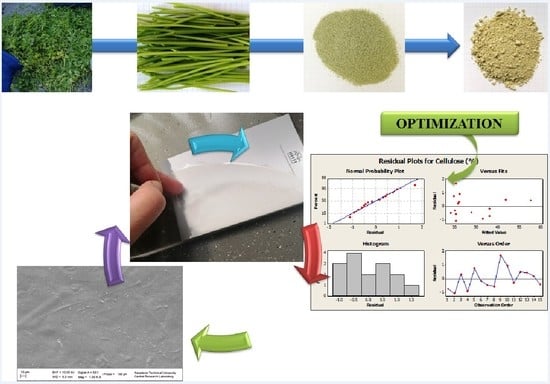
Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
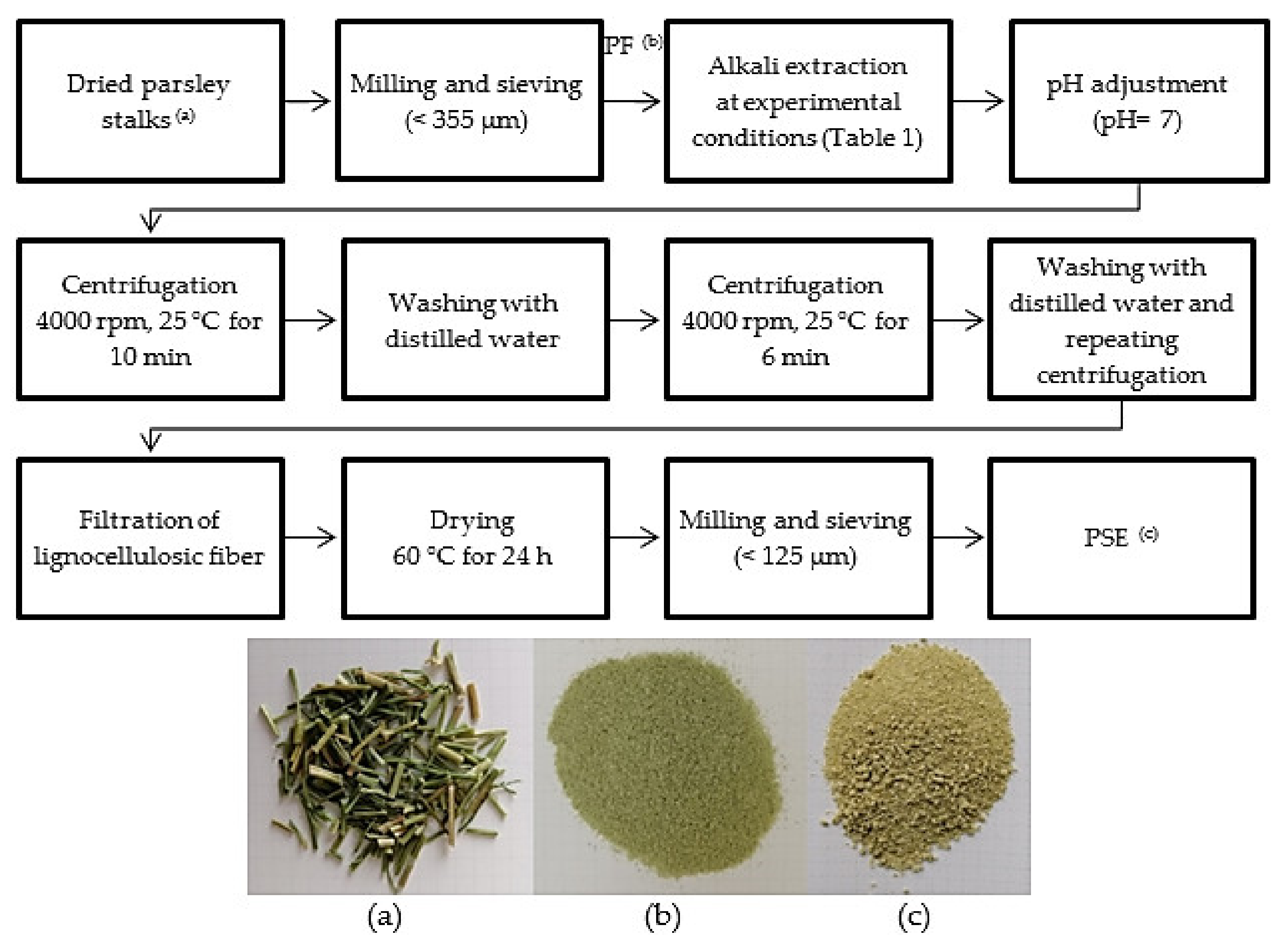
2.2. Production of Alkali Treated Parsley Stalks Fiber Extract (PSE) According to Response Surface Design
2.3. Physicochemical Properties of PF and PSE
2.4. Production of Biodegradable Films with PF and PSE
2.5. Characterization of the GA–NA Composite Films
2.5.1. The Water Vapor Permeability (WVP)
2.5.2. Optical Properties of the Films
2.5.3. Color Measurement
2.5.4. Thermogravimetric Analysis (TGA)
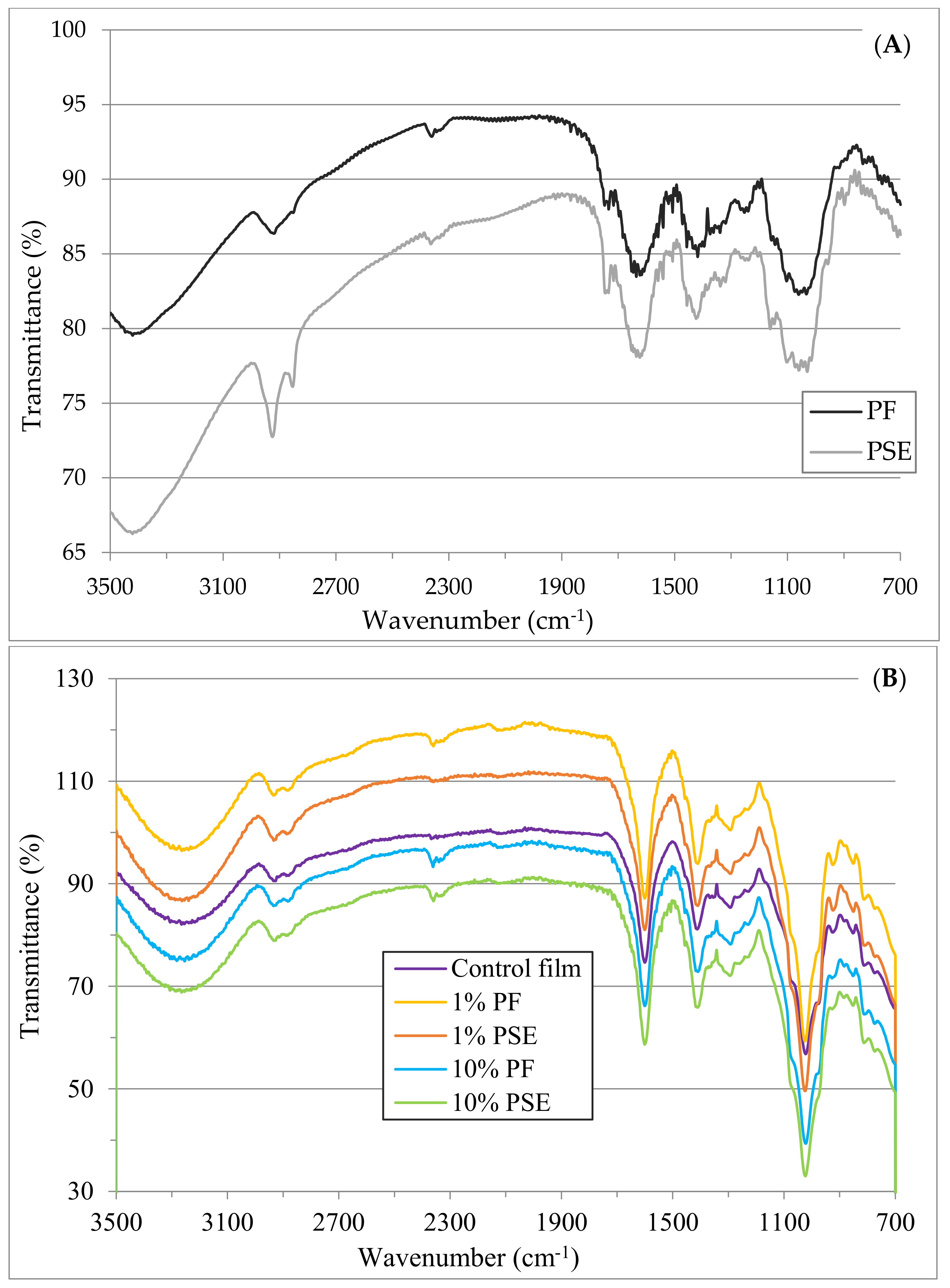
2.5.5. Fourier Transform Infrared (FTIR)
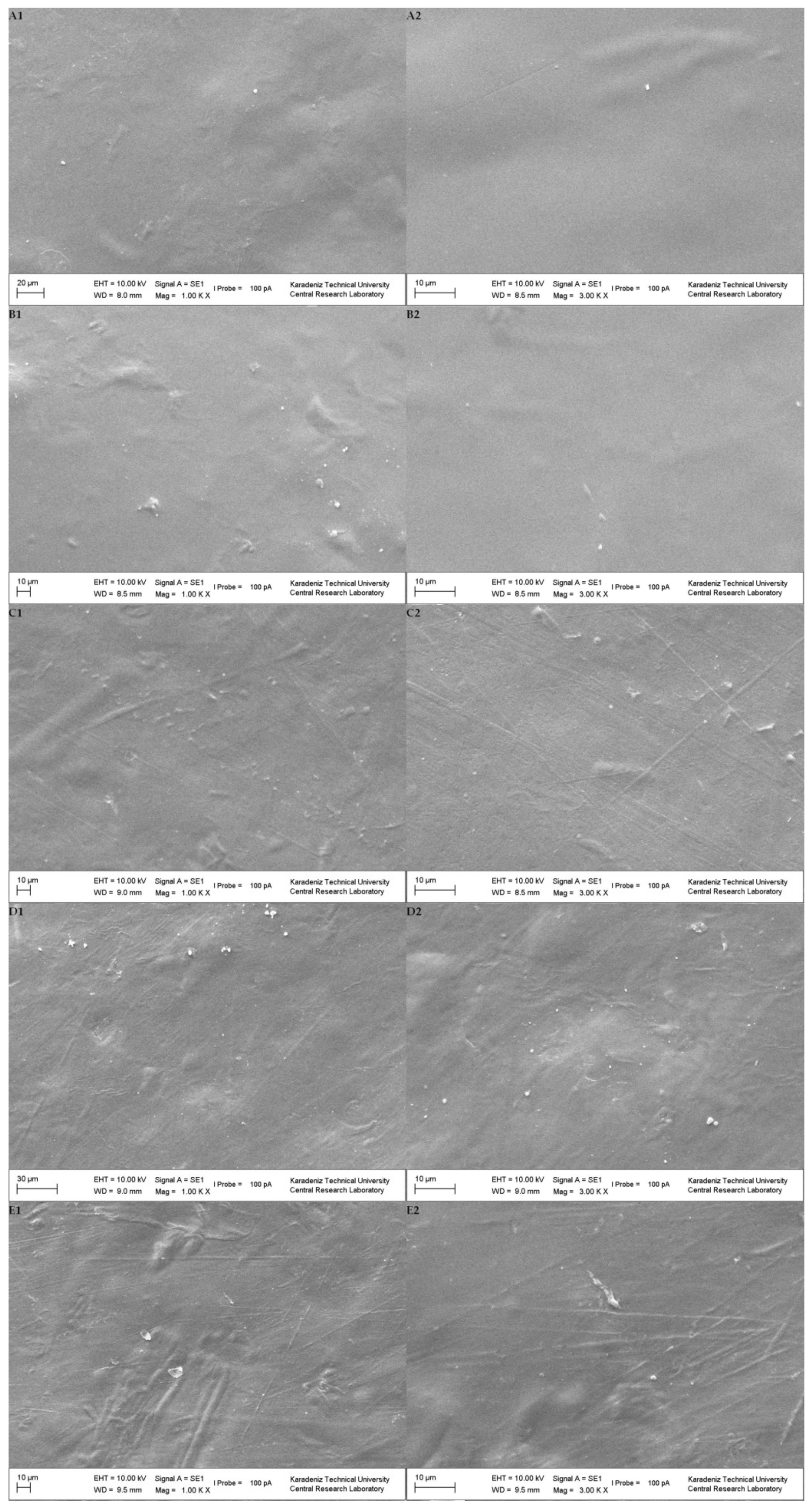
2.5.6. SEM Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of PF and Evaluation of the Experimental Design
3.2. Characterization of Composite GA–NA Films with PF and PSE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.Y.; Law, M.C.; Chan, Y.S. The potentials of corn waste lignocellulosic fibre as an improved reinforced bioplastic composites. J. Polym. Environ. 2021, 29, 363–381. [Google Scholar] [CrossRef]
- Kuila, A.; Sharma, V. (Eds.) Lignocellulosic Biomass Production and Industrial Applications; Scrivener Publishing LLC.: Beverly, MA, USA, 2017; pp. 5–40. [Google Scholar]
- Bian, H.; Gao, Y.; Luo, J.; Jiao, L.; Wu, W.; Fang, G.; Dai, H. Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: From agricultural waste to cellulose nanomaterials. Waste Manag. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Lim, C.J.; Arumugam, M.; Lim, C.K.; Ee, G.C.L. Mercerizing extraction and physicochemical characterizations of lignocellulosic fiber from the leaf waste of Mikania micrantha Kunth ex HBK. J. Nat. Fibers 2020, 17, 726–737. [Google Scholar]
