Microbiological Quality of Pig Carcasses in a Slaughterhouse under Risk-Based Inspection System
Abstract
1. Introduction
2. Materials and Methods
2.1. Adaptation of the Production Process
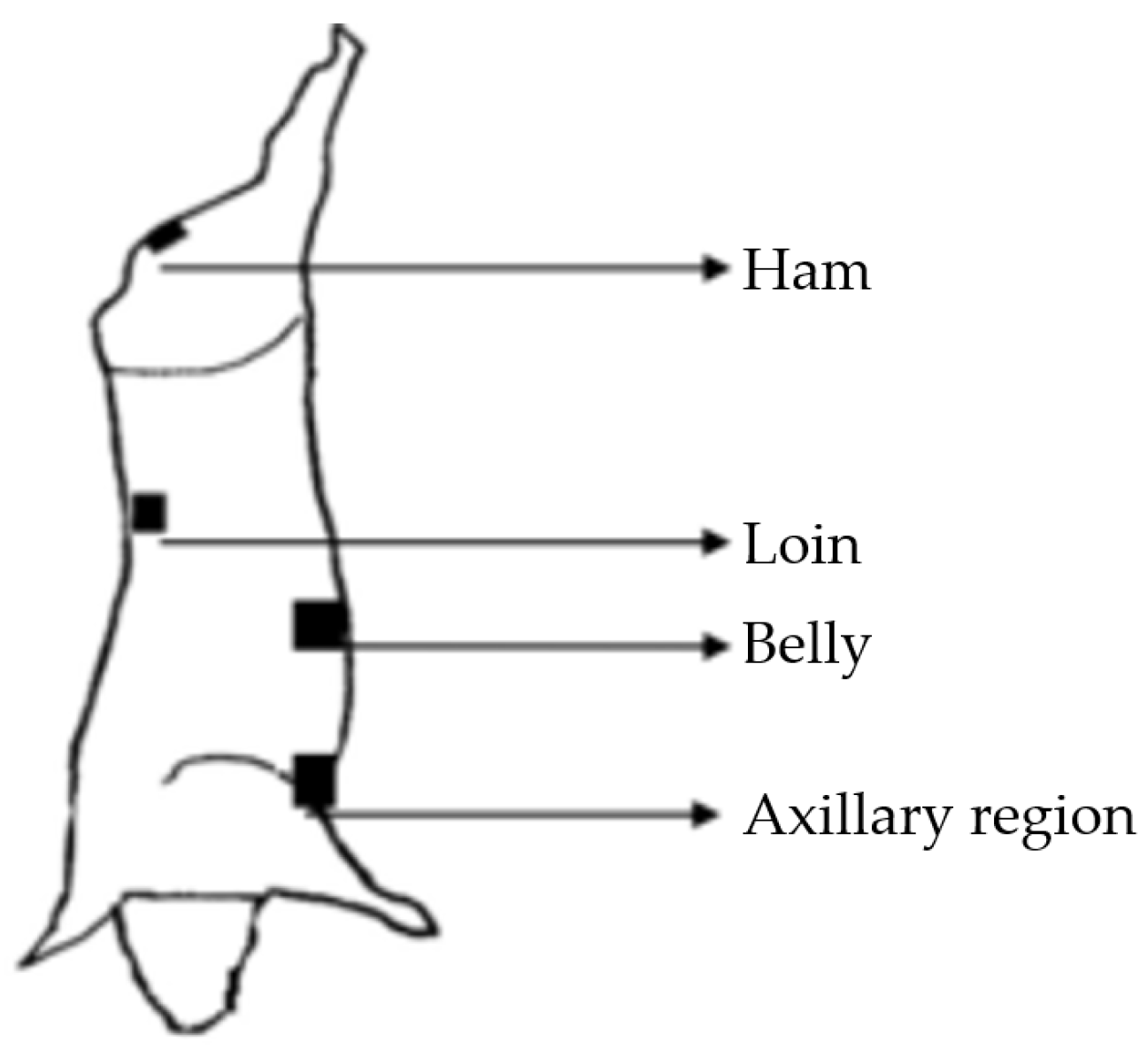
2.2. Sampling Plan
2.3. Sample Collection and Analysis Procedure
2.4. Statistical Analysis
3. Results
3.1. Frequency of Salmonella enterica Occurrence
3.2. Quantification of Enterobacteriaceae and Mesophilic Aerobic Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Risk-Based Food Inspection Manual; FAO Food and Nutrition Paper; FAO: Rome, Italy, 2008; Volume 89, p. 85. Available online: http://www.fao.org/3/i0096e/i0096e00.pdf (accessed on 30 November 2022).
