Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Mastic Extraction (AMGE and NMGE)
2.2. Stability Assessment of Liposomes for Gastrointestinal Stress
2.3. Cytotoxic Activity
2.4. Statistical Analysis
2.5. Antimicrobial Activity
3. Results and Discussion
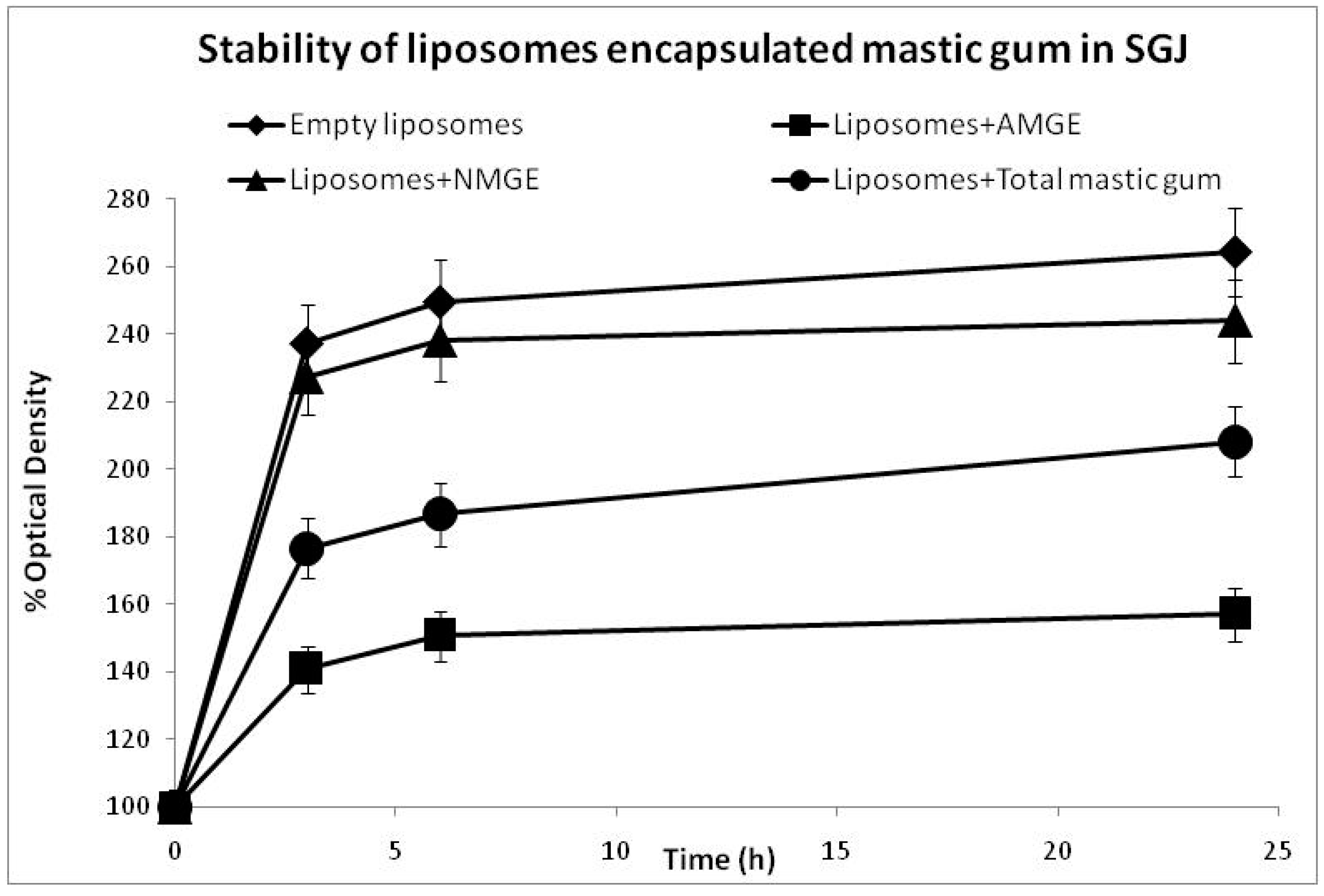
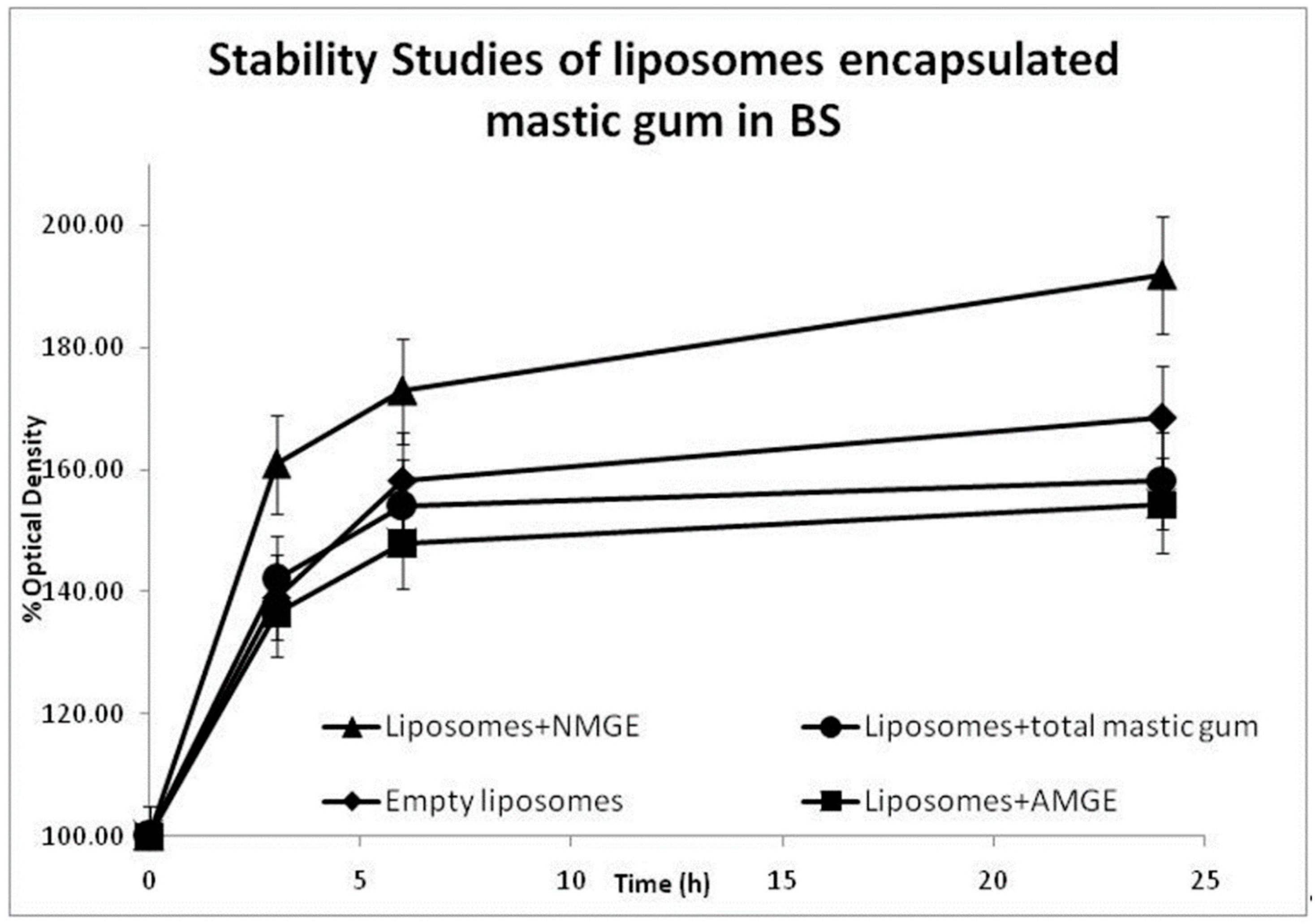
3.1. Stability Assessment of Liposomes for Gastrointestinal Stress
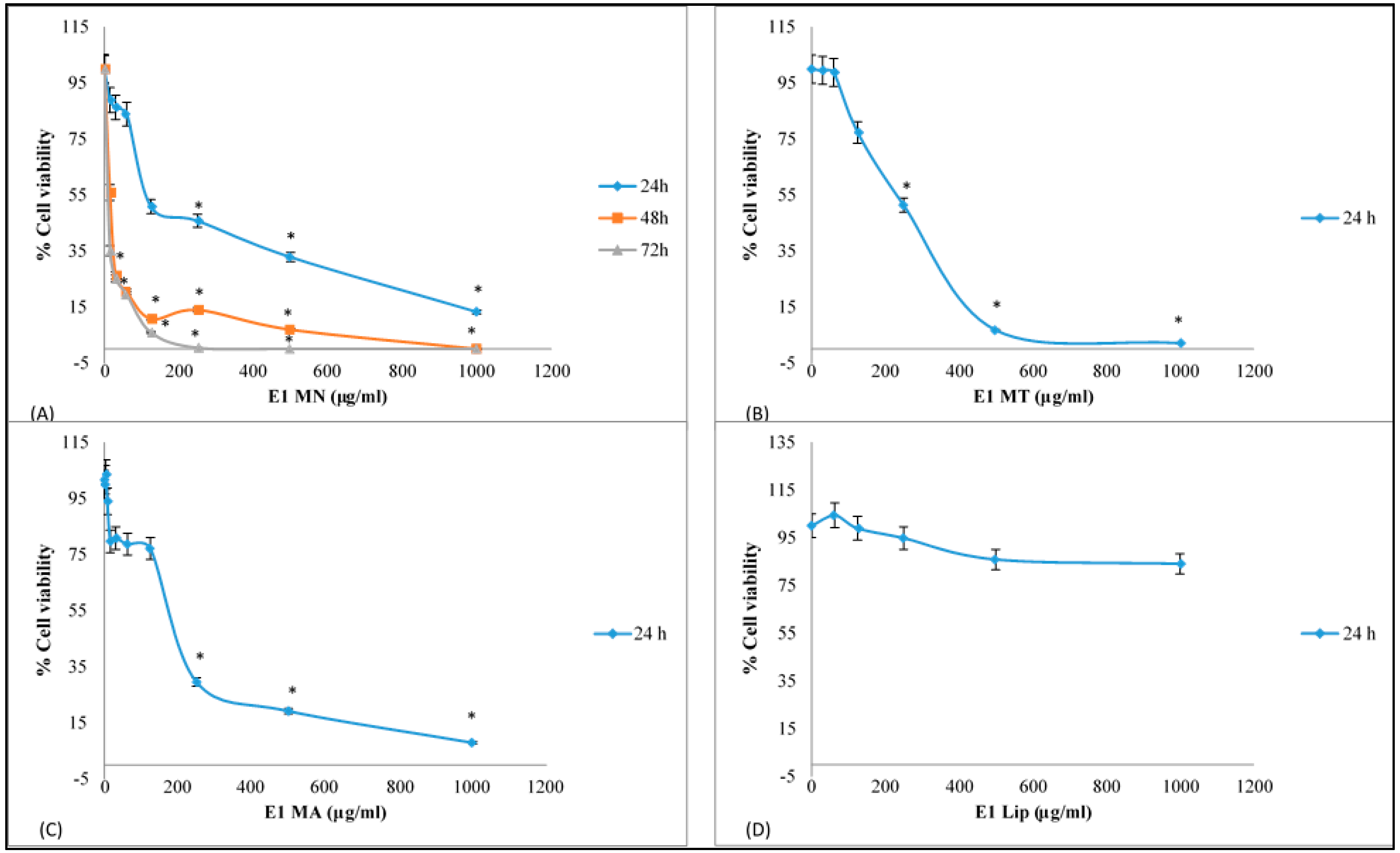
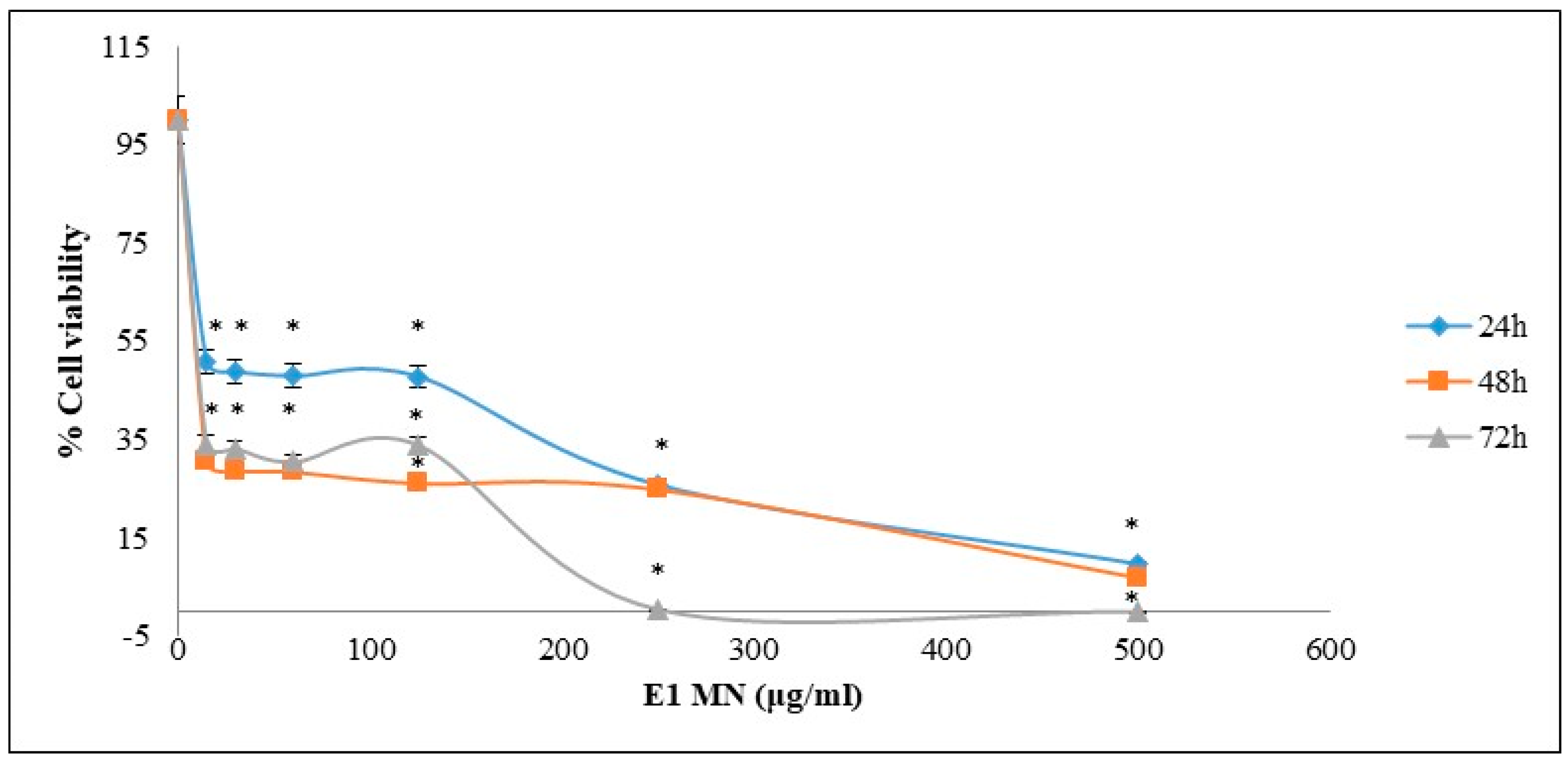
3.2. Cytotoxic Activity
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Said, M.S.; Ageel, A.M.; Parmar, N.S.; Tariq, M. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J. Ethnopharmacol. 1986, 15, 271–278. [Google Scholar] [CrossRef]
- Huwez, F.U.; Thirlwell, D.; Cockayne, A.; Ala’Aldeen, D.A.A. Mastic gum kills Helicobacter pylori. N. Engl. J. Med. 1998, 339, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Nychas, G.J.E. Antimicrobial activity of the essential oil of mastic gum (Pistacia lentiscus var. chia) on Gram positive and Gram negative bacteria in broth and in Model Food System. Int. Biodeterior. Biodegrad. 1995, 36, 411–420. [Google Scholar] [CrossRef]
