Modeling Tool for Studying the Influence of Operating Conditions on the Enzymatic Hydrolysis of Milk Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolysis Curves
2.3. Experimental Design and Statistics
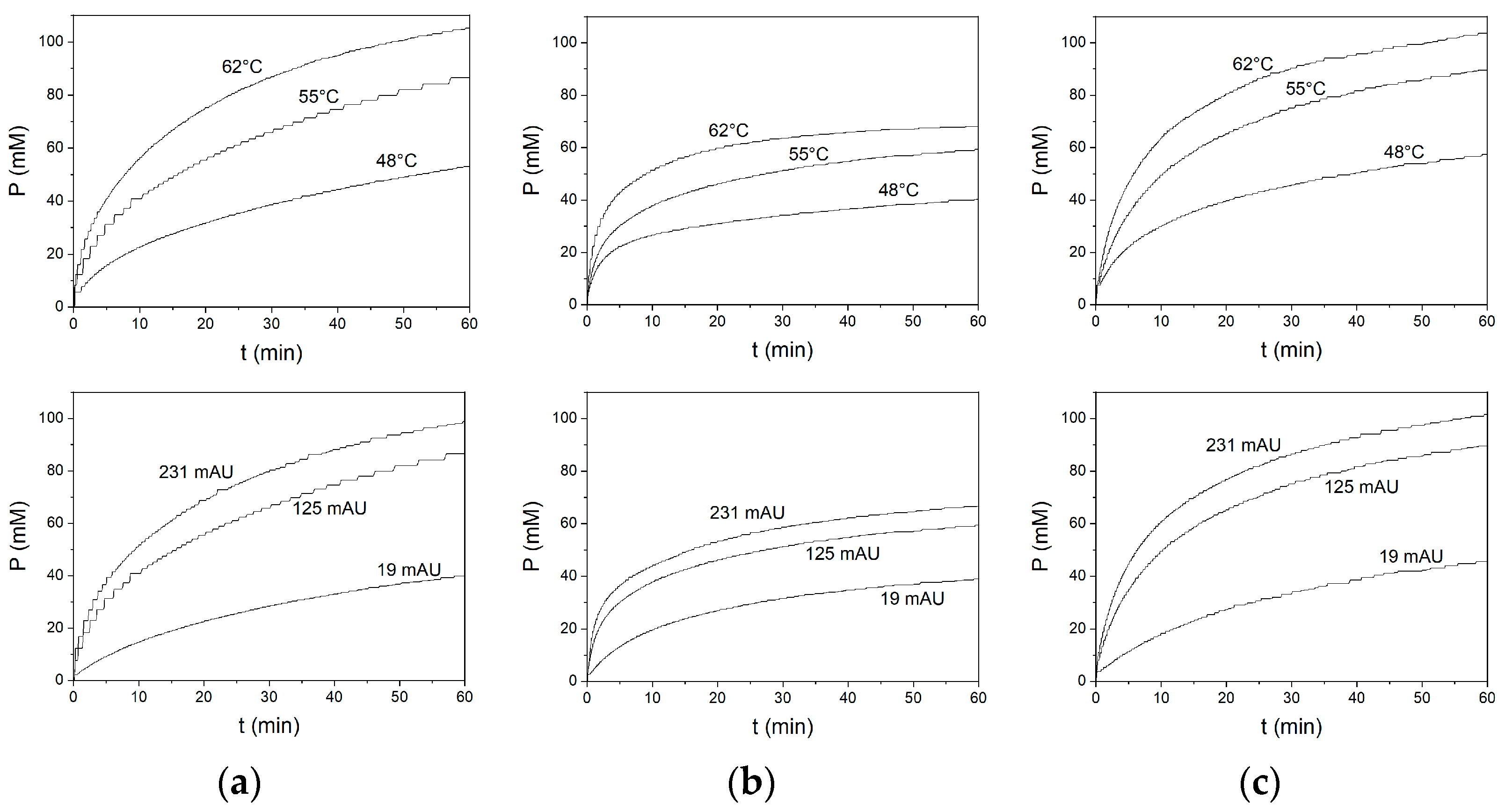
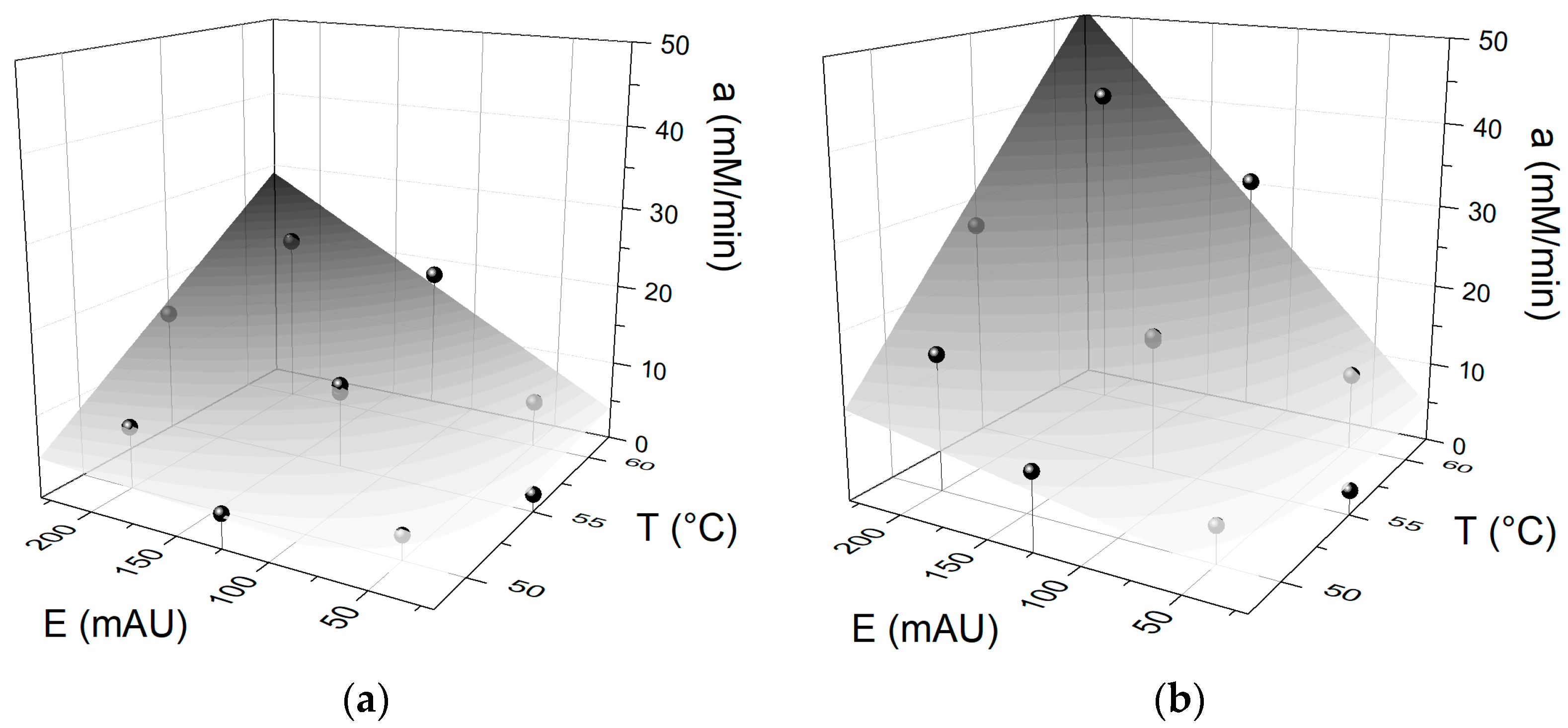
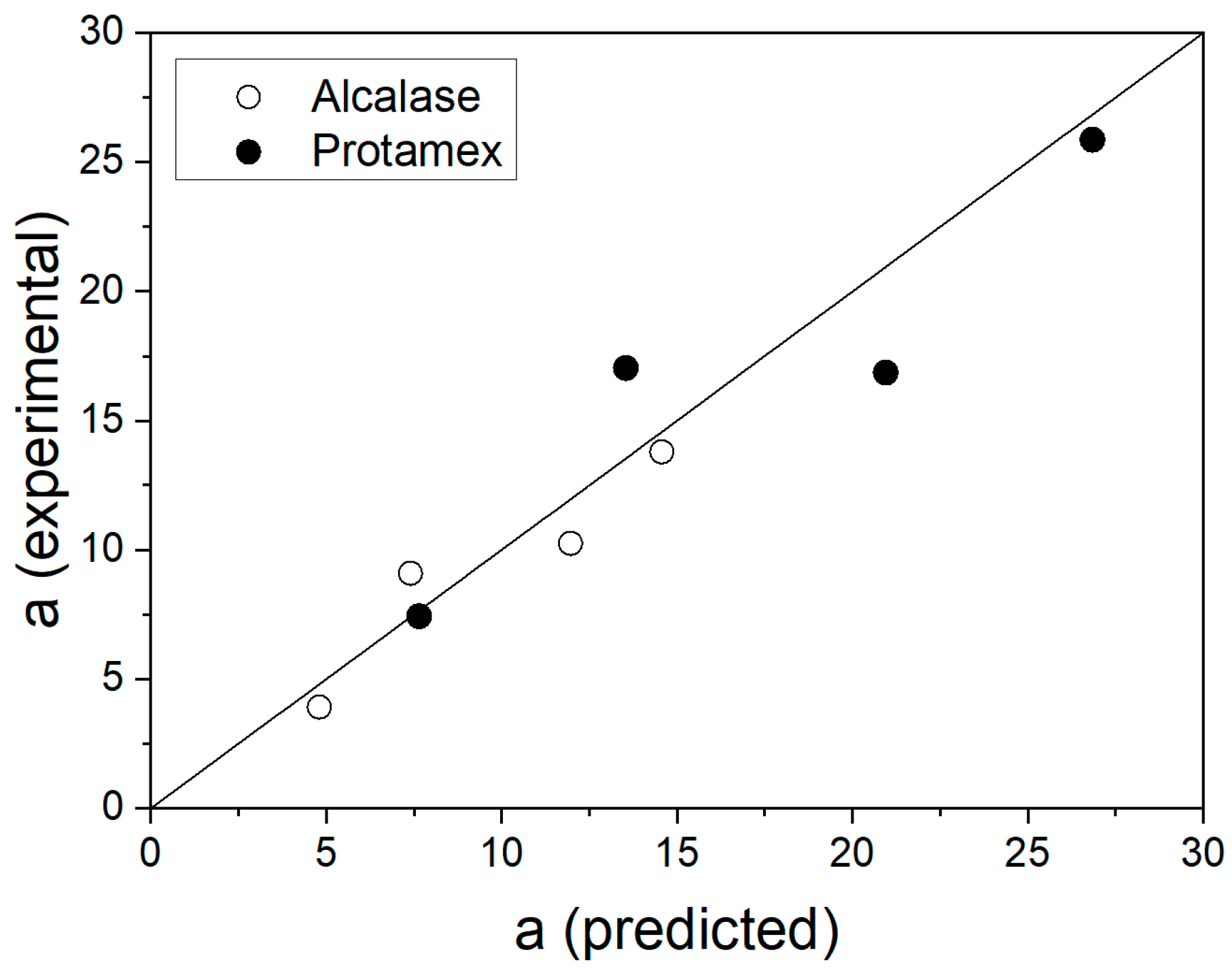
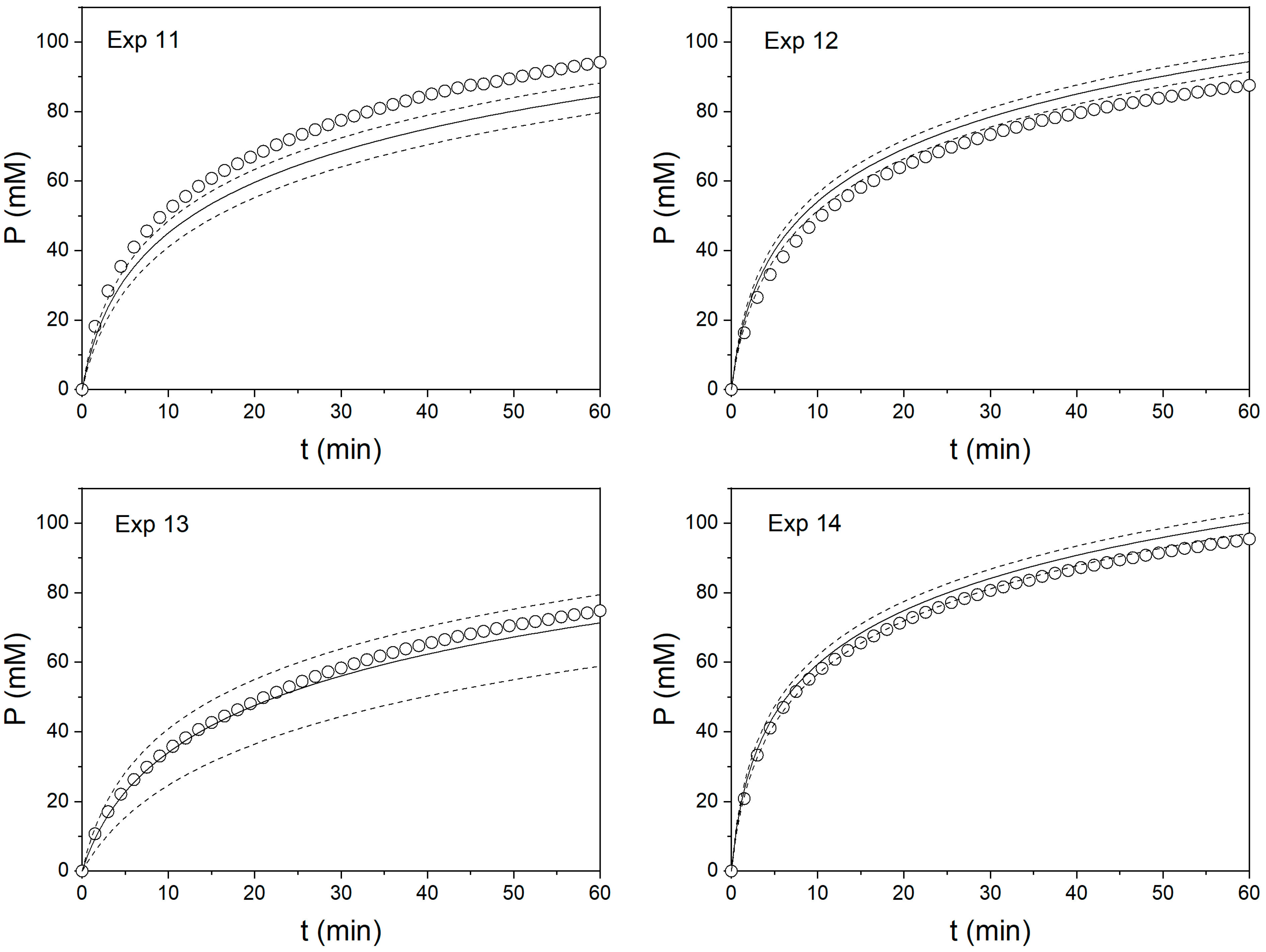
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Bu, G.H.; Luo, Y.K.; Chen, F.S.; Liu, K.L.; Zhu, T.W. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.; Bajzert, J.; Babij, K.; Szołtysik, M.; Stefaniak, T.; Willak-Janc, E.; Chrzanowska, J. Reduced IgE and IgG antigenic response to milk proteins hydrolysates obtained with the use of noncommercial serine protease from Yarrowia lipolytica. Food Chem. 2020, 302, 125350. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, H.; Sun, J.; Cheng, J.; Luo, X.; Wang, Z.; Yang, M.; Bing Tao, D.; Yue, X.; Zheng, Y. Effects of enzymatic treatments on the hydrolysis and antigenicity reduction of natural cow milk. Food Sci. Nutr. 2021, 9, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, B.; Jedrychowski, L.; Szabo, E.; Hajos, G. The reduction of cow milk proteins immunoreactivity by two-step enzymatic hydrolysis. Acta Aliment. 2005, 34, 307–315. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation, properties, and uses of enzymatic milk protein hydrolysates. Crit. Rev. Food Sci. Nutr. 2017, 57, 1119–1132. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Barking, UK, 1986. [Google Scholar]
- Apar, D.K.; Özbek, B. A Kinetic Study on Corn Gluten Hydrolysis. Chem. Eng. Technol. 2009, 32, 673–675. [Google Scholar] [CrossRef]
