Biovalorization of Market Surplus Bread for Development of Probiotic-Fermented Potential Functional Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of Bread Slurries as Fermentation Media
2.3. Microbial Enumeration
2.4. Fermentation of Bread Slurries with Probiotics
2.5. Fermentation of Bread Slurries Made from Different Bread Variants
2.6. Fermentation of Bread Slurries Supplemented with Sweeteners and Stabiliser
2.7. Shelf Life Monitoring
2.8. Non-Volatile Compounds Analysis
2.9. Volatile Organic Compounds Analysis
2.10. Ethanol Contents Analysis
2.11. Statistical Analysis
3. Results and Discussion
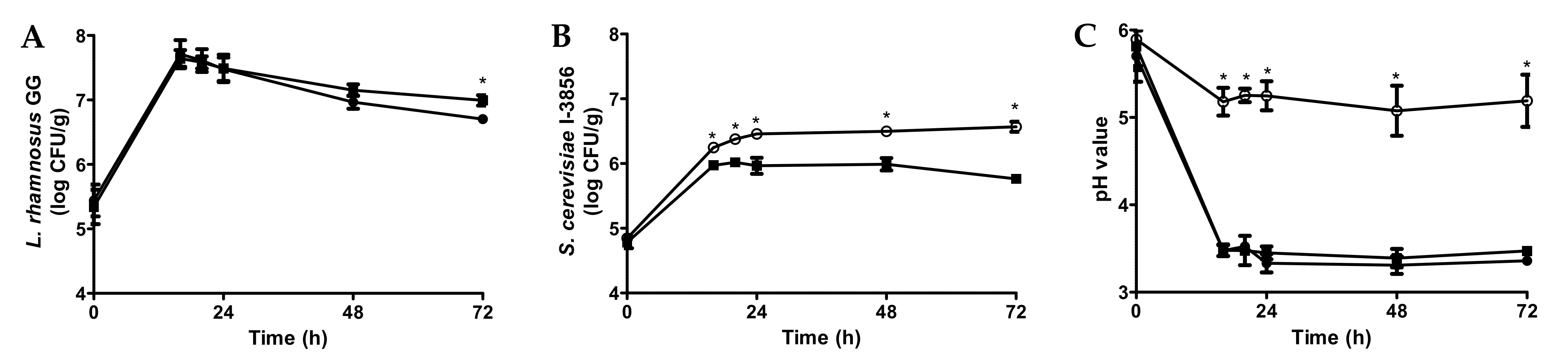
3.1. Fermentation of Bread Slurries with Probiotics
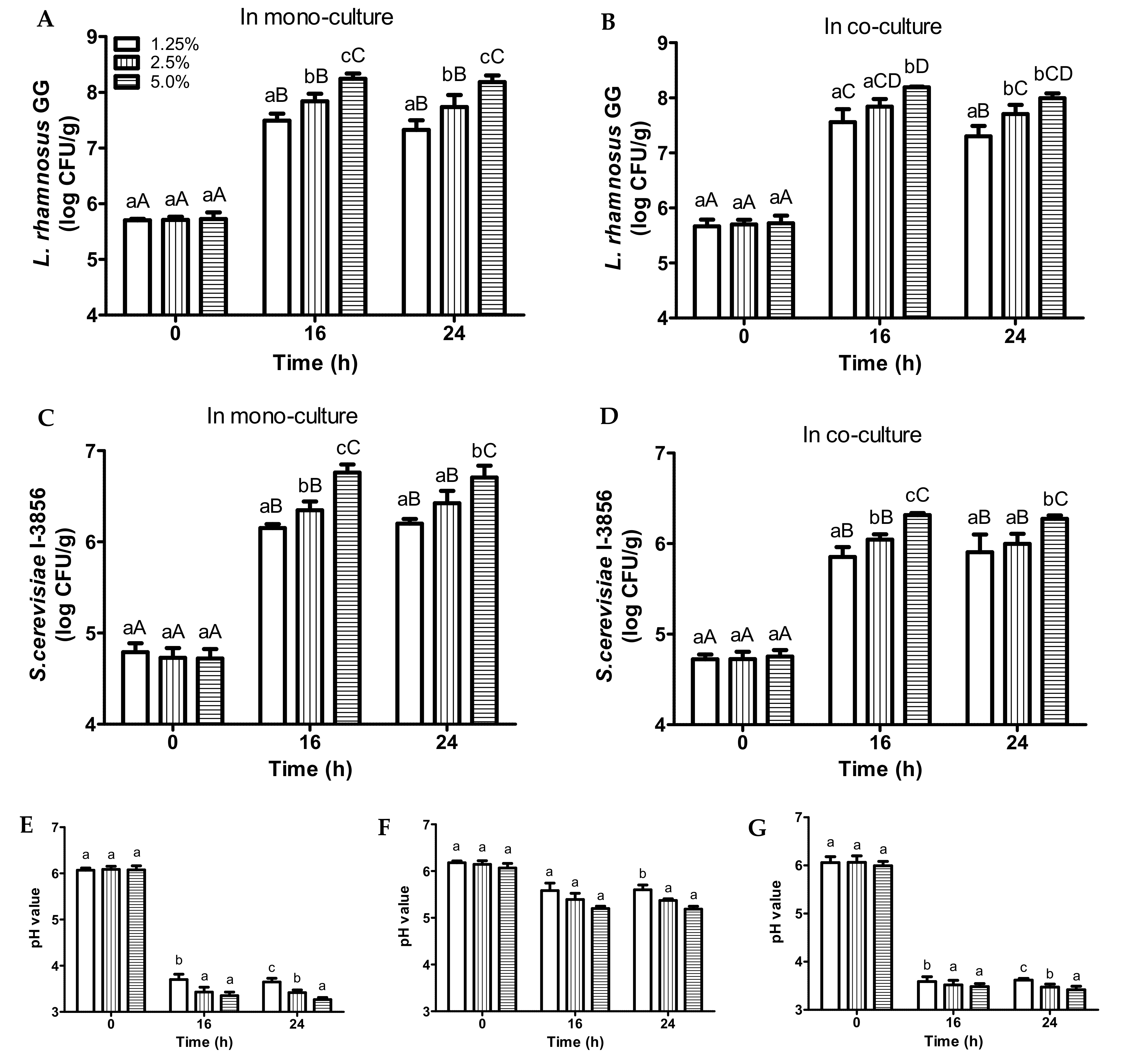
3.2. Fermentation of Bread Slurries of Different Bread Concentrations
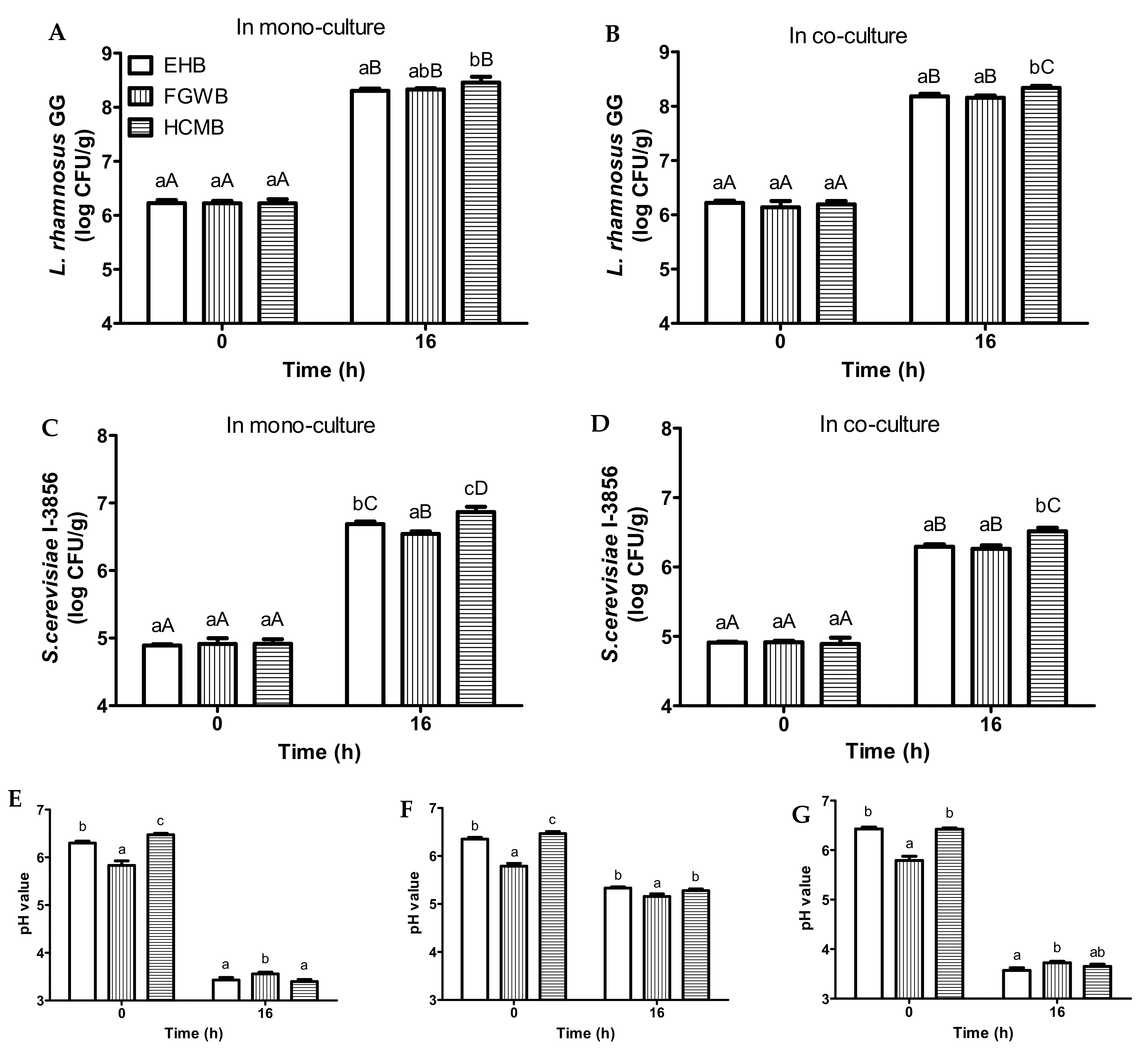
3.3. Fermentation of Bread Slurries Made from Different Bread Variants
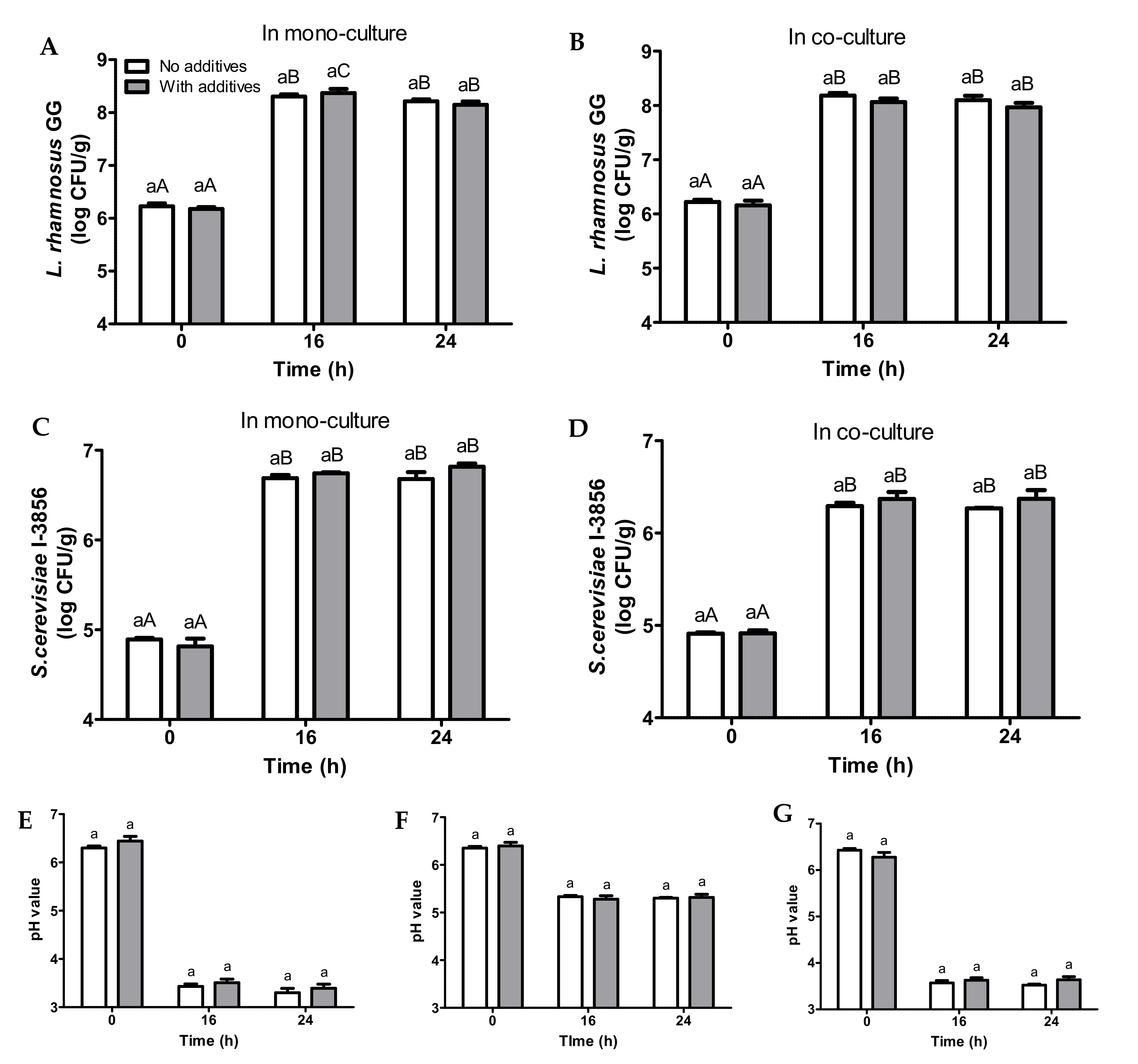
3.4. Fermentation of Bread Slurries Supplemented with Additives
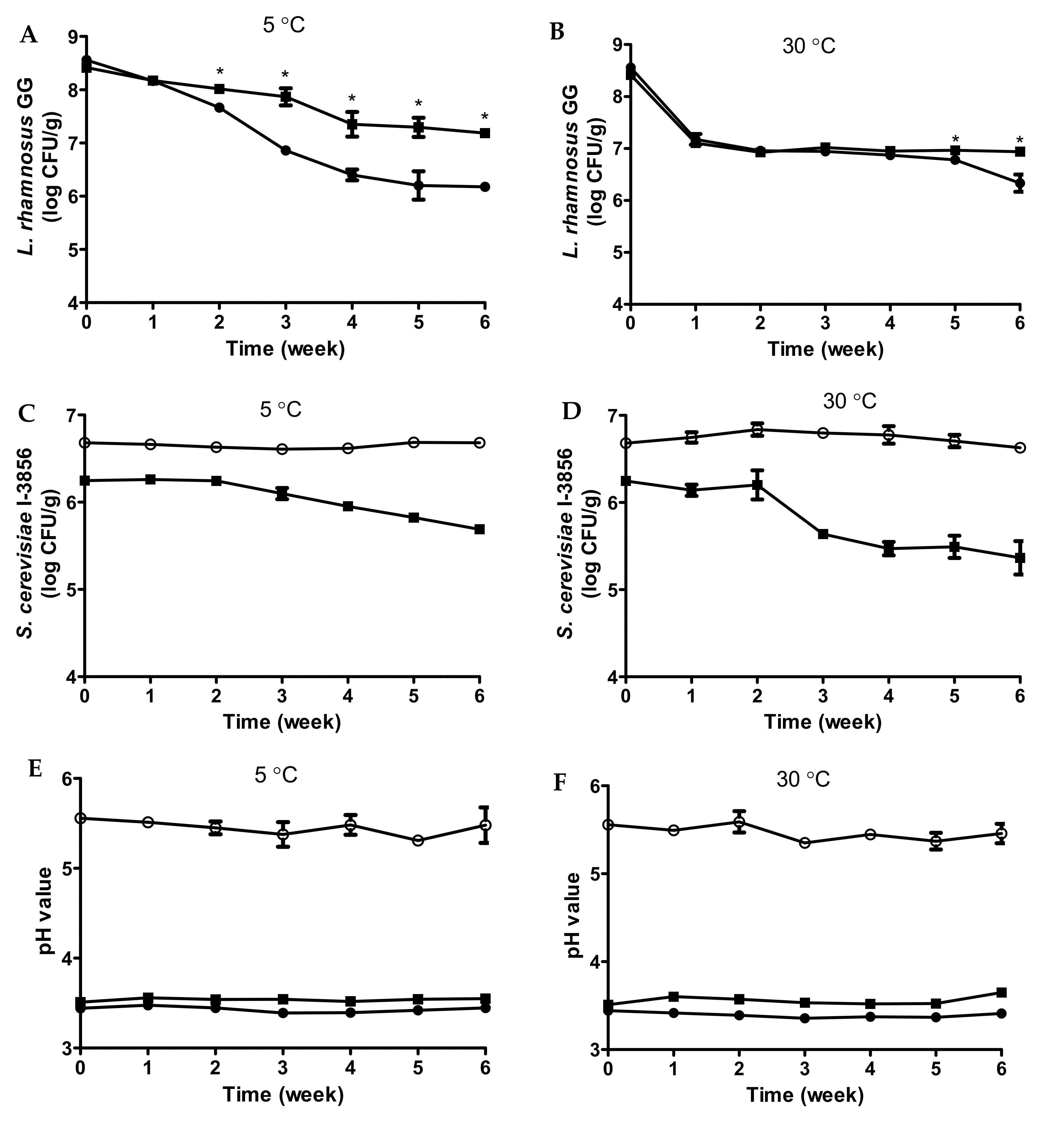
3.5. Shelf Life Monitoring of Fermented Bread Beverages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Toy, J.Y.H.; Lu, Y.; Huang, D.; Matsumura, K.; Liu, S.-Q. Enzymatic treatment, unfermented and fermented fruit-based products: Current state of knowledge. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef]
- FAO/WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome Italy, 2006. [Google Scholar]
