Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleogels
2.3. Manufacture of Meat Patties
2.4. Cooking of the Meat Patties
2.5. Methods of Analysis
2.5.1. Proximate Analysis
2.5.2. Oxidative Stability of Lipids
2.5.3. Oxidative Stability Index (OSI)
2.5.4. Fatty Acid Composition
2.5.5. Conjugated Diene Value
2.5.6. Malondialdehyde Value
2.6. Physicochemical and Sensory Properties of Meat Patties
2.6.1. Texture Analysis
2.6.2. Sensory Evaluation
2.6.3. Technological Properties
2.7. Statistical Analysis
3. Results and Discussions
3.1. Properties of Oleogels
3.2. Properties of Meat Patties
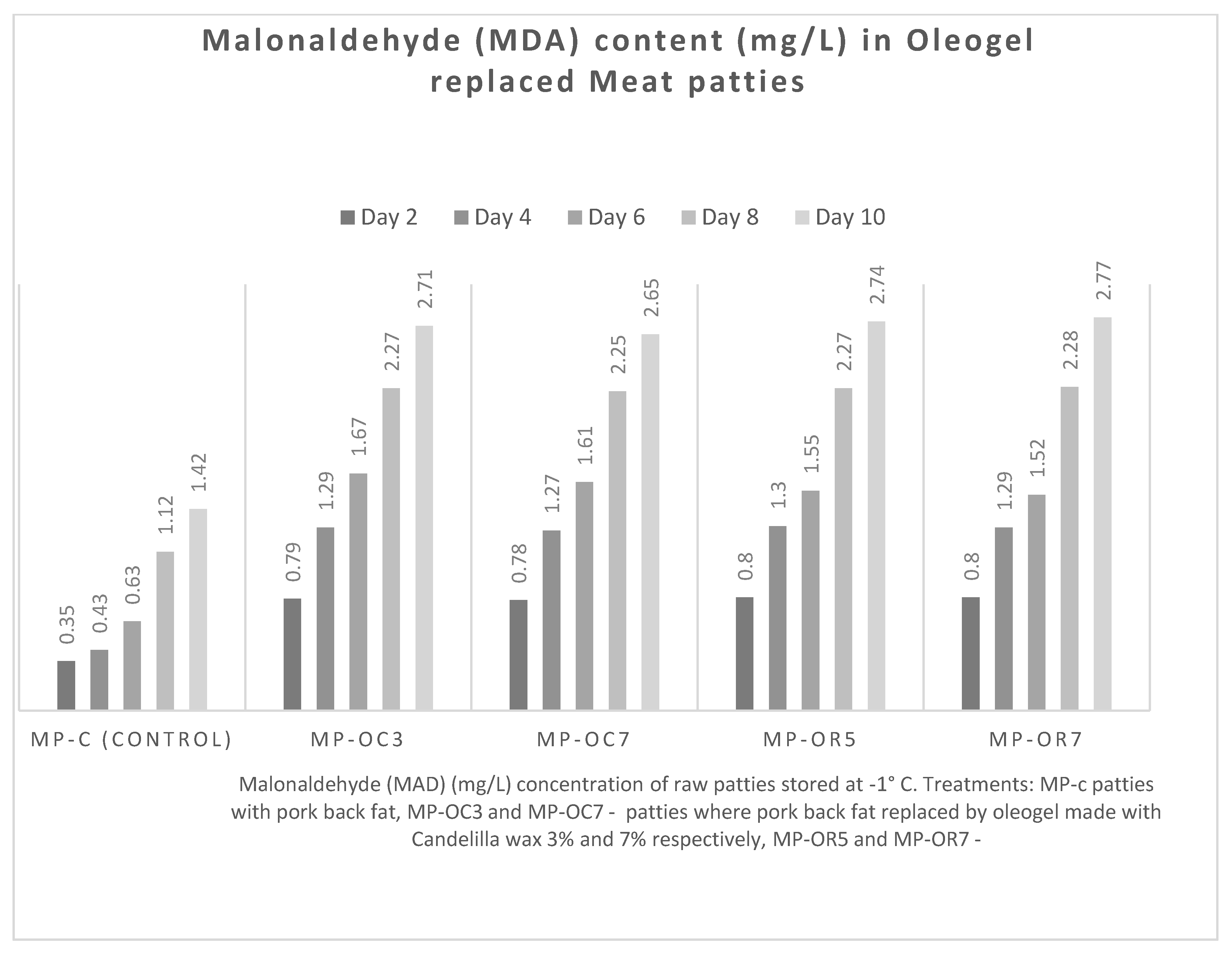
3.2.1. Malondialdehyde Value
3.2.2. Proximate Composition and Technological Properties
3.2.3. Fatty Acid Profile
3.2.4. Physicochemical and Sensory Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO—World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 4 December 2022).