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Portaria 711, de 1 de Novembro de 1995. Aprova as Normas Técnicas de Instalações e Equipamentos Para Abate e Industrialização de Suínos. Diário Oficial Da União. 1995. Available online: https://www.defesa.agricultura.sp.gov.br/legislacoes/portaria-mapa-711-de-01-11-1995,755.html (accessed on 30 November 2022). (In Portuguese)
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Decreto 9013, de 29 de março de 2017. Regulamenta a inspeção industrial e sanitária de produtos de origem animal, que disciplina a fiscalização e a inspeção industrial e sanitária de produtos de origem. Diário Oficial Da União 2017, 77. Available online: https://www.gov.br/agricultura/pt-br/assuntos/aquicultura-e-pesca/legislacao/legislacao-geral-da-pesca/decreto-no-9-013-de-29-03-2017.pdf/view (accessed on 30 November 2022). (In Portuguese).
- Herenda, D.; Chambers, P.G.; Ettriqui, A.; Seneviratna, P.; da Silva, T.J.P. Manual on Meat Inspection for Developing Countries; FAO: Rome, Italy, 1994; Available online: http://www.fao.org/3/t0756e/T0756E00.htm#TOC (accessed on 30 November 2022).
- Buncic, S.; Alban, L.; Blagojevic, B. From traditional meat inspection to development of meat safety assurance programs in pig abattoirs–The European situation. Food Control 2019, 106, 106705. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA J. 2011, 10, 198. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2351 (accessed on 30 November 2022).
- Riess, L.E.; Hoelzer, K. Implementation of Visual-Only Swine Inspection in the European Union: Challenges, Opportunities, and Lessons Learned. J. Food Prot. 2020, 83, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- EFSA/ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Biasino, W.; De Zutter, L.; Mattheus, W.; Bertrand, S.; Uyttendaele, M.; Van Damme, I. Correlation between slaughter practices and the distribution of Salmonella and hygiene indicator bacteria on pig carcasses during slaughter. Food Microbiol. 2018, 70, 192–199. [Google Scholar] [CrossRef]
- European Commission. Opinion of the Scientific Committee on Veterinary Measures Relating to Public Health on Revision of Meat Inspection Procedures. In Scientific Health Opinions; European Commission: Brussels, Belgium, 2000; p. 31. Available online: https://food.ec.europa.eu/system/files/2020-12/sci-com_scv_out30_en.pdf (accessed on 30 November 2022).
- Hamilton, D.R.; Gallas, P.; Lyall, L.; McOrist, S.; Hathaway, S.C.; Pointon, A.M.; Lester, S. Risk- based evaluation of postmortem inspection procedures for pigs in Australia. Vet. Rec. 2002, 151, 110–116. [Google Scholar] [CrossRef]
- Pointon, A.; Hamilton, D.; Kiermeier, A. Assessment of the post-mortem inspection of beef, sheep, goats and pigs in Australia: Approach and qualitative risk-based results. Food Control 2018, 90, 222–232. [Google Scholar] [CrossRef]
- de Freitas Costa, E.; Corbellini, L.G.; da Silva, A.P.S.P.; Nauta, M. A stochastic model to assess the effect of meat inspection practices on the contamination of the pig carcasses. Risk Anal. 2017, 37, 1849–1864. [Google Scholar] [CrossRef]
- Canadian, Food Inspection Agency. Post-Mortem Examination Program 2020; Canadian, Food Inspection Agency: Burnaby, BC, Canada, 2020; p. 92. Available online: https://www.inspection.gc.ca/food-safety-for-industry/industry-guidance/food-guidance/post-mortem-examination-program/eng/1578083999811/1578084000263#a11 (accessed on 30 November 2022).