- Lu, Y.; Putra, S.D.; Liu, S.-Q. A novel non-dairy beverage from durian pulp fermented with selected probiotics and yeast. Int. J. Food Microbiol. 2018, 265, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tan, C.-W.; Chen, D.; Liu, S.-Q. Potential of three probiotic lactobacilli in transforming star fruit juice into functional beverages. Food Sci. Nutr. 2018, 6, 2141–2150. [Google Scholar] [CrossRef]
- FAO. Food Loss and Food Waste. Available online: http://www.fao.org/ (accessed on 18 November 2018).
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Kinetic studies on the multi-enzyme solution produced via solid state fermentation of waste bread by Aspergillus awamori. Biochem. Eng. J. 2013, 80, 76–82. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Stepwise optimisation of enzyme production in solid state fermentation of waste bread pieces. Food Bioprod. Process. 2013, 91, 638–646. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Ethanol fermentation of waste bread using granular starch hydrolyzing enzyme: Effect of raw material pretreatment. Fuel 2014, 134, 250–256. [Google Scholar] [CrossRef]
- Kumar, A.; Roy, B.; Lakhani, G.P.; Jain, A. Evaluation of dried bread waste as feedstuff for growing crossbred pigs. Vet. World 2014, 7, 698–701. [Google Scholar] [CrossRef]
- Han, W.; Huang, J.; Zhao, H.; Li, Y. Continuous biohydrogen production from waste bread by anaerobic sludge. Bioresour. Technol. 2016, 212, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.C.J.; Cheung, A.S.Y.; Zhang, A.Y.-Z.; Lam, K.F.; Lin, C.S.K. Utilisation of waste bread for fermentative succinic acid production. Biochem. Eng. J. 2012, 65, 10–15. [Google Scholar] [CrossRef]