- Hooper, L.; Abdelhamid, A.; Bunn, D.; Brown, T.; Summerbell, C.D.; Skeaff, C.M. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015, 8, CD0118. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of Utilizing Healthy Fats in Foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef] [PubMed]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Zetzl, A.K.; Marangoni, A.G.; Barbut, S. Mechanical properties of ethylcellulose oleogels and their potential for saturated fat reduction in frankfurters. Food Funct. 2012, 3, 327. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on foodgrade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 7659–7676. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. The Effect of Shear on the Microstructure and Oil Binding Capacity of Wax Crystal Networks. Food Biophys. 2015, 10, 403–415. [Google Scholar] [CrossRef]
- Szymańska, I.; Żbikowska, A.; Onacik-Gür, S. Candelilla wax-based oleogels versus palm oil: Evaluation of physical properties of innovative and conventional lipids using optical techniques. J. Sci. Food Agric. 2022, 102, 2309–2320. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2020, 15, 42–49. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Flöter, E. Wax based oleogels and their application in sponge cakes. Food Funct. 2022, 13, 9419. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Dobson, S.; Marangoni, A.G. Hardness, plasticity, and oil binding capacity of binary mixtures of natural waxes in olive oil. Curr. Res. Food Sci. 2022, 5, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Hwang, H.S.; Singh, M.; Winkler-Moser, J.K.; Bakota, E.L.; Liu, S.X. Preparation of Margarines from Organogels of Sunflower Wax and Vegetable Oils. J. Food Sci. 2014, 79, C1926–C1932. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Asensi, G.; García-Alonso, F.J.; Martínez, E.; Santaella, M.; Martín-Pozuelo, G.; Bravo, S.; Periago, M.J. Effect of the addition of tomato paste on the nutritional and sensory properties of mortadella. Meat Sci. 2013, 93, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; Binyamin, H.; Aserin, A. Stabilization of water-in-oil emulsions by submicrocrystalline α-form fat particles. J. Amer. Oil. Chem. Soc. 1998, 75, 1825–1831. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Van Nostrand’s Encyclopedia of Chemistry; AOAC: Rockville, MD, USA, 2005. [Google Scholar] [CrossRef]
- ISO 3960:2010; Animal and Vegetable Fats and Oils. Determination of Peroxide Value. Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2010. [CrossRef]
- AACC Approved Methods of Analysis, 11th ed.; 58-15.01 Determination of Free Fatty Acids. AACC International Approved Methods; Cereals & Grains Association: St. Paul, MN, USA, 2009. [CrossRef]
- Läubli, M.W.; Bruttel, P. A Determination of the oxidative stability of fats and oils: Comparison between the active oxygen method (AOCS Cd 12-57) and the rancimat method. J. Am. Oil Chem. Soc. 1986, 63, 792–795. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Preparation of Methyl esters of Fatty Acids. ISO: Geneva, Switzerland, 2017. [CrossRef]