- European Commission. Commission Regulation No 218, of 7 March 2014. Amending Annexes to Regulations No 853/2004 and No 854/2004 of the European Parliament and of the Council and Commission Regulation (EC) No 2074/2005. Off. J. Eur. Union 2014, 218, 95–98. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0218&from=EN (accessed on 30 November 2022).
- Kich, J.D.; Coldebella, A.; Albuquerque, E.R.; Cardoso, M.R.d.I.; Corbellini, L.G.; Costa, E.d.F. Modernization of Swine Slaughter Inspection in Swine Slaughter Establishments—Risk-Based Inspection, 182th ed.; EMBRAPA Suínos e Aves: Concórdia, Brazil, 2021; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/222393/1/final9663.pdf (accessed on 30 November 2022).
- USDA. Modernization of Swine Slaughter Inspection: Proposed rule. Fed. Regist. 2018, 83, 4780–4823. Available online: https://www.federalregister.gov/documents/2018/02/01/2018-01256/modernization-of-swine-slaughter-inspection (accessed on 30 November 2022).
- USDA. Modernization of swine slaughter inspection: Final Rule. Fed. Regist. 2019, 84, 52300–52349. Available online: https://www.federalregister.gov/documents/2019/10/01/2019-20245/modernization-of-swine-slaughter-inspection (accessed on 30 November 2022).
- Antunović, B.; Blagojević, B.; Johler, S.; Guldimann, C.; Vieira-Pinto, M.; Vågsholm, I.; Meemken, D.; Alvseike, O.; Georgiev, M.; Alban, L. Challenges and opportunities in the implementation of new meat inspection systems in Europe. Trends Food Sci. Technol. 2021, 116, 460–467. [Google Scholar] [CrossRef]
- Edwards, D.S.; Johnston, A.M.; Mead, G.C. Meat inspection: An overview of present practices andfuture trends. Vet. J. 1997, 154, 135–147. [Google Scholar] [CrossRef]
- FAO. The Future of Food Safety. 2019. Available online: http://www.fao.org/3/ca4289en/CA4289EN.pdf (accessed on 11 April 2021).
- FAO; WHO. Food safety risk analysis: A guide for national food safety authorities. Food Nutr. Pap. 2006, 87, 50. [Google Scholar]
- Blagojevic, B.; Nesbakken, T.; Alvseike, O.; Vågsholm, I.; Antic, D.; Johler, S.; Houf, K.; Meemken, D.; Nastasijevic, I.; Pinto, M.V.; et al. Drivers, opportunities, and challenges of the European risk-based meat safety assurance system. Food Control 2021, 124, 107870. [Google Scholar] [CrossRef]
- Alban, L.; L’eger, A.; Veldhuis, A.; Schaik, G.V. Modernizing the antimicrobial residue monitoring programs for pig meat in Europe—the balance between flexibility and harmonization. Food Control 2018, 86, 403–414. [Google Scholar] [CrossRef]
- Alban, L.; Petersen, J.V.; Bækbo, A.K.; Pedersen T, Ø.; Kruse, A.B.; Pacheco, G.; Larsen, M.H. Modernising meat inspection of pigs–A review of the Danish process from 2006–2020. Food Control 2021, 119, 107450. [Google Scholar] [CrossRef]
- Alban, L.; Vieira-Pinto, M.; Meemken, D.; Maurer, P.; Ghidini, S.; Santos, S.; Laguna, J.G.; Laukkanen-Ninios, R.; Alvseike, O.; Langkabel, N. Differences in code terminology and frequency of findings in meat inspection of finishing pigs in seven European countries. Food Control 2022, 132, 108394. [Google Scholar] [CrossRef]