- Reddy, K.O.; Ashok, B.; Reddy, K.R.N.; Feng, Y.E.; Zhang, J.; Rajulu, A.V. Extraction and characterization of novel lignocellulosic fibers from Thespesia lampas plant. Int. J. Polym. Anal. Charact. 2014, 19, 48–61. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, R.C. Recent advances in alkaline pretreatment of lignocellulosic biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–459. [Google Scholar]
- Al-Oqla, F.M.; Thakur, V.K. Toward chemically treated low-cost lignocellulosic parsley waste/polypropylene bio-composites for resourceful sustainable bio-products. Int. J. Environ. Sci. Technol. 2022, 19, 6681–6690. [Google Scholar] [CrossRef]
- Sidana, A.; Yadav, S.K. Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Sogut, E.; Cakmak, H. Using of lignocellulosic fiber extracted from artichoke outer petal leaves as filler in potato starch-based composite films. Waste Biomass Valoriz. 2022, 13, 4861–4868. [Google Scholar] [CrossRef]
- Manna, S.; Saha, P.; Chowdhury, S.; Thomas, S.; Sharma, V. Alkali treatment to improve physical, mechanical and chemical properties of lignocellulosic natural fibers for use in various applications. In Lignocellulosic Biomass Production and Industrial Applications; Kuila, A., Sharma, V., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2017; pp. 47–63. [Google Scholar]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. B Eng. 2018, 145, 215–225. [Google Scholar]
- Bartos, A.; Anggono, J.; Farkas, Á.E.; Kun, D.; Soetaredjo, F.E.; Móczó, J.; Antoni; Purwaningsih, H.; Pukánszky, B. Alkali treatment of lignocellulosic fibers extracted from sugarcane bagasse: Composition, structure, properties. Polym. Test. 2020, 88, 106549. [Google Scholar] [CrossRef]