- Barros, R.M.; Xavier Malcata, F. A kinetic model for hydrolysis of whey proteins by cardosin A extracted from Cynara cardunculus. Food Chem. 2004, 88, 351–359. [Google Scholar] [CrossRef]
- Demirhan, E.; Apar, D.K.; Ozbek, B. A kinetic study on sesame cake protein hydrolysis by Alcalase. J. Food Sci. 2011, 76, C64–C67. [Google Scholar] [CrossRef]
- Sousa, R.; Lopes, G.P.; Tardioli, P.W.; Giordano, R.L.C.; Almeida, P.I.F.; Giordano, R.C. Kinetic model for whey protein hydrolysis by alcalase multipoint-immobilized on agarose gel particles. Braz. J. Chem. Eng. 2004, 21, 147–153. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Sousa, R.; Giordano, R.C.; Giordano, R.L.C. Kinetic model of the hydrolysis of polypeptides catalyzed by Alcalase® immobilized on 10% glyoxyl-agarose. Enzym. Microb. Technol. 2005, 36, 555–564. [Google Scholar] [CrossRef]
- Trusek-Holownia, A. Production of protein hydrolysates in an enzymatic membrane reactor. Biochem. Eng. J. 2008, 39, 221–229. [Google Scholar] [CrossRef]
- O’Meara, G.M.; Munro, P.A. Kinetics of the hydrolysis of lean meat protein by alcalase: Derivation of two alternative rate equations and their fit to experimental data. Biotechnol. Bioeng. 1985, 27, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.; Pinto, M.; Almonacid, S. Identification of the key mechanisms involved in the hydrolysis of fish protein by Alcalase. Process Biochem. 2014, 49, 258–264. [Google Scholar] [CrossRef]
- Márquez-Moreno, M.C.; Fernández-Cuadrado, V. Enzymic hydrolysis of vegetable proteins: Mechanism and kinetics. Process Biochem. 1993, 28, 481–490. [Google Scholar] [CrossRef]