- Polanowska, K.; Varghese, R.; Kuligowski, M.; Majcher, M. Carob kibbles as an alternative raw material for production of kvass with probiotic potential. J. Sci. Food Agric. 2021, 101, 5487–5497. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Factories 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weese, J.S.; Anderson, M.E. Preliminary evaluation of Lactobacillus rhamnosus strain GG, a potential probiotic in dogs. Can. Vet. J. 2002, 43, 771–774. [Google Scholar] [PubMed]
- Talwalkar, A.; Kailasapathy, K. The role of oxygen in the viability of probiotic bacteria with reference to Lactobacillus acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol. 2004, 5, 1–8. [Google Scholar] [PubMed]
- Toh, M.; Liu, S.Q. Influence of commercial inactivated yeast derivatives on the survival of probiotic bacterium Lactobacillus rhamnosus HN001 in an acidic environment. AMB Express 2017, 7, 156–167. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Tsao, M. Enhancement of survival of probiotic and non-probiotic lactic acid bacteria by yeasts in fermented milk under non-refrigerated conditions. Int. J. Food Microbiol. 2009, 135, 34–38. [Google Scholar] [CrossRef]
- Cayzeele-Decherf, A.; Pélerin, F.; Leuillet, S.; Douillard, B.; Housez, B.; Cazaubiel, M.; Jacobson, G.; Jüsten, P.; Desreumaux, P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J. Gastroenterol. 2017, 23, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Roussel, C.; Sivignon, A.; de Vallee, A.; Garrait, G.; Denis, S.; Tsilia, V.; Ballet, N.; Vandekerckove, P.; Van de Wiele, T.; Barnich, N.; et al. Anti-infectious properties of the probiotic Saccharomyces cerevisiae CNCM I-3856 on enterotoxigenic E. coli (ETEC) strain H10407. Appl. Microbiol. Biotechnol. 2018, 102, 6175–6189. [Google Scholar] [CrossRef]
- Gabrielli, E.; Pericolini, E.; Ballet, N.; Roselletti, E.; Sabbatini, S.; Mosci, P.; Decherf, A.; Pélerin, F.; Perito, S.; Jüsten, P.; et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef. Microbes 2018, 9, 219–230. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Mosci, P.; Decherf, A.C.; Pélerin, F.; Perito, S.; Scarpelli, P.; Vecchiarelli, A. Saccharomyces cerevisiae-based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis. Virulence 2018, 9, 954–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatini, S.; Monari, C.; Ballet, N.; Decherf, A.C.; Bozza, S.; Camilloni, B.; Perito, S.; Vecchiarelli, A. Anti-biofilm properties of Saccharomyces cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 probiotics against Gardnerella vaginalis. Microorganisms 2020, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; López, P.; Capozzi, V.; De Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-Glucans Improve Growth, Viability and Colonization of Probiotic Microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yuan, W.; Lu, Y.; Liu, S.Q. Biotransformation of spent coffee grounds by fermentation with monocultures of Saccha-romyces cerevisiae and Lachancea thermotolerans aided by yeast extracts. LWT Food Sci. Technol. 2021, 138, 110751. [Google Scholar] [CrossRef]