- Corbellini, L.G.; Costa, E.d.F.; Torres, M.; Castro, S.; Kich, J.D. Avaliação Qualitativa de Riscos para Priorização de Perigos Biológicos à Saúde Pública na Cadeia de Produção de Suínos Industriais, 186th ed.; Embrapa Suínos e Aves: Concórdia, Brazil, 2017; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1082710/1/Doc186.pdf (accessed on 30 November 2022). (In Portuguese)
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa, 79 de 14 de Dezembro de 2018. Aprova os Procedimentos de Inspeção Ante e Post Mortem de Suínos Com Base em Risco; Diário Oficial Da União: Brasília, Brazil, 2018. Available online: www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/55444279/do1-2018-12-17 (accessed on 30 November 2022). (In Portuguese)
- Costa, E.d.F.; Cardoso, M.; Kich, J.D.; Corbellini, L.G. A qualitative risk assessment approach to microbial foodborne hazards in Brazilian intensive pork production: A step towards risk prioritization. Microb. Risk Anal. 2020, 15, 100105. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Rasschaert, G.; De Zutter, L.; Mattheus, W.; De Reu, K. Identification of the source for Salmonella contamination of carcasses in a large pig slaughterhouse. Pathogens 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol. Infect. 2017, 145, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, L.G.; Bianco, A.; Costa, E.d.F.; Duarte AS, R.; Albuquerque, E.R.; Kich, J.D.; Cardoso, M.; Nauta, M. Effect of slaughterhouse and day of sample on the probability of a pig carcass being Salmonella-positive according to the Enterobacteriaceae count in the largest Brazilian pork production region. Int. J. Food Microbiol. 2016, 228, 58–66. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Carter, M.E.; Donnelly, W.J.; Leonard, F.C. Microbiologia veterinária e doenças infecciosas. Artmed Ed. 2005, 4, 503. (In Portuguese) [Google Scholar]
- Franco, B.D.G.D.M.; Landgraf, M. Microbiologia dos alimentos. Atheneu Ed. 2008, 2, 196. (In Portuguese) [Google Scholar]
- USDA. Livestock and Poultry: World Markets and Trade; United States Department of Agriculture: Washington, DC, USA, 2022; p. 31. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/73666448x/n5840036r/v979w6960/livestock_poultry.pdf (accessed on 30 November 2022).
- ICMSF. International Comission on Microbiological Specifications for Foods: Microorganisms in Foods 8. In Microorganisms in Foods 7; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. In Instrução Normativa 60, de 23 de Dezembro de 2018. Estabelece o Controle Microbiológico em Carcaça de Suínos e em Carcaça e Carne de Bovinos em Abatedouros Frigoríficos; Diário Oficial Da União: Brasília, Brazil, 2018. Available online: http://www.in.gov.br/en/web/dou/-/instrucao-normativa-n-60-de-23-de-dezembro-de-2019-235332356 (accessed on 30 November 2022). (In Portuguese)
- European Commission. Commission Regulation No 2017/1495, of 23 August 2017 amending Regulation No 2073/2005 as regards Campylobacter in broiler carcases. Off. J. Eur. Union 2017, 218, 6. Available online: https://www.fsai.ie/uploadedFiles/Reg2017_1495.pdf (accessed on 30 November 2022).