- He, M.L.; Chen, W.W.; Zhang, P.J.; Jiang, A.L.; Fan, W.; Yuan, H.Q.; Liu, W.W.; Zhang, J.Y. Gum mastic increases maspin expression in prostate cancer cells. Acta Pharmacol. Sin. 2007, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Gousia, P.; Economou, V.; Sakkas, H.; Papadopoulou, C.; Skaltsounis, A.L. Chemical investigation and antimicrobial properties of mastic water and its major constituents. Food Chem. 2011, 129, 907–911. [Google Scholar] [CrossRef]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Mouhajir, F.; Hudson, J.B.; Rejdali, M.; Towers, G.H.N. Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharm. Biol. 2001, 39, 364–374. [Google Scholar] [CrossRef]
- Marner, F.J.; Freyer, A.; Lex, J. Triterpenoids from gum mastic, the resin of Pistacia lentiscus. Phytochemistry 1991, 30, 3709–3712. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Bakola-Christianopoulou, M.N.; Apazidou, K.K.; Psarros, E.E. Gas chromatographic-mass spectroscopic analysis of the acidic triterpenic fraction of mastic gum. J. Chromatogr. A 1997, 769, 263–273. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Zlatanos, S.N.; Papageorgiou, V.P. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chem. 2005, 92, 721–727. [Google Scholar] [CrossRef]
- Ko, S.; Lee, S.C. Effect of nanoliposomes on the stabilization of incorporated retinol. Afr. J. Biotechnol. 2010, 9, 6158–6161. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. A Clin. J. 2016, 15, 33–36. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Corrigendum: Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Loutrari, H.; Magkouta, S.; Pyriochou, A.; Koika, V.; Kolisis, F.N.; Papapetropoulos, A.; Roussos, C. Mastic oil from Pistacia lentiscus var. chia inhibits growth and survival of human K562 leukemia cells and attenuates angiogenesis. Nutr. Cancer 2006, 55, 86–93. [Google Scholar] [CrossRef]
