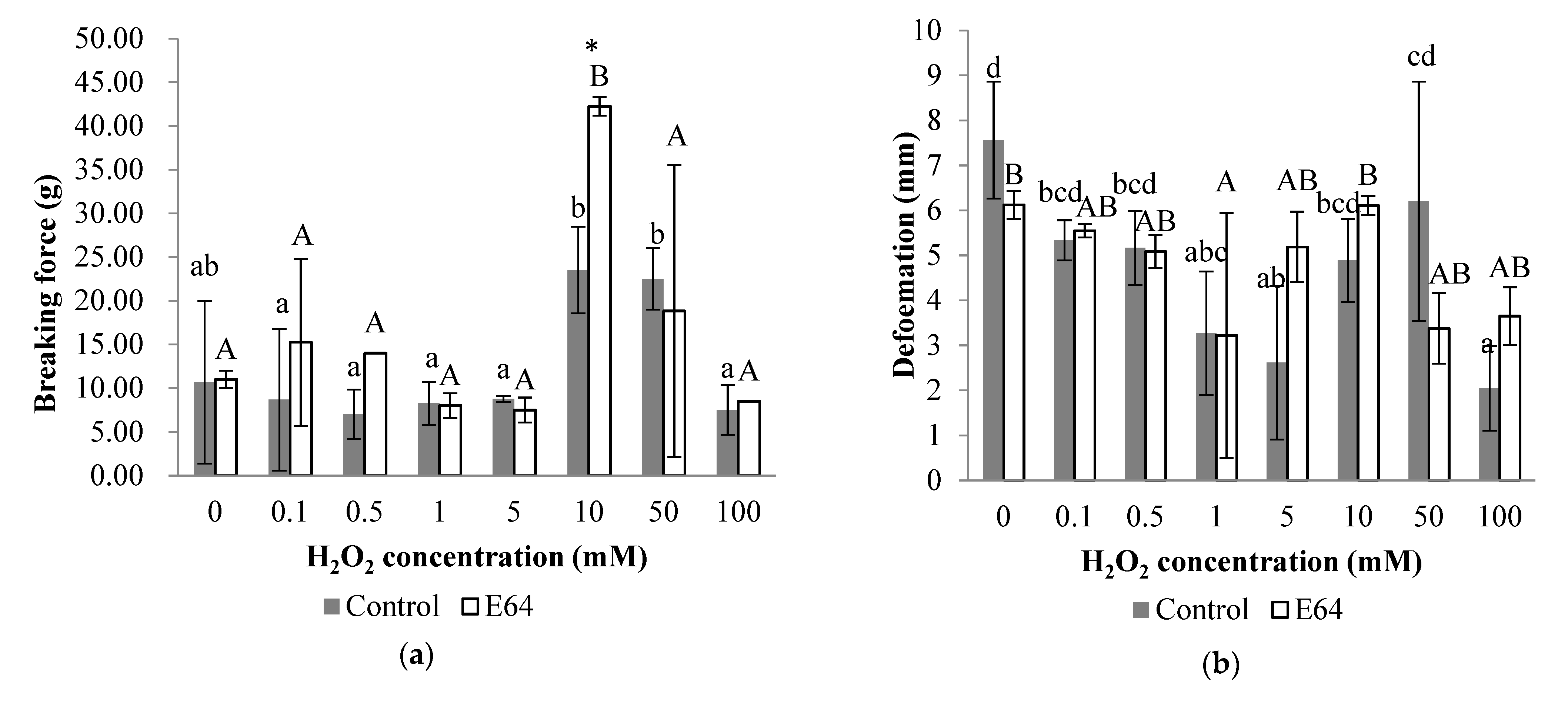
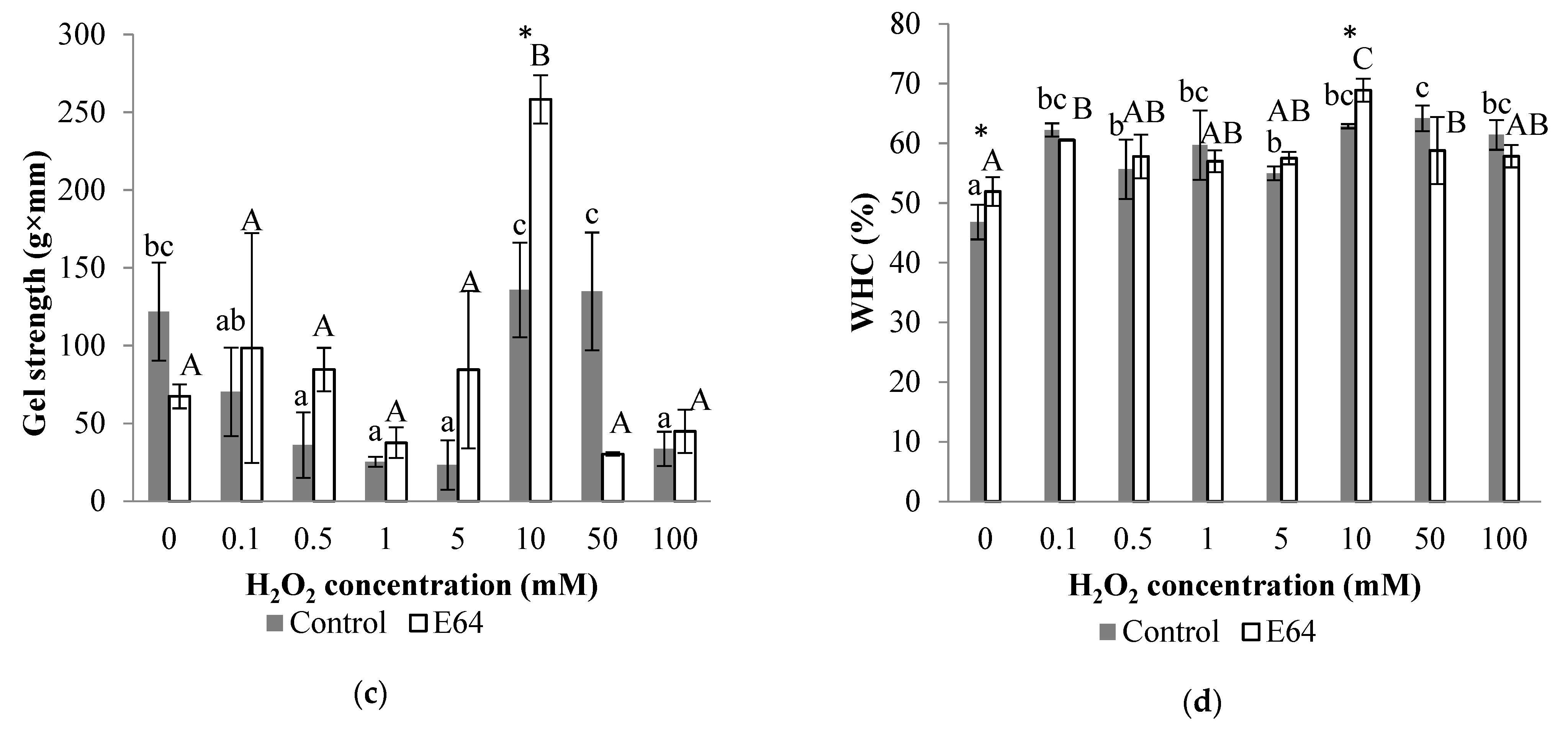
2.7.2. Gel Strength
2.7.3. WHC
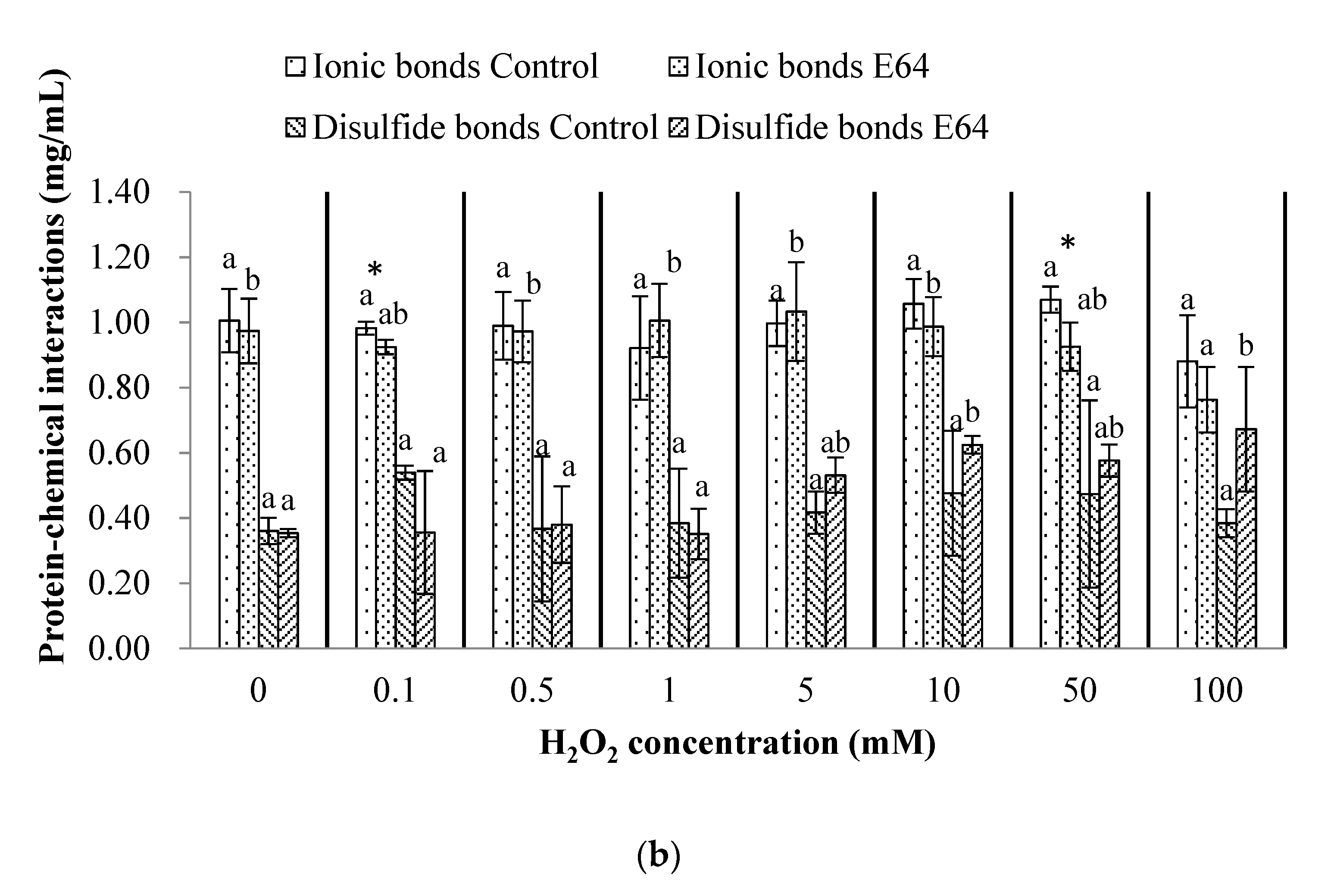
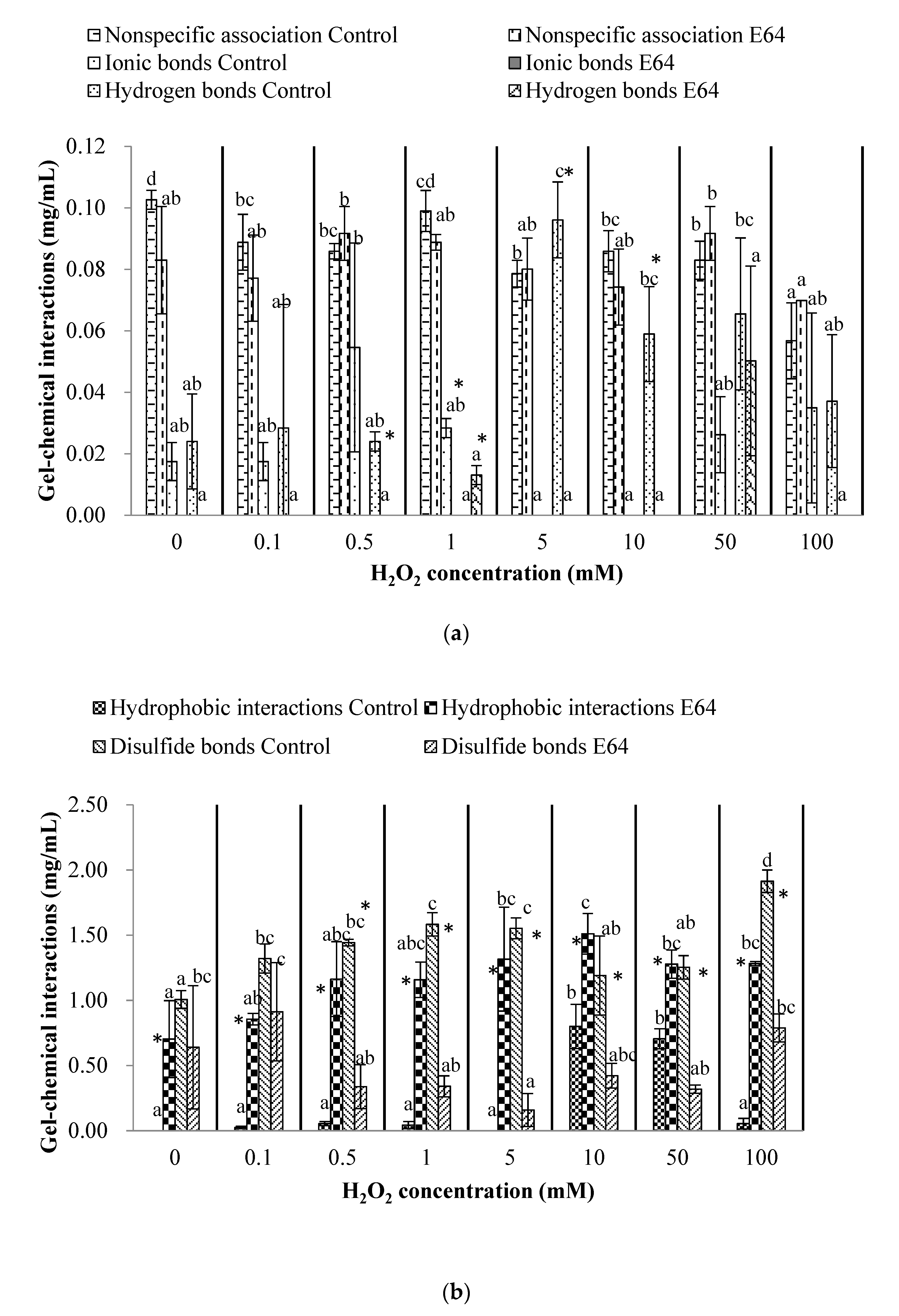
2.8. Chemical Interactions between Myofibrillar Protein/Gels
2.9. Statistical Analysis
3. Results
3.1. Cathepsin B and Cathepsin L Activity
3.2. SDS-PAGE
3.3. Protein Oxidation Parameters
3.3.1. Carbonyl Content
3.3.2. Total Sulfhydryl Content
3.4. Chemical Interactions of Myofibrillar Protein (Before Heating) and Gel (After Heating)
3.5. Gel Properties
3.5.1. Gel Strength
3.5.2. WHC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toyohara, H.; Shimizu, Y. Relation between the modori phenomenon and myosin heavy chain breakdown in Threadfin-bream gel. Agric. Biol. Chem. 1988, 52, 255–257. [Google Scholar] [CrossRef]
- Ge, L.; Xu, Y.; Xia, W. The function of endogenous cathepsin in quality deterioration of grass carp (Ctenopharyngodon idella) fillets stored in chilling conditions. Int. J. Food Sci. Technol. 2014, 50, 797–803. [Google Scholar] [CrossRef]
- Kirschke, H.; Barrett, A.J. Chemistry of Lysosomal Proteases; Academic Press Inc.: New York, NY, USA, 1987. [Google Scholar]
- Koga, H.; Yamada, H.; Nishimura, Y.; Kato, K.; Imoto, T. Comparative study on specificities of rat cathepsin L and papain: Amino acid differences at substrate-binding sites are involved in their specificities. J. Biochem. 1990, 108, 976. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Konagaya, S. Hydrolytic action of Salmon cathepsins B and L to muscle structural proteins in respect of muscle softening. Nippon Suisan Gakkaishi 1991, 57, 1917–1922. [Google Scholar] [CrossRef]