- Wang, S.-A.; Li, F.-L. Invertase SUC2 Is the Key Hydrolase for Inulin Degradation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 403–406. [Google Scholar] [CrossRef] [Green Version]
- Viscione, L. Fibre-enriched beverages. In Fibre-Rich and Wholegrain Food; Delcour, J.A., Poutanen, K., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 369–388. [Google Scholar]
- Nabais, R.C.; Sá-Correia, I.; Viegas, C.A.; Novais, J.M. Influence of Calcium Ion on Ethanol Tolerance of Saccharomyces bayanus and Alcoholic Fermentation by Yeasts. Appl. Environ. Microbiol. 1988, 54, 2439–2446. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, A.; Watanabe, T. The network structure of gellan gum hydrogels based on the structural parameters by the analysis of the restricted diffusion of water. Carbohydr. Polym. 1996, 30, 135–140. [Google Scholar] [CrossRef]
- Könönen, E. Anaerobic cocci and anaerobic Gram-positive nonsporulating Bacilli. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Philadelphia: Elsevier Saunders, PA, USA, 2015; pp. 2781–2786. [Google Scholar]
- Russell, J.B.; Diez-Gonzalez, F. The Effects of Fermentation Acids on Bacterial Growth. Adv. Microb. Physiol. 1998, 39, 205–234. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Surface physiological changes induced by lactic acid on pathogens in consideration of pKa and pH. Food Control. 2014, 46, 525–531. [Google Scholar] [CrossRef]