- Balan, K.V.; Demetzos, C.; Prince, J.; Dimas, K.; Cladaras, M.; Han, Z.; Wyche, J.H.; Pantazis, P. Induction of apoptosis in human colon cancer HCT116 cells treated with an extract of the plant product, chios mastic gum. In Vivo 2005, 19, 93–102. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Ball, S.; Arevalo, M.; Juarez, E.; Payne, J.D.; Jones, C. Breast cancer chemoprevention: An update on current practice and opportunities for primary care physicians. Prev. Med. 2019, 129, 105834. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.R. Discovery of the anticancer drug vinblastine from the endophytic Alternaria alternata and yield improvement by gamma irradiation mutagenesis. J. Appl. Microbiol. 2021, 131, 2886–2898. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, H.M.; Malogolowkin, M.H.; Krailo, M.D.; Piao, J.; Towbin, A.J.; McCarville, M.B.; Tiao, G.M.; Dunn, S.P.; Langham, M.R.; McGahren, E.D.; et al. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: A Children’s Oncology Group study. Cancer 2021, 11. [Google Scholar] [CrossRef]
- He, M.L.; Li, A.; Xu, C.S.; Wang, S.L.; Zhang, M.J.; Gu, H.; Yang, Y.Q.; Tao, H.H. Mechanisms of antiprostate cancer by gum mastic: NF-κB signal as target. Acta Pharmacol. Sin. 2007, 28, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Moulos, P.; Papadodima, O.; Chatziioannou, A.; Loutrari, H.; Roussos, C.; Kolisis, F.N. A transcriptomic computational analysis of mastic oil-treated Lewis lung carcinomas reveals molecular mechanisms targeting tumor cell growth and survival. BMC Med. Genom. 2009, 2, 68. [Google Scholar] [CrossRef]
- He, M.L.; Yuan, H.Q.; Jiang, A.L.; Gong, A.Y.; Chen, W.W.; Zhang, P.J.; Young, C.Y.; Zhang, J.Y. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer 2006, 106, 2547–2555. [Google Scholar] [CrossRef]
- Sakagami, H.; Kishino, K.; Kobayashi, M.; Hashimoto, K.; Iida, S.; Shimetani, A.; Nakamura, Y.; Takahashi, K.; Ikarashi, T.; Fukamachi, H.; et al. Selective Antibacterial and Apoptosis-modulating Activities of Mastic. In Vivo 2009, 23, 215–223. [Google Scholar]
- WHO. Foodborne Listeriosis. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 1 December 2021).