- MAPA. Procedimentos Para Coleta de Amostras em Superfície de Carcaças de Suínos; MAPA: Brasília, Brazil, 2019. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/arquivos-publicacoesMAPA-dipoa/folder_inspecao_carcaca_suinos_01-07-2019-2.pdf (accessed on 30 November 2022). (In Portuguese)
- AFNOR. Certification BIO 12/32-10/11. VIDAS UP Salmonella SPT; Agence Francaise de Normalisation: Paris, France, 2011. [Google Scholar]
- AFNOR. Certification 3MTM PetrifilmTM Enterobacteriaceae Count Plate 01/06-09/97; Agence Francaise de Normalisation: Paris, France, 1997. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International. AOAC Official Method 990.12. Aerobic Plate Count in Foods, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- ISO. 6579-1; Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2017; Volume 2017. Available online: https://www.sis.se/api/document/preview/921516/ (accessed on 30 November 2022).
- ISO. 7218; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations, 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2007.
- SAS Institute Inc. System for Microsoft Windows, Release 9.4.; cd rom; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Brown, M.; Stringer, M. Microbiological Risk Assessment in Food Processing; Woodhead Publishing Limited: Sawston, UK, 2002; Available online: https://books.google.com.br/books?hl=en&lr=&id=m_cMwApNmq0C&oi=fnd&pg=PR15&dq=Principles+and+guidelines+for+the+conduct+of+microbiological+risk+management&ots=DH18ZJOVa_&sig=AdNBzD4y_ZSs7UA8IWwG-4mheA8&redir_esc=y#v=onepage&q=Principles (accessed on 30 November 2022).
- AUSTRÁLIA. Food Act 2008 (WA) Fact Sheet 20: Australian Standard Alternative Equivalent Procedure—Risk-Based Review of Routine Visual Inspection of Pigs; Department of Health: Canberra, Australia, 2019; Volume 2008. Available online: https://ww2.health.wa.gov.au/-/media/Files/Corporate/general-documents/food/PDF/Factsheet-20-Visual-Inspections-of-Pigs.pdf (accessed on 30 November 2022).
- European Commission. Commission Regulation No 219, of 7 March 2014. Specific requirements for post-mortem inspection of domestic swine. Off. J. Eur. Union 2014, 219, 99–100. [Google Scholar]
- Da Silva RO, S.; Gonçalves, G.G.; Lazzari, A.M.; Mulinari, F. Prevalence of Salmonella spp. In slaughtered swine in the Federal District of Brazil, as determined by the PCR technique. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Neitzke, D.C.; Da Roza, C.R.; Weber, F.H. Segurança dos alimentos: Contaminação por Salmonella sp. no abate de suínos. Braz. J. Food Technol. 2017, 20, 7. [Google Scholar] [CrossRef]
- Seixas, F.N.; Tochetto, R.; Ferraz, S.M. Presença de Salmonella sp. em carcaças suínas amostradas em diferentes pontos da linha de processamento. Ciência Anim. Bras. 2009, 10, 634–640. Available online: https://www.revistas.ufg.br/vet/article/view/3996/4858 (accessed on 30 November 2022). (In Portuguese).