- Apar, D.K.; Ozbek, B. Hydrolysis and solubilization of corn gluten by Neutrase. J. Chem. Technol. Biotechnol. 2007, 82, 1107–1114. [Google Scholar] [CrossRef]
- Apar, D.K.; Ozbek, B. Corn gluten hydrolysis by Alcalase: Effects of process parameters on hydrolysis solubilization and enzyme inactivation. Chem. Biochem. Eng. Q. 2008, 22, 203–212. [Google Scholar]
- Demirhan, E.; Apar, D.K.; Özbek, B. Sesame cake protein hydrolysis by alcalase: Effects of process parameters on hydrolysis, solubilization, and enzyme inactivation. Korean J. Chem. Eng. 2011, 28, 195–202. [Google Scholar] [CrossRef]
- González-Tello, P.; Camacho, F.; Jurado, E.; Páez, M.P.; Guadix, E.M. Enzymatic hydrolysis of whey proteins: I. Kinetic models. Biotechnol. Bioeng. 1994, 44, 523–528. [Google Scholar] [CrossRef]
- Márquez, M.C.; Vázquez, M.A. Modeling of enzymatic protein hydrolysis. Process Biochem. 1999, 35, 111–117. [Google Scholar] [CrossRef]
- Qi, W.; He, Z. Enzymatic hydrolysis of protein: Mechanism and kinetic model. Front. Chem. China 2006, 1, 308–314. [Google Scholar] [CrossRef]
- Martinez-Araiza, G.; Castano-Tostado, E.; Amaya-Llano, S.L.; Regalado-Gonzalez, C.; Martinez-Vera, C.; Ozimek, L. Modeling of Enzymatic Hydrolysis of Whey Proteins. Food Bioprocess Technol. 2012, 5, 2596–2601. [Google Scholar] [CrossRef]
- Ruan, C.Q.; Chi, Y.J.; Zhang, R.D. Kinetics of Hydrolysis of Egg White Protein by Pepsin. Czech J. Food Sci. 2010, 28, 355–363. [Google Scholar] [CrossRef]
- Bhaskar, N.; Benila, T.; Radha, C.; Lalitha, R.G. Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 2008, 99, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, N.; Mahendrakar, N.S. Protein hydrolysate from visceral waste proteins of Catla (Catla catla): Optimization of hydrolysis conditions for a commercial neutral protease. Bioresour. Technol. 2008, 99, 4105–4111. [Google Scholar] [CrossRef]