- Mumy, K.L. Salmonella. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 211–212. [Google Scholar]
- Gortzi, O.; Athanasiadis, V.; Lalas, S.; Chinou, I.; Tsaknis, J. Study of antioxidant and antimicrobial activity of chios mastic gum fractions (neutral, acidic) before and after encapsulation in liposomes. J. Food Process. Technol. 2014, 5, 1000355. [Google Scholar] [CrossRef]
- Dua, S.J.; Rana, A.C.; Bhandari, A.K. Liposome: Methods of preparation and applications. Int. J. Clin. Pharmacol. Res. 2012, 3, 14–20. [Google Scholar]
- Dwiecki, K.; Górnas, P.; Wilk, A.; Nogala-Kałucka, M.; Polewski, K. Spectroscopic studies of D-α-tocopherol concentration-induced transformation in egg phosphatidylcholne vesicles. Cell. Mol. Biol. Lett. 2007, 12, 51–69. [Google Scholar] [CrossRef]
- Pak, D.; Muthaiyan, A.; Story, R.S.; Bryan, C.A.; Lee, S.-O.; Crandall, P.G.; Ricke, S.C. Fermentative Capacity of Three Strains of Lactobacillus Using Different Sources of Carbohydrates: In Vitro Evaluation of Synbiotic Effects, Resistance and Tolerance to Bile and Gastric Juices. J. Food Res. 2013, 2, 158. [Google Scholar] [CrossRef][Green Version]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Michalakea, E.; Graikou, K.; Aligiannis, N.; Panoutsopoulos, G.; Kalpoutzakis, E.; Roussakis, C.; Chinou, I. Isolation and structure elucidation of secondary metabolites of two Greek endemic Inula species. Biological activities. Phytochem. Lett. 2019, 31, 155–160. [Google Scholar] [CrossRef]
- Wu, W.; Lu, Y.; Qi, J. Oral delivery of liposomes. Ther. Deliv. 2015, 6, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Nacka, F.; Cansell, M.; Méléardb, P.; Combe, N. Incorporation of α-tocopherol in marine lipid-based liposomes: In vitro and in vivo studies. Lipids 2001, 36, 1313–1320. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, K.E.; Kim, J.J.; Lim, S.H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef]
- Şirin, N.; Elmas, L.; Seçme, M.; Dodurga, Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene 2020, 737, 144428. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Batista, H.R.F.; Estevam, E.B.B.; Alves, C.C.F.; Forim, M.R.; Nicolella, H.D.; Furtado, R.A.; Tavares, D.C.; Silva, T.S.; Martins, C.H.G.; et al. Chemical composition and in vitr antibacterial and antiproliferative activities of the essential oil from the leaves of Psidium myrtoides O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 33, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.; Bazaid, S.; Shohayeb, M.; El-Sayed, M.; El-Wakil, E.A. Phytochemical Studies and Evaluation of Antioxidant, Anticancer and Antimicrobial Properties of Conocarpus erectus L. Growing in Taif, Saudi Arabia. Eur. J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Wageesha, N.D.A.; Soysa, P.; Choudhary, M.; Ekanayake, M. Evaluation of antioxidant and polyphenolic content of a Sri Lankan poly herbal formulae and assessment of its in vitro antiproliferative activity on RD and MCF-7 cancer cells compared to healthy CC 1 cells. Asian J. Ethnopharmacol. Med. Foods 2017, 4, 17–25. [Google Scholar]