- White, P. Conjugated Diene, Anisidine Value, and Carbonyl Value Analyses. In Methods to Access Quality and Stability of Oils and Fat-Containing Foods; The American Oil Chemists Society: Champaign, IL, USA; AOCS Publishing: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; pp. 302–310. [Google Scholar] [CrossRef]
- Bourne, M.C.; Kenny, J.F.; Barnard, J. computer-assisted readout of data from texture profile analysis curves. J. Texture Stud. 1978, 9, 481–494. [Google Scholar] [CrossRef]
- Kouba, M. Quality of organic animal products. Livest. Prod. Sci. 2003, 80, 33–40. [Google Scholar] [CrossRef]
- El-Magoli, S.B.; Laroia, S.; Hansen, P.T.M. Flavour and texture characteristics of low fat ground beef patties formulated with whey protein concentrate. Meat Sci. 1996, 42, 179–193. [Google Scholar] [CrossRef]
- Murphy, E.W.; Criner, P.E.; Grey, B.C. Comparison of methods for calculating retentions of nutrients in cooked foods. J. Agric. Food Chem. 1975, 23, 1153–1157. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Ferrer-González, B.M.; García-Martínez, I.; Totosaus, A. Textural properties, sensory acceptance and fatty acid profile of cooked meat batters employing pumpkin seed paste or soybean oil oleogel as fat replacers. Grasas Aceites 2019, 70, e320. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed Oil in New Application: Assessment of Structure of Oleogels Based on their Physicochemical Properties and Microscopic Observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chem. Soc. 2011, 89, 639–647. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.B.; Cunha, R.L. Tailoring Properties of Mixed-Component Oleogels: Wax and Monoglyceride Interactions Towards Flaxseed Oil Structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Kodali, D.R. The utilization of rice bran wax to stabilize long chain ω-3 polyunsaturated fatty acid esters. Lipid Technol. 2009, 21, 254–256. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yılmaz, E. Preparation and Characterization of Virgin Olive Oil-Beeswax Oleogel Emulsion Products. J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Scott, J.D.; Rouzer, C.A.; Rachel, E.; Marnett, G.L.J. Malondialdehyde, a Product of Lipid Peroxidation, Is Mutagenic in Human Cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef]
- Serdaroğlu, M. Improving low fat meatball characteristics by adding whey powder. Meat Sci. 2006, 72, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Soncu, E.D.; Kolsar, N.; Çiçek, N.; Öztürk, G.S.; Lu, T.A.; Arici, Y.K. The Comparative Effect of Carrot and Lemon Fiber as a Fat Replacer on Physico-chemical, Textural, and Organoleptic Quality of Low-fat Beef Hamburger. Korean J. Food Sci. Anim. Resour. 2015, 35, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.; Barbut, S. Effects of protein level and fat/oil on emulsion stability, texture, microstructure, and color of meat batters. Meat Sci. 2009, 82, 228–233. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Hemp Oil (Control) | O-C3 | O-C7 | O-R5 | O-R7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of Storage, Days | |||||||||||||||