- Chabeaud, A.; Dutournie, P.; Guerard, F.; Vandanjon, L.; Bourseau, P. Application of response surface methodology to optimize the antioxidant activity of a saithe (Pollachius virens) hydrolysate. Mar. Biotechnol. 2009, 11, 445–455. [Google Scholar] [CrossRef]
- Liaset, B.; Nortvedt, R.; Lied, E.; Espe, M. Studies on the nitrogen recovery in enzymic hydrolysis of Atlantic salmon (Salmo salar, L.) frames by Protamex™ protease. Process Biochem. 2002, 37, 1263–1269. [Google Scholar] [CrossRef]
- Ovissipour, M.; Kenari, A.A.; Motamedzadegan, A.; Nazari, R.M. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food Bioprocess Technol. 2012, 5, 696–705. [Google Scholar] [CrossRef]
- Valencia, P.; Espinoza, K.; Ceballos, A.; Pinto, M.; Almonacid, S. Novel modeling methodology for the characterization of enzymatic hydrolysis of proteins. Process Biochem. 2015, 50, 589–597. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Valencia, P.L.; Solis, T.; Rojas, P.; Ibañez, F.; Astudillo-Castro, C.; Pinto, M.; Almonacid, S. Proteolytic susceptibility of food byproduct proteins: An evaluation by means of a quantitative index. Process Biochem. 2019, 77, 63–69. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]

| Variable | Levels | ||||
|---|---|---|---|---|---|
| −1.4 | −1.0 | 0 | +1.0 | +1.4 | |
| E (mUA) | 19 | 50 | 125 | 200 | 231 |
| T (°C) | 48 | 50 | 55 | 60 | 62 |