- Ponce, J.; da Silva Andrade, J.G.; dos Santos, L.N.; Bulla, M.K.; Barros, B.C.B.; Favaro, S.L.; Hioka, N.; Caetano, W.; Batistela, V.R. Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes. Carbohydr. Polym. Technol. Appl. 2021, 2, 100061. [Google Scholar]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [PubMed]
- Shanmugasundaram, N.; Rajendran, I.; Ramkumar, T. Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 2018, 195, 566–575. [Google Scholar]
- Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Hajiali, H.; Canale, C.; Scarpellini, A.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Mele, E. Low-cost and effective fabrication of biocompatible nanofibers from silk and cellulose-rich materials. ACS Biomater. Sci. Eng. 2016, 2, 526–534. [Google Scholar] [CrossRef]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct transformation of edible vegetable waste into bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar]
- Boccalon, E.; Gorrasi, G. Functional bioplastics from food residual: Potentiality and safety issues. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3177–3204. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Mirzaei, H.; Hosseini, S.M.H.; Khalesi, M. Gum arabic improves the mechanical properties of wild almond protein film. Carbohydr. Polym. 2019, 222, 114994. [Google Scholar] [PubMed]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef] [PubMed]
- Abdin, M.; El-Beltagy, A.E.; El-Sayed, M.E.; Naeem, M.A. Production and characterization of sodium alginate/Gum Arabic based films enriched with Syzygium cumini seeds extracts for food application. J. Polym. Environ. 2022, 30, 1615–1626. [Google Scholar]