- Liu, H.; Yin, L.; Zhang, N.; Li, S.; Ma, C. Isolation of cathepsin B from the muscle of silver carp (Hypophthalmichthys molitrix) and comparison of cathepsins B and L actions on surimi gel softening. Food Chem. 2008, 110, 310–318. [Google Scholar] [CrossRef]
- Luo, Y.K.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Comparison of gel properties of surimi from Alaska Pollock and three freshwater fish species: Effects of thermal processing and protein concentration. J. Food Sci. Technol. 2001, 66, 548–554. [Google Scholar] [CrossRef]
- Hu, Y.; Ji, R.; Jiang, H.; Zhang, J.; Chen, J.; Ye, X. Participation of cathepsin L in modori phenomenon in carp (Cyprinus carpio) surimi gel. Food Chem. 2012, 134, 2014–2020. [Google Scholar] [CrossRef]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Oxidative environments decrease tenderization of beef steaks through inactivation of u-calpain. J. Anim. Sci. 2004, 82, 3254–3266. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.P.; Zhang, W.G.; Fu, Q.Q.; Liu, N.; Zhou, G.H. Activity and expression of nitric oxide synthase in pork skeletal muscles. Meat Sci. 2015, 99, 25–31. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Xia, X.; Liu, Q.; Diao, X. Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process Biochem. 2013, 48, 863–870. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2013, 96, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A.; Yeh, J.I.; Mallett, T.C.; Luba, J.; Rd, C.E.; Charrier, V.; Parsonage, D. Protein-sulfenic acids: Diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 1999, 38, 15407–15416. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, D.; Pearce, J.; Burley, R.W. Protein Gelation; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Lin, X.; Yang, W.; Xu, D.; Wang, L. Effect of electron irradiation and heat on the structure of hairtail surimi. Radiat. Phys. Chem. 2015, 114, 50–54. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mayer, S.G.; Park, J.W. FT-IR and Raman spectroscopies determine structural changes of tilapia fish protein isolate and surimi under different comminution conditions. Food Chem. 2017, 226, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Wang, Z.; He, F.; Wang, L.; Pan, H.; Li, X.; Wang, Q.; Zhang, D. Gel properties and molecular forces of lamb myofibrillar protein during heat induction at different pH values. Process Biochem. 2014, 49, 631–636. [Google Scholar] [CrossRef]
- Wan, J.; Ikuo, K.; Mikio, S.; Nobuo, S. Effect of calcium ion concentration on the gelling properties and transglutaminase activity of Walleye Pollack surimi paste. Fish. Sci. 1994, 60, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef]
- Carlin, K.R.M.; Hufflonergan, E.; Rowe, L.J.; Lonergan, S.M. Effect of oxidation, pH, and ionic strength on calpastatin inhibition of u- and m-calpain. J. Anim. Sci. 2006, 84, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food. Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef]
- Barrett, A.J.; Kirschke, H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981, 80, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Shen, H. Effect of frozen storage on thermal stability of sarcoplasmic protein and myofibrillar protein from common carp (Cyprinus carpio) muscle. Int. J. Food Sci. Technol. 2013, 48, 1962–1969. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Lu, H.; Luo, Y.; Feng, L. Effects of hydrolysates from silver carp (Hypophthalmichthys molitrix) scales on rancidity stability and gel properties of fish products. Food Bioprocess Technol. 2014, 7, 2178–2188. [Google Scholar] [CrossRef]