- Singhal, B.; Chaudhary, N. Metabiotics as functional metabolites of probiotics: An emerging concept and its potential application in food and health. In Biotechnical Processing in the Food Industry: New Methods, Techniques, and Applications; Verma, D.K., Tatel, A.R., Ashish-Baldi, K.S.S., Garcia, S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 207–230. [Google Scholar]
- Landete, J.M.; Ferrer, S.; Monedero, V.; Zúñiga, M. Malic enzyme and malolactic enzyme pathways are functionally linked but independently regulated in Lactobacillus BL23. Appl. Environ. Microbiol. 2013, 79, 5509–5518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Song, H.Y.; Yu, R.-C. Optimization of culture conditions for gamma-aminobutyric acid production in fermented adzuki bean milk. J. Food Drug Anal. 2018, 26, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Gonzalez, R.; Martínez-Moreno, R.; Beltran, G.; Mas, A. Biomass production and alcoholic fermentation perfor-mance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 2012, 12, 477–485. [Google Scholar]
- White, W.H.; Gunyuzlu, P.L.; Toyn, J.H. Saccharomyces cerevisiae is capable of de novo pantothenic acid biosynthesis in-volving a novel pathway of beta-alanine production from spermine. J. Biol. Chem. 2001, 276, 10794–10800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FEMA. Flavour Library. Available online: https://www.femaflavor.org/ (accessed on 7 March 2019).
