Iodine Bioavailability and Accumulation of Arsenic and Cadmium in Rats Fed Sugar Kelp (Saccharina latissima)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sugar Kelp
2.3. Experimental Diets
2.4. Energy Intake, Feed Efficiency and Iodine Bioavailability
2.5. Blood Haematology
2.6. Liver Metabolomic Profiling and Data Analysis
2.7. Plasma Measurements of Iodine-Regulated Thyroid Hormones
2.8. Statistical Analysis
3. Results
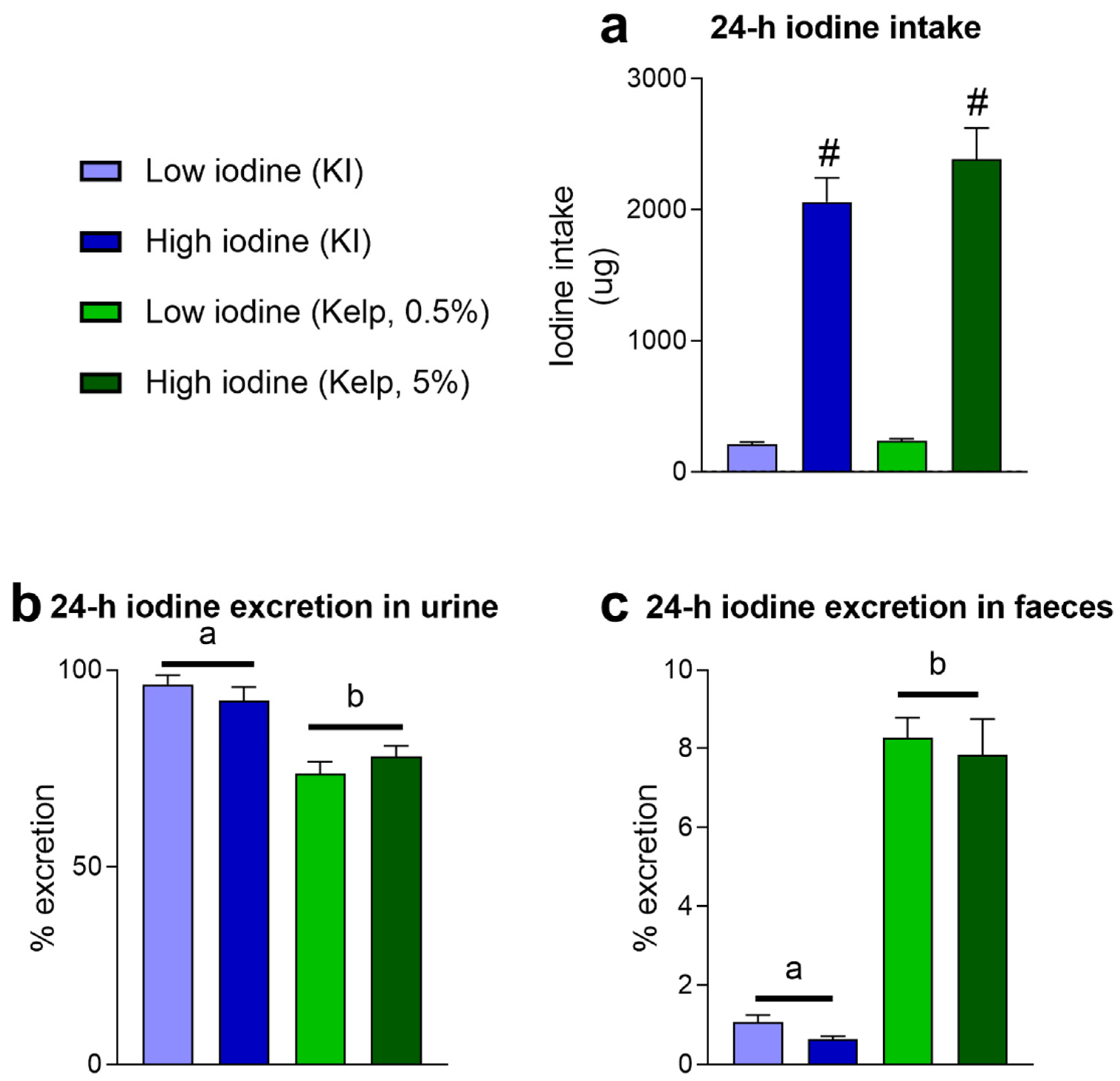
3.1. Iodine from Sugar Kelp (Saccharina latissima) Has Lower Bioavailability Than Iodine from KI
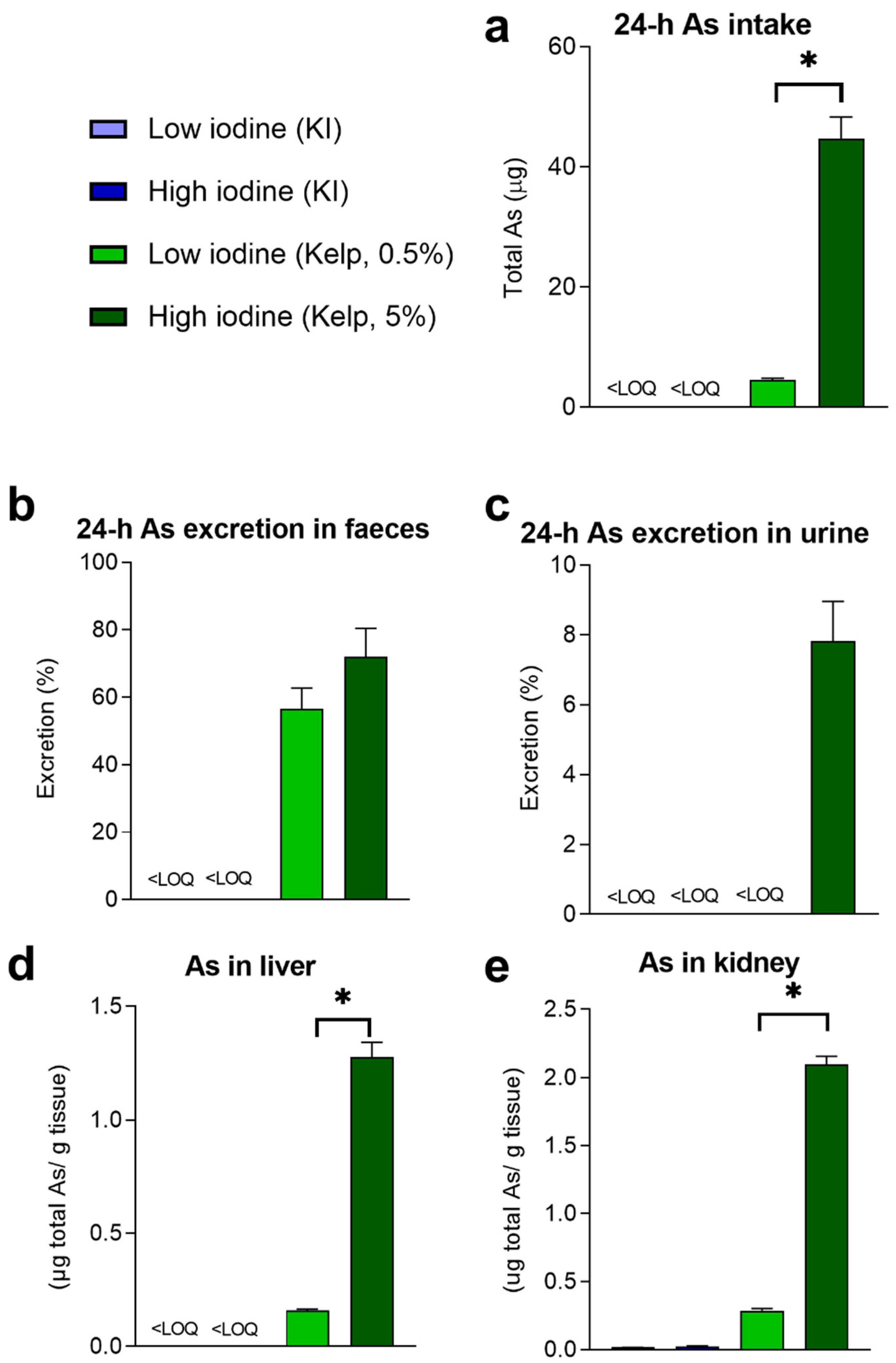