- Hultmann, L.; Rustad, T. Textural changes during iced storage of salmon (Salmo salar) and cod (Gadus morhua). J. Aquat. Food Prod. Technol. 2002, 11, 105–123. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Borderίas, A.J.; Montero, P. Chemical interactions of nonmuscle proteins in the network of Sardine (Sardina pilchardus) muscle gels. LWT-Food Sci. Technol 1997, 30, 602–608. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Liu, X.; Zhang, Y.; Wang, H.; Luo, Y. Effects of chilling and partial freezing on rigor mortis changes of bighead carp (Aristichthys nobilis) fillets: Cathepsin activity, protein degradation and microstructure of myofibrils. J. Food Sci. 2016, 80, C2725–C2731. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.L.; Chen, J. Oxidation-induced unfolding facilitates myosin cross-linking in myofibrillar protein by microbial transglutaminase. J. Agric. Food. Chem. 2012, 60, 8020–8027. [Google Scholar] [CrossRef] [PubMed]
- Aranishi, F.; Ogata, H.; Hara, K.; Osatomi, K.; Ishihara, T. Susceptibility of opioid peptides and myofibrillar proteins to carp cathepsin L. J. Agric. Food. Chem. 1998, 46, 388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.T.; Lee, J.J.; Chen, H.C. Proteolysis of actomyosin by cathepsins B, L, L-like, and X from mackerel (Scomber australasicus). J. Agric. Food. Chem. 1996, 44, 769–773. [Google Scholar] [CrossRef]
- Ogata, H.; Aranishi, F.; Hara, K.; Osatomi, K.; Ishihara, T. Proteolytic degradation of myofibrillar components by carp cathepsin L. J. Sci. Food Agric. 1998, 76, 499–504. [Google Scholar] [CrossRef]