| D1 | D15 | D35 | D1 | D15 | D35 | D1 | D15 | D35 | D1 | D15 | D35 | D1 | D15 | D35 | |
| C16:0 | 5.60 | 5.31 | 5.35 | 5.42 | 5.29 | 5.39 | 5.46 | 5.40 | 5.37 | 5.70 | 5.42 | 5.33 | 5.66 | 5.32 | 5.31 |
| C18:0 | 2.29 | 2.13 | 2.09 | 2.14 | 2.15 | 2.10 | 2.16 | 2.14 | 2.10 | 2.29 | 2.14 | 2.11 | 2.24 | 2.12 | 2.07 |
| C18:1 | 9.77 | 9.18 | 9.27 | 9.43 | 9.15 | 9.34 | 9.49 | 9.40 | 9.30 | 9.94 | 9.25 | 9.25 | 9.69 | 9.16 | 9.17 |
| C18:2cis | 56.81 | 57.40 | 56.55 | 57.11 | 57.69 | 56.94 | 56.85 | 57.14 | 56.95 | 56.43 | 57.05 | 57.31 | 56.72 | 57.44 | 57.12 |
| C18:3a | 18.16 | 18.53 | 18.35 | 18.49 | 18.26 | 18.70 | 18.47 | 18.36 | 18.71 | 18.22 | 18.34 | 18.48 | 18.30 | 18.42 | 18.81 |
| C18:3 | 4.12 | 4.24 | 4.27 | 4.24 | 4.24 | 4.38 | 4.33 | 4.23 | 4.40 | 4.21 | 4.21 | 4.27 | 4.26 | 4.25 | 4.33 |
| C20:2 | 1.35 | 1.38 | 1.34 | 1.32 | 1.39 | 1.32 | 1.35 | 1.37 | 1.33 | 1.33 | 1.36 | 1.36 | 1.34 | 1.38 | 1.38 |
| Trans Total | 0.02 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 0.12 | 0.01 | 0.02 | 0.11 | 0.01 | 0.02 | 0.01 | 0.01 |
| SFAs | 9.22 | 8.75 | 8.96 | 8.85 | 8.78 | 8.78 | 8.91 | 8.85 | 8.74 | 9.29 | 9.16 | 8.77 | 9.13 | 9.16 | 8.67 |
| MUFAs | 10.28 | 9.65 | 9.98 | 9.91 | 9.61 | 9.82 | 9.97 | 9.89 | 9.80 | 10.47 | 9.73 | 9.73 | 10.19 | 9.64 | 9.64 |
| PUFAs | 80.47 | 81.59 | 81.45 | 81.21 | 81.61 | 81.38 | 81.08 | 81.13 | 81.44 | 80.22 | 80.99 | 81.47 | 80.65 | 81.53 | 81.67 |
| n-3 PUFA | 18.20 | 18.57 | 18.93 | 18.54 | 18.29 | 18.74 | 18.55 | 18.40 | 18.75 | 18.25 | 18.38 | 18.52 | 18.34 | 18.42 | 18.84 |
| n-6 PUFA | 62.28 | 63.02 | 62.52 | 62.67 | 63.32 | 62.64 | 62.54 | 62.74 | 62.69 | 61.97 | 62.61 | 62.95 | 62.32 | 63.07 | 62.83 |
| n-9 PUFA | 10.14 | 9.54 | 9.81 | 9.79 | 9.51 | 9.72 | 9.85 | 9.77 | 9.70 | 10.31 | 9.61 | 9.62 | 10.06 | 9.53 | 9.53 |
| Omega 6/3 | 3.422 | 3.394 | 3.303 | 3.380 | 3.462 | 3.343 | 3.371 | 3.410 | 3.343 | 3.396 | 3.406 | 3.399 | 3.398 | 3.424 | 3.335 |
| Parameter | Storage Duration, Days | Hemp Oil (Control) | O-C3 | O-C7 | O-R5 | O-R7 |
|---|---|---|---|---|---|---|
| Induction Period (hours) | - | 3.1 a | 3.65 b | 3.45 b | 3.95 c | 3.75 c |
| Peroxide Value (meq/kg) | 1 | 2.18 | 2.17 | 2.31 | 2.19 | 2.18 |
| 6 | 7.73 b | 6.92 ab | 7.10 ab | 6.30 a | 6.87 ab | |
| 12 | 10.70 b | 8.65 a | 9.30 a | 8.10 a | 8.22 a | |
| 20 | 13.51 b | 11.34 a | 12.47 a | 11.96 a | 10.24 a | |
| 33 | 16.76 b | 13.85 a | 14.10 a | 12.44 a | 13.75 a | |
| 50 | 13.72 | 12.31 | 13.80 | 13.34 | 12.78 | |
| Acidity Value (KOH/g oil) | 1 | 0.55 | 0.52 | 0.51 | 0.50 | 0.53 |
| 6 | 0.92 b | 0.64 a | 0.68 a | 0.62 a | 0.63 a | |