- Teixeira, S.R. Detecção de Salmonella spp. em Amostras de Fezes, Linfonodos e Carcaças de Suínos no Momento do Abate; Universidade de São Paulo: São Paulo, Brazil, 2006; Available online: https://teses.usp.br/teses/disponiveis/10/10134/tde-14052007-133108/publico/Solange_Rosa_Teixeira.pdf (accessed on 30 November 2022). (In Portuguese)
- Zhou, Z.; Jin, X.; Zheng, H.; Li, J. The prevalence and load of Salmonella, and key risk points of Salmonella contamination in a swine slaughterhouse in Jiangsu province, China. Food Control 2018, 87, 153–160. [Google Scholar] [CrossRef]
- Paim, D.S.; Pissetti, C.; Vieira, T.R.; Werlang, G.O.; de Freitas Costa, E.; Kich, J.D.; Cardoso, M. Enumeration, antimicrobial resistance and typing of Salmonella enterica: Profile of strains carried in the intestinal contents of pigs at slaughter in Southern Brazil. Acta Sci. Vet. 2019, 47, 1–11. [Google Scholar] [CrossRef]
- Kich, J.D.; Coldebella, A.; Morés, N.; Nogueira, M.G.; Cardoso, M.; Fratamico, P.M.; Call, J.E.; Fedorka-Cray, P.; Luchansky, J.B. Prevalence, distribution, and molecular characterization of Salmonella recovered from swine finishing herds and a slaughter facility in Santa Catarina, Brazil. Int. J. Food Microbiol. 2011, 151, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Cê, E.R. Influência das Etapas do Processo de abate de Suínos na Prevalência de Patógenos e Níveis de Microrganismos Indicadores de Qualidade e Higiene; Universidade Tecnológica Federal Do Paraná: Londrina, Brazil, 2016; Available online: https://repositorio.utfpr.edu.br/jspui/bitstream/1/1665/1/LD_PPGTAL_M_C%C3%AA%2C%20Elton%20Rodrigo_2016.pdf (accessed on 30 November 2022). (In Portuguese)
- Swanenburg, M.; Urlings HA, P.; Snijders JM, A.; Keuzenkamp, D.A.; Van Knapen, F. Salmonella in slaughter pigs: Prevalence, serotypes and critical control points during slaughter in two slaughterhouses. Int. J. Food Microbiol. 2001, 70, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Fois, F.; Mazza, R.; Putzolu, M.; Delogu, M.L.; Lochi, P.G.; Pani, S.P.; Mazzette, R. Salmonella prevalence and microbiological contamination of pig carcasses and slaughterhouse environment. Ital. J. Food Saf. 2014, 3, 210–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baptista, F.M.; Dahl, J.; Nielsen, L.R. Factors influencing Salmonella carcass prevalence in Danish pig abattoirs. Prev. Vet. Med. 2010, 95, 231–238. [Google Scholar] [CrossRef]
- MAPA. Anuário dos Programas de Controle de Alimentos de Origem Animal do DIPOA; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2020; Volume 6. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/anuario-dos-programas-de-controle-de-alimentos-de-origem-animal-do-dipoa/anuario-dos-programas-de-controle-de-alimentos-de-origem-animal-volume-6.pdf (accessed on 30 November 2022). (In Portuguese)
- EFSA. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- USDA. The Nationwide Microbiological Baseline Data Collection Program: Market Hogs Survey; United States Department of Agriculture: Washington, DC, USA, 2011. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-07/Baseline_Data_Market_Hogs_2010-2011.pdf (accessed on 30 November 2022).
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Ofício Circular No 522 de 22 de Julho de 2014. Certificado Sanitário Internacional para Exportação de Carne Suína Resfriada ou Congelada Destinada ao Chile; Sistema Eletrônico de Informações: Brasília, Brazil, 2014. (In Portuguese)
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Ofício Circular No 243 de 09 de Novembro de 2020. Certificado Sanitário Para Exportação de Carne do Brasil para a República Popular da China; Sistema Eletrônico de Informações: Brasília, Brazil, 2020; p. 2. (In Portuguese)
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Ofício Circular No 97 de 18 de Maio de 2020. Certificado Sanitário Internacional para Exportação de Carne e Produtos Cárneos de Bovinos e Suínos para Hong Kong; Sistema Eletrônico de Informações: Brasília, Brazil, 2020; p. 2. (In Portuguese)
- European Commission. Commission regulation No 1441/2007, of 5 December 2007, amending Regulation No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2007, 322, 18. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF (accessed on 30 November 2022).