| Compounds | Unfermented Bread Slurry | L. rhamnosus GG | S. cerevisiae CNCM I-3856 | L. rhamnosus GG + S. cerevisiae CNCM I-3856 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 6 (5 °C) | Week 6 (30 °C) | Week 0 | Week 6 (5 °C) | Week 6 (30 °C) | Week 0 | Week 6 (5 °C) | Week 6 (30 °C) | ||
| Sugars (g/L) | ||||||||||
| Fructose | 3.21 ± 0.11 b | 0.85 ± 0.21 a | 0.61 ± 0.32 a | ND | 0.62 ± 0.34 a | ND | ND | 0.48 ± 0.25 a | ND | ND |
| Glucose | 2.46 ± 0.13 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Organic acids (g/L) | ||||||||||
| Oxalic acid | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Malic acid | 0.19 ± 0.03 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Lactic acid | ND | 2.98 ± 0.20 b | 3.17 ± 0.23 bc | 3.33 ± 0.13 c | ND | ND | ND | 2.50 ± 0.15 a | 2.42 ± 0.16 a | 2.32 ± 0.41 a |
| Acetic acid | 0.11 ± 0.02 a | 0.17 ± 0.02 b | 0.15 ± 0.02 b | 0.45 ± 0.02 d | 0.16 ± 0.05 b | 0.15 ± 0.03 b | 0.30 ± 0.00 c | 0.15 ± 0.03 b | 0.15 ± 0.02 b | 0.30 ± 0.05 c |
| Propionic acid | 0.18 ± 0.02 a | 0.19 ± 0.02 a | 0.17 ± 0.03 a | 0.19 ± 0.00 a | 0.18 ± 0.02 a | 0.17 ± 0.01 a | 0.18 ± 0.00 a | 0.17 ± 0.02 a | 0.17 ± 0.01 a | 0.18 ± 0.02 a |
| Free amino acids (μg/mL) | ||||||||||
| Ammonia | 2.72 ± 0.19 b | 2.61 ± 0.52 b | 3.26 ± 0.46 b | 7.76 ± 0.66 d | 1.28 ± 0.10 a | 1.10 ± 0.04 a | 0.94 ± 0.07 a | 1.49 ± 0.09 a | 1.59 ± 0.15 a | 6.93 ± 1.09 c |
| Serine | 2.00 ± 0.53 a | 2.97 ± 0.14 b | 3.13 ± 0.16 b | 5.63 ± 0.28 c | ND | ND | ND | 2.61 ± 0.20 b | 2.61 ± 0.39 b | 6.67 ± 0.74 d |
| Glutamic acid | 10.13 ± 0.47 a | 44.73 ± 6.66 c | 43.53 ± 6.49 c | 71.34 ± 8.50 c | 2.47 ± 0.82 a | 1.88 ± 0.35 a | 5.37 ± 1.66 a | 19.02 ± 2.18 b | 19.06 ± 2.46 b | 47.96 ± 5.63 c |
| Glycine | 2.04 ± 0.07 b | 2.23 ± 0.16 b | 2.47 ± 0.20 bc | 3.37 ± 0.19 d | ND | ND | 1.56 ± 0.74 a | 2.08 ± 0.09 b | 2.87 ± 0.09 c | 4.55 ± 0.28 e |
| Histidine | ND | ND | ND | 1.81 ± 0.20 b | ND | ND | ND | ND | ND | 1.60 ± 0.17 a |
| Arginine | 3.51 ± 0.35 b | 4.97 ± 0.62 cd | 4.88 ± 0.23 cd | 5.40 ± 0.39 d | ND | ND | 2.04 ± 1.15 a | 2.32 ± 0.23 a | 2.40 ± 0.29 a | 4.36 ± 0.67 bc |
| Threonine | 1.47 ± 0.04 a | ND | ND | ND | ND | ND | ND | ND | ND | 3.90 ± 0.61 b |