| Exp | Variables | Alcalase | Neutrase | Protamex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 (S) | x2 (E) | x3 (T) | a ± se (mM/min) | b ± se (mM−1) | R2 | a ± se (mM/min) | b ± se (mM−1) | R2 | a ± se (mM/min) | b ± se (mM−1) | R2 | |
| 1 | −1.4 | 0.0 | −1.4 | 4.06 ± 0.015 | 0.0494 ± 1.36 × 10−4 | 0.9940 | 25.6 ± 0.137 | 0.135 ± 2.02 × 10−4 | 0.9967 | 9.15 ± 0.030 | 0.0631 ± 9.98 × 10−5 | 0.9966 |
| 2 | −1.0 | 1.0 | −1.0 | 7.56 ± 0.027 | 0.0432 ± 9.01 × 10−5 | 0.9955 | 30.2 ± 0.196 | 0.104 ± 1.93 × 10−4 | 0.9923 | 16.5± 0.042 | 0.0509 ± 5.38 × 10−5 | 0.9983 |
| 3 | −1.0 | −1.0 | −1.0 | 2.89 ± 0.009 | 0.0498 ± 1.34 × 10−4 | 0.9955 | 8.76 ± 0.011 | 0.105 ± 5.28 × 10−5 | 0.9996 | 4.36 ± 0.010 | 0.0542 ± 8.37 × 10−5 | 0.9980 |
| 4 | 0.0 | 1.4 | 0.0 | 15.2 ± 0.038 | 0.0356 ± 4.39 × 10−5 | 0.9980 | 42.7 ± 0.122 | 0.080 ± 6.38 × 10−5 | 0.9985 | 26.6 ± 0.050 | 0.0409 ± 2.99 × 10−5 | 0.9991 |
| 5 | 0.0 | 0.0 | 0.0 | 9.46 ± 0.042 | 0.0359 ± 9.36 × 10−5 | 0.9928 | 26.3 ± 0.048 | 0.082 ± 4.68 × 10−5 | 0.9993 | 16.1 ± 0.030 | 0.0407 ± 3.44 × 10−5 | 0.9990 |
| 6 | 0.0 | −1.4 | 0.0 | 2.15 ± 0.003 | 0.0508 ± 8.14 × 10−5 | 0.9987 | 4.96 ± 0.007 | 0.082 ± 6.66 × 10−5 | 0.9993 | 2.93 ± 0.006 | 0.0498 ± 8.65 × 10−5 | 0.9981 |
| 7 | 1.0 | 1.0 | 1.0 | 21.7 ± 0.042 | 0.0361 ± 2.96 × 10−5 | 0.9990 | 86.5 ± 0.593 | 0.078 ± 1.28 × 10−4 | 0.9933 | 40.7 ± 0.126 | 0.0386 ± 4.12 × 10−5 | 0.9979 |
| 8 | 1.0 | −1.0 | 1.0 | 5.76 ± 0.015 | 0.0353 ± 6.53 × 10−5 | 0.9974 | 22.0 ± 0.124 | 0.085 ± 1.58 × 10−4 | 0.9934 | 9.44 ± 0.014 | 0.0392 ± 3.19 × 10−5 | 0.9993 |
| 9 | 1.4 | 0.0 | 1.4 | 17.8 ± 0.045 | 0.0343 ± 4.01 × 10−5 | 0.9981 | 105.8 ± 1.08 | 0.090 ± 2.04 × 10−4 | 0.9866 | 30.2 ± 0.113 | 0.0411 ± 5.69 × 10−5 | 0.9967 |