- Zweifel, C.; Baltzer, D.; Stephan, R. Microbiological contamination of cattle and pig carcasses at five abattoirs determined by swab sampling in accordance with EU Decision 2001/471/EC. Meat Sci. 2005, 69, 559–566. [Google Scholar] [CrossRef]
- Lindblad, M. Microbiological sampling of swine carcasses: A comparison of data obtained by swabbing with medical gauze and data collected routinely by excision at Swedish abattoirs. Intern. J. Food Microbiol. 2007, 118, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Delhalle, L.; De Sadeleer, L.; Bollaerts, K.; Farnir, F.; Saegerman, C.; Korsak, N.; Dewulf, J.; De Zutter, L.; Daube, G. Risk factors for Salmonella and hygiene indicators in the 10 largest Belgian pig slaughterhouses. J. Food Prot. 2008, 71, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Ghafir, Y.; China, B.; Dierick, K.; De Zutter, L.; Daube, G. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meats in Belgium. J. Food Prot. 2008, 71, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, D.; Duggan, S.; Fanning, S.; Cormican Barron, U.G.; Butler, F.; Duffy, G. Prevalence and numbers of Salmonella spp. and Enterobacteriaceae on pork cuts in abattoirs in the Republic of Ireland. J. Appl. Microbiol. 2008, 105, 1209–1219. Available online: https://sfamjournals.onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2672.2008.03854.x?casa_token=ulLaPvsaCKYAAAAA%3AuSsv68Jh0kLzK1vf0qOauTVAZk39Rs_pgqXepNbU-rlPK8k66blWDQdKrPz4zdXIyPgO6JWuIFsEBxXi (accessed on 30 November 2022). [CrossRef]
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Circular No 130, de 13 de Fevereiro de 2007. Exportações de Carne Suína Para os Estados-Membros da União Europeia; Diário Oficial Da União: Brasília, Brazil, 2007; pp. 1–14. (In Portuguese)
- USDA. FSIS Directive: Salmonella and Campylobacter Verification Program for Raw Meat and Poultry Products; United States Department of Agriculture: Washington, DC, USA, 2013. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-02/10250.1.pdf (accessed on 30 November 2022).
- Fasanmi, O.G.; Makinde, G.E.O.; Popoola, M.A.; Fasina, O.F.; Matere, J.; Kehinde, O.O.; Balogun, F.A.; Ogundare, S.T. Potential risk factors associated with carcass contamination in slaughterhouse operations and hygiene in Oyo state, Nigeria. Int. J. Livest. Prod. 2018, 9, 211–220. [Google Scholar] [CrossRef]
- Gomes-Neves, E.; Cardoso, C.S.; Araújo, A.C.; da Costa, J.M.C. Meat handlers training in Portugal: A survey on knowledge and practice. Food Control 2011, 22, 501–507. [Google Scholar] [CrossRef]
- Abas, S.N.H.H.; Yussof, N.H.H.M.; Yusra, F.N.; Idris, P.S.R.P.H. Effects of Training and Motivation Practices on Performance and Task Efficiency: The Case of Brunei Meat Slaughterhouses. Int. J. Asian Bus. Inf. Manag. 2021, 12, 59–74. [Google Scholar] [CrossRef]
- Cook, E.A.J.; de Glanville, W.A.; Thomas, L.F.; Kariuki, S.; Bronsvoort BM, D.C.; Fèvre, E.M. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health 2017, 17, 14. [Google Scholar] [CrossRef]
| Traditional | Risk-Based | ||
|---|---|---|---|
| Inspection Line | Procedure | Evaluation and Classification | Procedure |
| A1—Head and jowl | Visual and incision | Head, jowl, and tongue | Visual |
| A—Uterus | Visual and palpation | Intestines, stomach, spleen, pancreas, bladder, and uterus * | Visual |