| Alanine | 12.62 ± 0.33 d | 8.79 ± 0.27 c | 9.02 ± 0.47 c | 13.83 ± 0.70 e | ND | ND | 3.74 ± 0.62 a | 7.00 ± 0.66 b | 8.40 ± 0.77 c | 14.00 ± 0.70 e |
| Proline | 1.81 ± 0.14 d | 16.70 ± 2.22 c | 17.01 ± 2.70 c | 25.26 ± 2.85 e | ND | ND | 1.78 ± 0.15 a | 14.52 ± 1.06 b | 13.73 ± 1.35 bc | 21.30 ± 1.37 d |
| Tyrosine | 2.29 ± 0.35 a | 3.29 ± 0.40 ab | 3.95 ± 0.70 b | 9.73 ± 0.85 c | ND | ND | 2.82 ± 0.35 ab | ND | 2.63 ± 0.17 ab | 14.79 ± 2.09 c |
| Valine | 1.45 ± 0.61 a | ND | ND | 2.72 ± 0.21 b | ND | ND | 2.97 ± 0.17 b | ND | ND | 7.01 ± 0.52 c |
| Lysine | 2.92 ± 0.27 b | ND | ND | ND | ND | ND | 2.38 ± 0.59 a | ND | ND | 2.16 ± 0.69 a |
| Isoleucine | ND | ND | ND | 2.52 ± 0.33 a | ND | ND | 2.94 ± 0.22 a | ND | ND | 6.46 ± 0.97 b |
| Leucine | 2.83 ± 0.16 ab | 1.44 ± 0.20 a | 1.85 ± 0.25 a | 5.84 ± 0.76 c | ND | ND | 3.64 ± 0.28 b | ND | 1.64 ± 0.18 a | 14.76 ± 2.23 c |
| Tryptophan | 5.10 ± 0.18 d | 4.38 ± 0.17 c | 4.76 ± 0.08 cd | ND | ND | ND | ND | 2.32 ± 0.77 a | 3.20 ± 0.44 b | ND |
| γ-ABA | 3.51 ± 0.35 cd | 2.54 ± 2.21 abc | 4.92 ± 0.46 e | 5.84 ± 0.74 e | 1.67 ± 0.35 ab | 1.39 ± 0.21 a | 2.70 ± 0.15 abc | 3.01 ± 0.45 d | 4.81 ± 0.24 e | 12.03 ± 0.17 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-L.; Toh, M.; Lu, Y.; Ku, S.; Liu, S.-Q. Biovalorization of Market Surplus Bread for Development of Probiotic-Fermented Potential Functional Beverages. Foods 2022, 11, 250. https://doi.org/10.3390/foods11030250
Nguyen T-L, Toh M, Lu Y, Ku S, Liu S-Q. Biovalorization of Market Surplus Bread for Development of Probiotic-Fermented Potential Functional Beverages. Foods. 2022; 11(3):250. https://doi.org/10.3390/foods11030250
Chicago/Turabian StyleNguyen, Thuy-Linh, Mingzhan Toh, Yuyun Lu, Sebastian Ku, and Shao-Quan Liu. 2022. "Biovalorization of Market Surplus Bread for Development of Probiotic-Fermented Potential Functional Beverages" Foods 11, no. 3: 250. https://doi.org/10.3390/foods11030250
APA StyleNguyen, T.-L., Toh, M., Lu, Y., Ku, S., & Liu, S.-Q. (2022). Biovalorization of Market Surplus Bread for Development of Probiotic-Fermented Potential Functional Beverages. Foods, 11(3), 250. https://doi.org/10.3390/foods11030250