| 10 | 0.0 | 0.0 | 0.0 | 10.4 ± 0.035 | 0.0401 ± 7.31 × 10−5 | 0.9962 | 37.7 ± 0.091 | 0.084 ± 5.79 × 10−5 | 0.9989 | 16.5 ± 0.031 | 0.0399 ± 3.38 × 10−5 | 0.9990 |
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 9.694 | 0.350 | 27.68 | 1.47 × 10−7 | |
| β1 | 4.563 | 0.392 | 11.65 | 2.41 × 10−5 | |
| β2 | 4.880 | 0.392 | 12.46 | 1.63 × 10−5 | |
| β12 | 2.820 | 0.554 | 5.093 | 0.00223 | |
| R2 = 0.9814 | R2 aj = 0.9721 | ||||
| Analysis of variance for kinetic constant a | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 4 | 388.8616 | 97.2154 | 66.04 | 0.000162 |
| Error | 5 | 7.3599 | 1.47198 | ||
| Total | 9 | 396.2215 | |||
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 0.04103 | 0.00109 | 37.75 | 2.38 × 10−9 | |
| β1 | −0.00537 | 0.00122 | 4.42 | 0.00310 | |
| β2 | −0.00344 | 0.00122 | 2.83 | 0.0253 | |
| R2 = 0.7972 | R2 aj = 0.7393 | ||||
| Analysis of variance kinetic constant b | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 3 | 0.0003251 | 0.000108 | 7.864 | 0.01679 |
| Error | 6 | 0.0000827 | 0.000014 | ||
| Total | 9 | 0.0004078 | |||
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 25.07 | 6.53 | 3.84 | 0.00855 | |
| β1 | 22.86 | 4.98 | 4.59 | 0.00375 | |
| β2 | 17.41 | 4.98 | 3.49 | 0.01291 | |
| β11 | 17.49 | 5.96 | 2.94 | 0.02610 | |
| R2 = 0.8746 | R2 aj = 0.8119 | ||||
| Analysis of variance for kinetic constant a | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 4 | 8322.2 | 2080.55 | 8.72 | 0.0177 |
| Error | 5 | 1193.2 | 238.63 | ||
| Total | 9 | 9515.4 | |||
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 0.08091 | 0.00188 | 42.98 | 9.64 × 10−10 | |
| β1 | −0.01352 | 0.00144 | 9.40 | 3.21 × 10−5 | |