- Xu, T.; Gao, C.; Yang, Y.; Shen, X.; Huang, M.; Liu, S.; Tang, X. Retention and release properties of cinnamon essential oil in antimicrobial films based on chitosan and gum arabic. Food Hydrocoll. 2018, 84, 84–92. [Google Scholar] [CrossRef]
- Amalraj, A.; Raj, K.K.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, characterization, and antimicrobial activity of chitosan/gum arabic/polyethylene glycol composite films incorporated with black pepper essential oil and ginger essential oil as potential packaging and wound dressing materials. Adv. Compos. Hybrid Mater. 2020, 3, 485–497. [Google Scholar] [CrossRef]
- Sogut, E.; Cakmak, H. Utilization of carrot (Daucus carota L.) fiber as a filler for chitosan based films. Food Hydrocoll. 2020, 106, 105861. [Google Scholar]
- AOAC International. Official Method 940.26; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Ayeni, A.O.; Adeeyo, O.A.; Oresegun, O.M.; Oladimeji, T.E. Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am. J. Eng. Res. 2015, 4, 14–19. [Google Scholar]
- American Society for Testing and Materials. ASTM E96/E96M-16: Standard Test Methods for Water Vapor Transmission of Materials; American Society for Testing & Materials: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef]
- Cakmak, H.; Unal, F. Reinforcement of Na-alginate based films with carrot juice processing wastes. Hittite J. Sci. Eng. 2020, 7, 199–204. [Google Scholar] [CrossRef]