- Sharif Sharifi, M.; Hazell, S.L. Fractionation of Mastic Gum in Relation to Antimicrobial Activity. Pharmaceuticals 2009, 2, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J.; Drulis-Kawa, Z.; Dorotkiewicz-Jach, A.; Doroszkiewicz, W.; Kozubek, A. In vitro Antimicrobial Activity of Liposomes Containing Ciprofloxacin, Meropenem and Gentamicin Against Gram-Negative Clinical Bacterial Strains. Lett. Drug Des. Discov. 2007, 4, 297–304. [Google Scholar] [CrossRef]
| Compounds | IC50 Values μg/mL | |||||
|---|---|---|---|---|---|---|
| HepG2 | MCF-7 | |||||
| 24 h | 48 h b | 72 h b | 24 h b | 48 h b | 72 h b | |
| Liposomes contained NMGE | 132 ± 6.7 | 17 ± 0.7 | 11 ± 0.9 | 18 ± 1.4 | 12 ± 1.1 | 10 ± 0.2 |
| Liposomes contained AMGE | 184 ± 20.2 | |||||
| Liposomes contained total mastic gum | 255 ± 17.9 | |||||
| Empty liposomes | - a | |||||
| Mastic Extract | Liposome Type | S. aureus | S. epidermidis | P. aeruginosa | E. cloacae | K. pnaumioniae | E. coli |
|---|---|---|---|---|---|---|---|
| Total mastic gum | 22/0.04 | 20/0.05 | 17/0.21 | 15/0.25 | 13/0.34 | 18/0.18 | |
| Free AMGE | 17/0.19 | 16/0.20 | 14/0.27 | 13/0.38 | 11/0.47 | 15/0.25 | |
| Free NMGE | 14/0.45 | 13/0.52 | 12/0.73 | 11/0.88 | 10/1.0 | 12/0.64 | |
| Liposome + AMGE | EI | 12/0.67 | 12/0.74 | 10/0.94 | 9/1.32 | 8/1.77 | 9/1.53 |
| Liposome + NMGE | EI | 10/1.38 | 10/1.50 | 9/1.71 | 9/1.94 | 8/1.98 | 8/1.94 |
| Netilmicin | 23/4 × 10−3 | 23/4 × 10−3 | 20/8.8 × 10−3 | 21/8 × 10−3 | 21/8 × 10−3 | 19/10 × 10−3 | |
| Amoxicillin | 25/2 × 10−3 | 24/2 × 10−3 | 22/2.4 × 10−3 | 21/2.2 × 10−3 | 20/2.8 × 10−3 | 24/2 × 10−3 |
| Mastic Extract | Liposome Type | Candida albicans | Candida tropicalis | Candida glabrata |
|---|---|---|---|---|
| Total mastic gum | 14/0.73 | 16/0.56 | 19/0.32 | |
| Free AMGE | 10/1.10 | 11/0.98 | 13/0.78 | |
| Free NMGE | 12/1.25 | 13/1.00 | 130.85 | |
| Liposome + AMGE | EI | 8/1.90 | 9/1.88 | 10/1.81 |
| Liposome + NMGE | EI | 9/1.98 | 9/2.00 | 10/1.87 |
| 5-Flucytosine | 21/1 × 10−3 | 22/1 × 10−3 | 25/0.5 × 10−3 | |
| AmphotericinΒ | 22/1 × 10−3 | 23/0.5 × 10−3 | 23/0.4 × 10−3 |
| Mastic Extract | Liposome Type | Listeria monocytogenes | Salmonella poona |
|---|---|---|---|
| Total mastic gum | 21/0.12 | 25/0.05 | |
| Free AMGE | 16/0.15 | 19/0.12 | |
| Free NMGE | 13/0.27 | 15/0.20 | |
| Liposome + AMGE | EI | 10/1.12 | 11/1.10 |
| Liposome + NMGE | EI | 10/1.27 | 10/1.24 |
| Netilmicin | 22/0.06 | 22/0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gortzi, O.; Rovoli, M.; Katsoulis, K.; Graikou, K.; Karagkini, D.-A.; Stagos, D.; Kouretas, D.; Tsaknis, J.; Chinou, I. Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods 2022, 11, 271. https://doi.org/10.3390/foods11030271
Gortzi O, Rovoli M, Katsoulis K, Graikou K, Karagkini D-A, Stagos D, Kouretas D, Tsaknis J, Chinou I. Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods. 2022; 11(3):271. https://doi.org/10.3390/foods11030271
Chicago/Turabian StyleGortzi, Olga, Magdalini Rovoli, Konstantinos Katsoulis, Konstantia Graikou, Despoina-Aikaterini Karagkini, Dimitrios Stagos, Dimitrios Kouretas, John Tsaknis, and Ioanna Chinou. 2022. "Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes" Foods 11, no. 3: 271. https://doi.org/10.3390/foods11030271
APA StyleGortzi, O., Rovoli, M., Katsoulis, K., Graikou, K., Karagkini, D.-A., Stagos, D., Kouretas, D., Tsaknis, J., & Chinou, I. (2022). Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods, 11(3), 271. https://doi.org/10.3390/foods11030271