| β11 | 0.01470 | 0.00172 | 8.56 | 5.92 × 10−5 | |
| R2 = 0.9585 | R2 aj = 0.9466 | ||||
| Analysis of variance kinetic constant b | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 3 | 0.002673 | 0.000891 | 46.17 | 0.000154 |
| Error | 6 | 0.000116 | 0.000019 | ||
| Total | 9 | 0.002788 | |||
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 17.25 | 0.818 | 21.09 | 7.41 × 10−7 | |
| β1 | 7.40 | 0.914 | 8.09 | 0.000191 | |
| β2 | 9.60 | 0.914 | 10.50 | 4.38 × 10−5 | |
| β12 | 4.79 | 1.293 | 3.71 | 0.0100 | |
| R2 = 0.9693 | R2 aj = 0.9540 | ||||
| Analysis of variance kinetic constant a | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 4 | 1267.3 | 316.83 | 39.47 | 0.000565 |
| Error | 5 | 40.1 | 8.03 | ||
| Total | 9 | 1307.4 | |||
| Regression analysis for kinetic constant | |||||
| Predictor | Coefficient | se | tcalc | P | |
| β0 | 0.0423 | 0.00157 | 27.01 | 2.44 × 10−8 | |
| β1 | −0.00731 | 0.00120 | 6.10 | 0.000489 | |
| β11 | 0.00436 | 0.00143 | 3.05 | 0.0186 | |
| R2 = 0.8693 | R2 aj = 0.8320 | ||||
| Analysis of variance kinetic constant b | |||||
| Source of variation | Degrees of freedom | Sum of squares | Mean square | F | P |
| Regression | 3 | 0.000534 | 0.000178 | 13.30 | 0.004637 |
| Error | 6 | 0.000080 | 0.000013 | ||
| Total | 9 | 0.000614 | |||
| Exp | Variables | Alcalase | Protamex | |||||
|---|---|---|---|---|---|---|---|---|
| x1 (T) | x2 (E) | a ± se | a ± se | |||||
| Predicted | Experimental | Error (%) | Predicted | Experimental | Error (%) | |||
| 11 | −0.5 | 0 | 7.41 ± 0.44 | 9.10 ± 0.02 | 18.6 | 13.6 ± 2.51 | 17.0 ± 0.01 | 20.4 |
| 12 | 0.5 | 0 | 12.0 ± 0.44 | 10.2 ± 0.01 | 16.8 | 21.0 ± 2.51 | 16.9 ± 0.03 | 24.2 |