- Khorrami, N.K.; Radi, M.; Amiri, S.; McClements, D.J. Fabrication and characterization of alginate-based films functionalized with nanostructured lipid carriers. Int. J. Biol. Macromol. 2021, 182, 373–384. [Google Scholar]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Bindhu, B.; Reena, V.L. A polymer blend from gum Arabic and sodium alginate-preparation and characterization. J. Polym. Res. 2020, 27, 154. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar]
- Soazo, M.; Báez, G.; Barboza, A.; Busti, P.A.; Rubiolo, A.; Verdini, R.; Delorenzi, N.J. Heat treatment of calcium alginate films obtained by ultrasonic atomizing: Physicochemical characterization. Food Hydrocoll. 2015, 51, 193–199. [Google Scholar] [CrossRef]

| Run Order | Point Type 1 | NaOH Concentration (%, w/v) | Sample/Alkali (S/A) (w/v) | Temperature (°C) |
|---|---|---|---|---|
| 1 | 2 | 6 | 0.020 | 100 |
| 2 | 0 | 6 | 0.035 | 70 |
| 3 | 2 | 10 | 0.020 | 70 |
| 4 | 2 | 10 | 0.050 | 70 |
| 5 | 2 | 6 | 0.050 | 40 |
| 6 | 2 | 2 | 0.035 | 100 |
| 7 | 2 | 6 | 0.020 | 40 |
| 8 | 0 | 6 | 0.035 | 70 |
| 9 | 0 | 6 | 0.035 | 70 |
| 10 | 2 | 2 | 0.020 | 70 |
| 11 | 2 | 2 | 0.050 | 70 |
| 12 | 2 | 6 | 0.050 | 100 |
| 13 | 2 | 10 | 0.035 | 100 |
| 14 | 2 | 10 | 0.035 | 40 |
| 15 | 2 | 2 | 0.035 | 40 |
| Run Order | NaOH (% w/v) | S/A (w/v) | Temperature (°C) | Fiber Yield (%) | Cellulose (%) | Cellulose /Hemicellulose | Cellulose /Lignin | Lignin (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0.020 | 100 | 25.81 | 35.34 | 0.8782 | 1.6139 | 21.90 |
| 2 | 6 | 0.035 | 70 | 30.65 | 19.62 | 0.3382 | 1.3244 | 14.81 |
| 3 | 10 | 0.020 | 70 | 32.59 | 22.35 | 0.3608 | 2.6827 | 8.33 |
| 4 | 10 | 0.050 | 70 | 29.50 | 32.20 | 0.5705 | 3.3049 | 9.74 |
| 5 | 6 | 0.050 | 40 | 46.74 | 20.25 | 0.3015 | 2.5171 | 8.05 |
| 6 | 2 | 0.035 | 100 | 34.00 | 35.94 | 0.7581 | 2.8772 | 12.49 |