| 12 | 1.31 b | 0.93 a | 0.91 a | 0.84 a | 0.87 a | |
| 20 | 2.69 b | 1.70 a | 1.77 a | 1.92 a | 1.95 a | |
| 33 | 3.44 b | 2.55 a | 2.85 a | 2.96 a | 3.10 a | |
| 50 | 2.12 | 1.96 | 2.10 | 1.86 | 2.00 | |
| Free Fatty Acids Content (% oleic acid) | 1 | 0.22 | 0.20 | 0.21 | 0.22 | 0.21 |
| 6 | 0.41 | 0.35 | 0.37 | 0.34 | 0.38 | |
| 12 | 0.52 | 0.42 | 0.46 | 0.43 | 0.46 | |
| 20 | 0.74 b | 0.48 a | 0.51 a | 0.47 a | 0.55 a | |
| 33 | 1.22 b | 0.91 a | 0.98 a | 0.83 a | 0.88 a | |
| 50 | 0.98 | 0.95 | 0.94 | 0.99 | 1.00 | |
| Conjugated Diene Value | 1 | 1.33 | 1.34 | 1.30 | 1.39 | 1.15 |
| 6 | 4.55 | 5.35 | 6.96 | 5.51 | 5.42 | |
| 12 | 6.46 | 6.98 | 7.86 | 6.94 | 6.74 | |
| 20 | 8.28 | 9.42 | 9.51 | 9.24 | 9.14 | |
| 33 | 9.24 a | 11.69 b | 12.32 b | 12.62 b | 11.09 b | |
| 50 | 12.75 | 14.15 | 13.08 | 14.26 | 13.54 |
| MP-C (Control) | MP-OC3 | MP-OC7 | MP-OR5 | MP-OR7 | |
|---|---|---|---|---|---|
| Moisture (%) | |||||
| Uncooked | 62.00 | 58.70 | 60.10 | 60.10 | 60.00 |
| Cooked | 50.00 | 53.90 | 50.20 | 49.00 | 51.50 |
| Ash (%) | |||||
| Uncooked | 2.45 b | 1.93 a | 1.99 a | 1.94 a | 1.93 a |
| Cooked | 2.00 a | 2.36 b | 2.44 b | 2.28 b | 2.74 b |
| Protein (%) | |||||
| Uncooked | 18.30 | 18.20 | 17.80 | 18.10 | 18.10 |
| Cooked | 25.00 | 27.30 | 26.60 | 26.90 | 27.00 |
| Cooking loss% | 24.48 a | 38.58 c | 33.69 b | 34.16 b | 40.35 c |
| Moisture retention (%) | 35.76 b | 33.11 a | 33.29 a | 32.26 a | 30.72 a |
| Fat retention (%) | 93.27 c | 43.54 a | 62.10 b | 68.17 b | 58.01 b |
| Uncooked Samples | Cooked Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MP-C (Control) | MP-OC3 | MP-OC7 | MP-OR5 | MP-OR7 | MP-C (Control) | MP-OC3 | MP-OC7 | MP-OR5 | MP-OR7 | |
| C14:0 | 1.19 | 0.12 | 0.48 | 0.13 | 0.17 | 1.62 | 0.20 | 0.23 | 0.20 | 0.17 |
| C16:1 | 1.83 | 0.18 | 0.53 | 0.21 | 0.23 | 2.17 | 0.31 | 0.35 | 0.27 | 0.26 |
| C16:0 | 24.96 | 6.25 | 9.30 | 6.31 | 6.77 | 25.35 | 7.26 | 7.43 | 7.01 | 6.92 |
| C18:0 | 13.02 | 2.41 | 4.13 | 2.50 | 2.72 | 13.46 | 2.93 | 2.98 | 2.81 | 2.74 |
| C18:1 | 42.27 | 9.00 | 12.38 | 9.81 | 9.70 | 41.13 | 10.66 | 10.83 | 10.54 | 10.26 |
| C18:2cis | 12.52 | 55.95 | 48.28 | 55.80 | 55.25 | 11.30 | 53.50 | 52.27 | 54.29 | 54.70 |
| C18:3a | 0.78 | 18.13 | 16.24 | 18.14 | 18.09 | 0.89 | 17.28 | 16.93 | 17.68 | 17.93 |
| C18:3 g | NA | 3.77 | 3.34 | 3.63 | 3.68 | 0.04 | 3.55 | 3.45 | 3.54 | 3.63 |
| C20:2 | 0.46 | 1.29 | 1.10 | 1.21 | 1.24 | 0.43 | 1.19 | 1.14 | 1.18 | 1.21 |
| Trans Total | 0.23 | 0.03 | 0.26 | 0.06 | 0.06 | 0.58 | 0.09 | 0.10 | 0.08 | 0.07 |
| SFAs | 40.08 | 10.19 | 17.35 | 10.52 | 11.11 | 41.54 | 11.82 | 14.14 | 11.63 | 11.26 |
| MUFAs | 45.53 | 9.58 | 13.43 | 10.51 | 10.48 | 44.72 | 11.52 | 11.66 | 11.33 | 11.01 |
| PUFAs | 14.15 | 80.19 | 68.96 | 78.89 | 78.34 | 13.14 | 76.57 | 74.08 | 76.95 | 77.66 |
| n-3 PUFA | 1.05 | 19.19 | 16.24 | 18.25 | 18.18 | 1.25 | 18.33 | 16.93 | 17.96 | 18.12 |
| n-6 PUFA | 13.10 | 61.00 | 52.72 | 60.65 | 60.16 | 11.89 | 58.24 | 57.16 | 59.00 | 59.54 |