- Estévez, M.; Ollilainen, V.; Heinonen, M. Analysis of protein oxidation markers alpha-aminoadipic and gamma-glutamic semialdehydes in food proteins using liquid chromatography (LC)-electrospray ionization (ESI)-multistage tandem mass spectrometry (MS). J. Agric. Food. Chem. 2009, 57, 3901–3910. [Google Scholar] [CrossRef]
- Li, Y.; Xia, L.; Wang, J.Z.; Zhang, C.H.; Sun, H.M.; Wang, C.Q.; Xie, X.L. Effects of oxidation on water distribution and physicochemical properties of porcine myofibrillar protein gel. Food Biophys. 2014, 9, 169–178. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xie, B.J.; Xiong, S.B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, H.; Wang, Q.; Fan, D.; Zhang, H. Intervention on activity and structure of cathepsin L during surimi gel degradation under microwave irradiation. Food Hydrocoll. 2020, 103, 105705. [Google Scholar] [CrossRef]
- Tang, S.; Feng, G.; Gao, R.; Ren, J.; Zeng, M. Thermal gel degradation (modori) in Sturgeon (Acipenseridae) surimi gels. J. Food Sci. 2019, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Yang, W. Chemical interactions and rheological properties of hairtail (Trichiurus haumela) surimi: Effects of chopping and pressure. Food Biosci. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Wu, M.; Cao, Y.; Lei, S.; Liu, Y.; Wang, J.; Hu, J.; Li, Z.; Liu, R.; Ge, Q.; Yu, H. Protein structure and sulfhydryl group changes affected by protein gel properties: Process of thermal-induced gel formation of myofibrillar protein. Int. J. Food Prop. 2019, 22, 1834–1847. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q. Physicochemical properties and microstructure of fish myofibrillar protein-lipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L.; Ren, Y.; Zhao, Q. Chemical interactions and protein conformation changes during silver carp (Hypophthalmichthys Molitrix) surimi gel formation. Int. J. Food Prop. 2014, 17, 1702–1713. [Google Scholar] [CrossRef] [Green Version]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Physicochemical properties, gel-forming ability and myoglobin content of sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) surimi produced by conventional method and alkaline solubilisation process. Eur. Food Res. Technol. Health Care 2006, 222, 58–63. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Liang, Y.; Zhang, X.; Wen, G. Effects of Cathepsins on Gel Strength and Water-Holding Capacity of Myofibrillar Protein Gels from Bighead Carp (Aristichthys nobilis) under a Hydroxyl Radical-Generation Oxidizing System. Foods 2022, 11, 330. https://doi.org/10.3390/foods11030330
Lu H, Liang Y, Zhang X, Wen G. Effects of Cathepsins on Gel Strength and Water-Holding Capacity of Myofibrillar Protein Gels from Bighead Carp (Aristichthys nobilis) under a Hydroxyl Radical-Generation Oxidizing System. Foods. 2022; 11(3):330. https://doi.org/10.3390/foods11030330
Chicago/Turabian StyleLu, Han, Yunhong Liang, Xiangmei Zhang, and Gang Wen. 2022. "Effects of Cathepsins on Gel Strength and Water-Holding Capacity of Myofibrillar Protein Gels from Bighead Carp (Aristichthys nobilis) under a Hydroxyl Radical-Generation Oxidizing System" Foods 11, no. 3: 330. https://doi.org/10.3390/foods11030330
APA StyleLu, H., Liang, Y., Zhang, X., & Wen, G. (2022). Effects of Cathepsins on Gel Strength and Water-Holding Capacity of Myofibrillar Protein Gels from Bighead Carp (Aristichthys nobilis) under a Hydroxyl Radical-Generation Oxidizing System. Foods, 11(3), 330. https://doi.org/10.3390/foods11030330