| 7 | 6 | 0.020 | 40 | 43.75 | 17.58 | 0.2616 | 2.3061 | 7.62 |
| 8 | 6 | 0.035 | 70 | 33.99 | 20.14 | 0.3313 | 1.6144 | 12.47 |
| 9 | 6 | 0.035 | 70 | 34.55 | 22.42 | 0.3642 | 1.7714 | 12.66 |
| 10 | 2 | 0.020 | 70 | 43.81 | 23.06 | 0.3699 | 2.9787 | 7.75 |
| 11 | 2 | 0.050 | 70 | 44.21 | 19.67 | 0.3069 | 2.1625 | 9.09 |
| 12 | 6 | 0.050 | 100 | 23.37 | 44.18 | 1.1910 | 2.8002 | 15.78 |
| 13 | 10 | 0.035 | 100 | 19.69 | 55.85 | 2.1023 | 3.6480 | 15.31 |
| 14 | 10 | 0.035 | 40 | 41.44 | 21.71 | 0.3358 | 2.9288 | 7.41 |
| 15 | 2 | 0.035 | 40 | 49.68 | 27.35 | 0.4298 | 3.8714 | 7.07 |
| Term * | Coefficient (Coded Value) | SE Coefficient | T | p |
|---|---|---|---|---|
| Constant | 34.12 | 0.75 | 34.82 | 0.000 |
| C | −6.06 | 0.70 | −10.42 | 0.000 |
| T | −9.84 | 0.70 | −16.39 | 0.000 |
| C2 | 2.74 | 1.02 | 3.36 | 0.020 |
| Model | <0.0001 | |||
| Lack of fit | 0.6463 |
| Term * | Coefficient (Coded Value) | SE Coefficient | T | p |
|---|---|---|---|---|
| Constant | 20.02 | 0.87 | 27.48 | 0.000 |
| C | 3.26 | 0.64 | 7.06 | 0.001 |
| S | 2.25 | 0.64 | 4.86 | 0.005 |
| T | 10.55 | 0.64 | 22.85 | 0.000 |
| C2 | 4.82 | 0.94 | 6.96 | 0.001 |
| T2 | 9.84 | 0.94 | 14.35 | 0.000 |
| C × S | 3.31 | 0.90 | 5.07 | 0.004 |
| C × T | 6.39 | 0.90 | 9.78 | 0.000 |
| Model | <0.0001 | |||
| Lack of fit | 0.4173 |
| Term * | Coefficient (Coded Value) | SE Coefficient | T | p |
|---|---|---|---|---|
| Constant | 0.38 | 0.07 | 3.21 | 0.024 |
| C | 0.19 | 0.07 | 2.86 | 0.035 |
| T | 0.45 | 0.07 | 6.84 | 0.001 |
| T2 | 0.40 | 0.10 | 4.22 | 0.008 |
| C × T | 0.36 | 0.10 | 3.86 | 0.012 |
| Model | <0.0001 | |||
| Lack of fit | 0.0065 |