| n-9 PUFA | 43.38 | 9.34 | 12.70 | 10.23 | 10.17 | 42.02 | 11.10 | 11.19 | 10.98 | 10.65 |
| Omega 6/3 | 12.48 | 3.18 | 3.25 | 3.32 | 3.31 | 9.51 | 3.18 | 3.38 | 3.29 | 3.29 |
| Sample | ||||||
|---|---|---|---|---|---|---|
| Matrices | Texture Parameter | MP-C (Control) | MP-OC3 | MP-OC7 | MP-OR5 | MP-OR7 |
| Raw | Hardness (N) | 7.73 b | 3.97 a | 10.38 c | 4.49 a | 7.60 b |
| Cohesiveness (Ratio) | 0.35 b | 0.28 ab | 0.23 a | 0.33 b | 0.32 b | |
| Gumminess | 2.77 c | 1.09 a | 2.32 b | 1.44 b | 2.39 c | |
| Springiness | 2.66 b | 1.90 a | 1.68 a | 1.85 a | 1.89 a | |
| Cooked | Hardness N. (N) | 52.05 a | 91.26 b | 90.43 b | 167.06 c | 169.96 c |
| Cohesiveness (Ratio) | 0.56 ab | 0.52 a | 0.62 b | 0.51 a | 0.48 a | |
| Chewiness | 30.83 a | 48.40 b | 55.10 b | 83.85 c | 96.11 d | |
| Springiness | 4.69 a | 4.76 a | 5.22 b | 5.11 ab | 4.47 a | |
| Sample | ||||||
|---|---|---|---|---|---|---|
| Sensory Attribute | MP-C (Control) | MP-OC3 | MP-OC7 | MP-OR5 | MP-OR7 | |
| Odor | Overall intensity | 7.25 | 5.88 | 6.38 | 7.25 | 6.25 |
| Meaty | 7.25 c | 6.00 b | 6.25 b | 5.63 a | 4.75 a | |
| Vegetal | 1.00 a | 3.50 b | 2.50 b | 4.00 c | 4.63 c | |
| Non-typical | 1.13 a | 2.63 b | 2.38 b | 2.25 b | 3.50 b | |
| Texture | Hardness | 4.13 a | 3.88 a | 3.75 a | 5.13 b | 4.88 b |
| Springiness | 5.88 | 4.88 | 5.38 | 5.13 | 4.88 | |
| Chewiness | 5.50 | 5.38 | 4.88 | 4.88 | 5.38 | |
| Greasiness | 1.50 a | 5.75 b | 6.28 c | 4.50 b | 5.63 b | |
| Juiciness | 4.88 c | 3.75 b | 4.18 b | 2.88 a | 2.63 a | |
| Mouth coating | 4.50 | 3.50 | 4.38 | 2.88 | 3.63 | |
| Taste | Overall intensity | 6.75 | 6.25 | 6.75 | 7.00 | 6.38 |
| Meaty taste | 6.88 b | 5.38 a | 5.88 a | 4.88 a | 5.50 a | |
| Vegetal | 1.38 a | 3.88 b | 4.00 b | 5.13 c | 5.25 c | |
| Aftertaste | 2.13 | 2.88 | 3.50 | 2.63 | 4.00 | |
| Non-typical | 1.00 a | 1.80 b | 1.80 b | 1.80 b | 1.90 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidioglu, I.; Alenčikienė, G.; Dzedulionytė, M.; Zabulionė, A.; Bali, A.; Šalaševičienė, A. Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties. Foods 2022, 11, 4030. https://doi.org/10.3390/foods11244030
Hamidioglu I, Alenčikienė G, Dzedulionytė M, Zabulionė A, Bali A, Šalaševičienė A. Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties. Foods. 2022; 11(24):4030. https://doi.org/10.3390/foods11244030
Chicago/Turabian StyleHamidioglu, Irfan, Gitana Alenčikienė, Miglė Dzedulionytė, Aelita Zabulionė, Aditya Bali, and Alvija Šalaševičienė. 2022. "Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties" Foods 11, no. 24: 4030. https://doi.org/10.3390/foods11244030
APA StyleHamidioglu, I., Alenčikienė, G., Dzedulionytė, M., Zabulionė, A., Bali, A., & Šalaševičienė, A. (2022). Characterization of the Quality and Oxidative Stability of Hemp-Oil-Based Oleogels as an Animal Fat Substitute for Meat Patties. Foods, 11(24), 4030. https://doi.org/10.3390/foods11244030