| 13 | 0 | −1 | 4.81 ± 0.58 | 3.92 ± 0.01 | 22.8 | 7.65 ± 3.29 | 7.44 ± 0.01 | 2.9 |
| 14 | 0 | 1 | 14.6 ± 0.58 | 13.8 ± 0.02 | 5.7 | 26.8 ± 3.29 | 25.9 ± 0.03 | 3.8 |
| Exp | Variables | DH | ||||||
|---|---|---|---|---|---|---|---|---|
| x1 (T) | x2 (E) | Alcalase | Protamex | |||||
| Predicted | Experimental | Error (%) | Predicted | Experimental | Error (%) | |||
| 11 | −0.5 | 0 | 21.8 | 25.7 | 15.3 | 25.5 | 29.6 | 14.0 |
| 12 | 0.5 | 0 | 25.2 | 24.8 | 1.5 | 28.5 | 27.5 | 3.8 |
| 13 | 0 | −1 | 18.8 | 19.6 | 3.9 | 21.6 | 23.4 | 7.9 |
| 14 | 0 | 1 | 26.6 | 27.2 | 2.2 | 30.3 | 30.1 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, P.; Espinoza, K.; Astudillo-Castro, C.; Salazar, F. Modeling Tool for Studying the Influence of Operating Conditions on the Enzymatic Hydrolysis of Milk Proteins. Foods 2022, 11, 4080. https://doi.org/10.3390/foods11244080
Valencia P, Espinoza K, Astudillo-Castro C, Salazar F. Modeling Tool for Studying the Influence of Operating Conditions on the Enzymatic Hydrolysis of Milk Proteins. Foods. 2022; 11(24):4080. https://doi.org/10.3390/foods11244080
Chicago/Turabian StyleValencia, Pedro, Karen Espinoza, Carolina Astudillo-Castro, and Fernando Salazar. 2022. "Modeling Tool for Studying the Influence of Operating Conditions on the Enzymatic Hydrolysis of Milk Proteins" Foods 11, no. 24: 4080. https://doi.org/10.3390/foods11244080
APA StyleValencia, P., Espinoza, K., Astudillo-Castro, C., & Salazar, F. (2022). Modeling Tool for Studying the Influence of Operating Conditions on the Enzymatic Hydrolysis of Milk Proteins. Foods, 11(24), 4080. https://doi.org/10.3390/foods11244080