| B—Intestines, stomach, spleen, pancreas, and bladder | Visual, palpation, and incision | ||
| Inspection of mesenteric lymph nodes | Visual and incision | ||
| C—Heart and tongue | Visual, palpation, and incision | Heart | Visual and incision |
| D—Lungs and liver | Visual, palpation, and incision | Lungs and liver | Visual and palpation |
| E—Carcass | Visual and incision of lymph nodes | Carcass | Visual |
| F—Kidneys | Visual, palpation, and incision | Kidneys * | Visual |
| G—Brain * | Visual | Brain * | Visual |
| Traditional System | Risk-Based System | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| 5:30 a.m. | 0 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 |
| 8:30 a.m. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 11:30 a.m. | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 2:30 p.m. | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 0 | 1 | 1 |
| Treatment | Sampling Time | Means | Pr > χ2 | |||
|---|---|---|---|---|---|---|
| 5:30 a.m. | 8:30 a.m. | 11:30 a.m. | 2:30 p.m. | |||
| Enterobacteriaceae—log (CFU/cm2) | ||||||
| Risk-Based | −3.293 ± 0.695 b | −0.592 ± 0.466 a | −1.621 ± 0.492 ab | −0.921 ± 0.473 ab | −1.607 ± 0.279 | 0.0079 |
| Traditional | 0.247 ± 0.457 | −0.697 ± 0.471 | −0.866 ± 0.472 | 0.593 ± 0.451 | −0.181 ± 0.235 | 0.0666 |
| Mean | −1.523 ± 0.420 b | −0.645 ± 0.333 ab | −1.244 ± 0.344 ab | −0.164 ± 0.328 a | 0.0319 | |
| Pr > χ2 | <0.0001 | 0.8735 | 0.2640 | 0.0200 | <0.0001 | |
| Mesophilic aerobic counts—log (CFU/cm2) | ||||||
| Risk-Based | 3.613 ± 0.353 | 3.509 ± 0.316 | 3.410 ± 0.316 | 3.449 ± 0.316 | 3.495 ± 0.163 | 0.9765 |
| Traditional | 4.599 ± 0.330 | 4.352 ± 0.327 | 4.296 ± 0.325 | 5.155 ± 0.347 | 4.601 ± 0.168 | 0.2579 |
| Mean | 4.106 ± 0.242 | 3.930 ± 0.227 | 3.853 ± 0.227 | 4.302 ± 0.234 | 0.5234 | |
| Pr > χ2 | 0.0414 | 0.0639 | 0.0505 | 0.0003 | <0.0001 | |
| Enterobacteriaceae | Mesophilic Aerobic Counts | |||
|---|---|---|---|---|
| CFU/cm2 | Traditional | Risk-Based | Traditional | Risk-Based |
| <0.25 | 34% | 49.5% | 0% | 1.1% |
| Between 0.25 and 250 | 66% | 50.5% | 67% | 98.9% |
| >250 | 0% | 0% | 33% | 0% |
| Total | 100% | 100% | 100% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalheiro, L.G.; Gené, L.A.; Coldebella, A.; Kich, J.D.; Ruiz, V.L.d.A. Microbiological Quality of Pig Carcasses in a Slaughterhouse under Risk-Based Inspection System. Foods 2022, 11, 3986. https://doi.org/10.3390/foods11243986
Cavalheiro LG, Gené LA, Coldebella A, Kich JD, Ruiz VLdA. Microbiological Quality of Pig Carcasses in a Slaughterhouse under Risk-Based Inspection System. Foods. 2022; 11(24):3986. https://doi.org/10.3390/foods11243986
Chicago/Turabian StyleCavalheiro, Luciana Giacometti, Luisa Aneiros Gené, Arlei Coldebella, Jalusa Deon Kich, and Vera Letticie de Azevedo Ruiz. 2022. "Microbiological Quality of Pig Carcasses in a Slaughterhouse under Risk-Based Inspection System" Foods 11, no. 24: 3986. https://doi.org/10.3390/foods11243986
APA StyleCavalheiro, L. G., Gené, L. A., Coldebella, A., Kich, J. D., & Ruiz, V. L. d. A. (2022). Microbiological Quality of Pig Carcasses in a Slaughterhouse under Risk-Based Inspection System. Foods, 11(24), 3986. https://doi.org/10.3390/foods11243986