| Sample | Film Thickness (µm) | WVP × 107 (g/kPa.h.m) | Opacity (AU.nm/mm) | Transmittance (%) | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| CF | 61 ± 22 a | 2.244 ± 0.132 a | 378 ± 104 a | 83.46 ± 2.02 d | 96.42 ± 0.24 d | −0.33 ± 0.03 f | 3.60 ± 0.30 a |
| 1% PF | 82 ± 28 a,b | 2.838 ± 0.265 b–d | 396 ± 34 a,b | 81.55 ± 2.26 d | 96.33 ± 0.75 d | −0.67 ± 0.10 e | 4.01 ± 0.79 a |
| 5% PF | 91 ± 31 b | 3.352 ± 0.281 d,e | 638 ± 112 b | 60.30 ± 2.26 c | 94.54 ± 0.99 b,c | −1.89 ± 0.42 b | 9.06 ± 2.53 c |
| 10% PF | 93 ± 30 b | 3.592 ± 0.366 e | 1559 ± 288 d | 51.32 ± 3.32 b | 92.65 ± 1.30 a | −2.44 ± 0.28 a | 15.94 ± 2.68 d |
| 1% PSE | 77 ± 27 a,b | 2.390 ± 0.184 a,b | 414 ± 40 a,b | 81.84 ± 2.11 d | 96.52 ± 0.37 d | −0.70 ± 0.11 e | 4.32 ± 0.86 a |
| 5% PSE | 87 ± 21 b | 2.743 ± 0.230 a–c | 900 ± 203 c | 58.63 ± 3.45 c | 95.33 ± 0.32 c | −1.14 ± 0.07 d | 6.84 ± 0.64 b |
| 10% PSE | 93 ± 25 b | 3.002 ± 0.136 c,d | 2269 ± 217 e | 39.09 ± 1.90 a | 94.16 ± 0.28 b | −1.62 ± 0.11 c | 9.60 ± 0.41 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakmak, H.; Dekker, M. Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films. Foods 2022, 11, 3932. https://doi.org/10.3390/foods11233932
Cakmak H, Dekker M. Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films. Foods. 2022; 11(23):3932. https://doi.org/10.3390/foods11233932
Chicago/Turabian StyleCakmak, Hulya, and Matthijs Dekker. 2022. "Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films" Foods 11, no. 23: 3932. https://doi.org/10.3390/foods11233932
APA StyleCakmak, H., & Dekker, M. (2022). Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films. Foods, 11(23), 3932. https://doi.org/10.3390/foods11233932