3.2. Excretion and Accumulation of Total As and iAs in Rats Fed Diets Containing Sugar Kelp
3.3. Excretion and Accumulation of Cd and Cu from Sugar Kelp
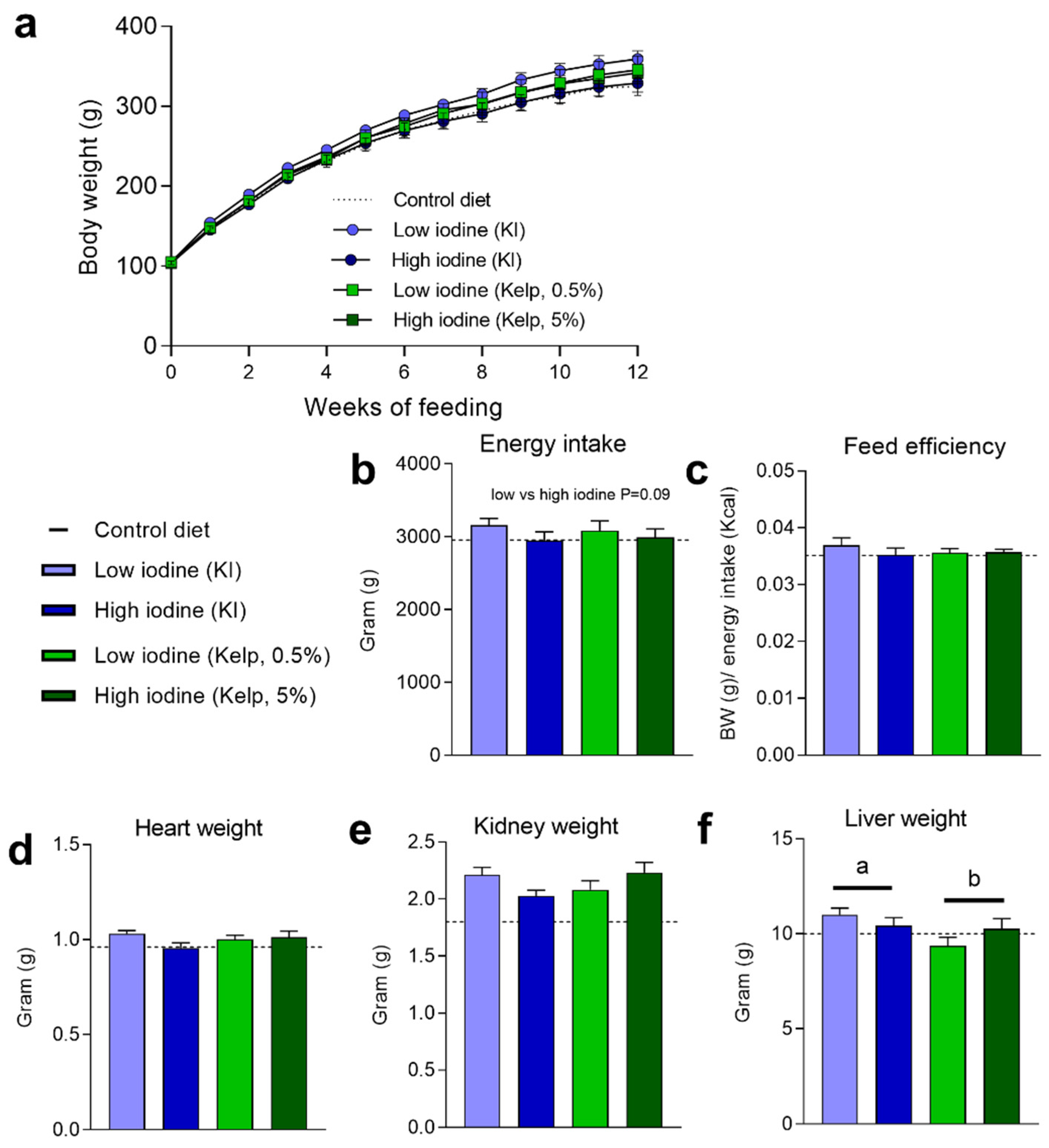
3.4. Levels of Iodine in the Diet Do Not Affect Body Weight Development and Feed Efficiency, but Modulate Energy Intake
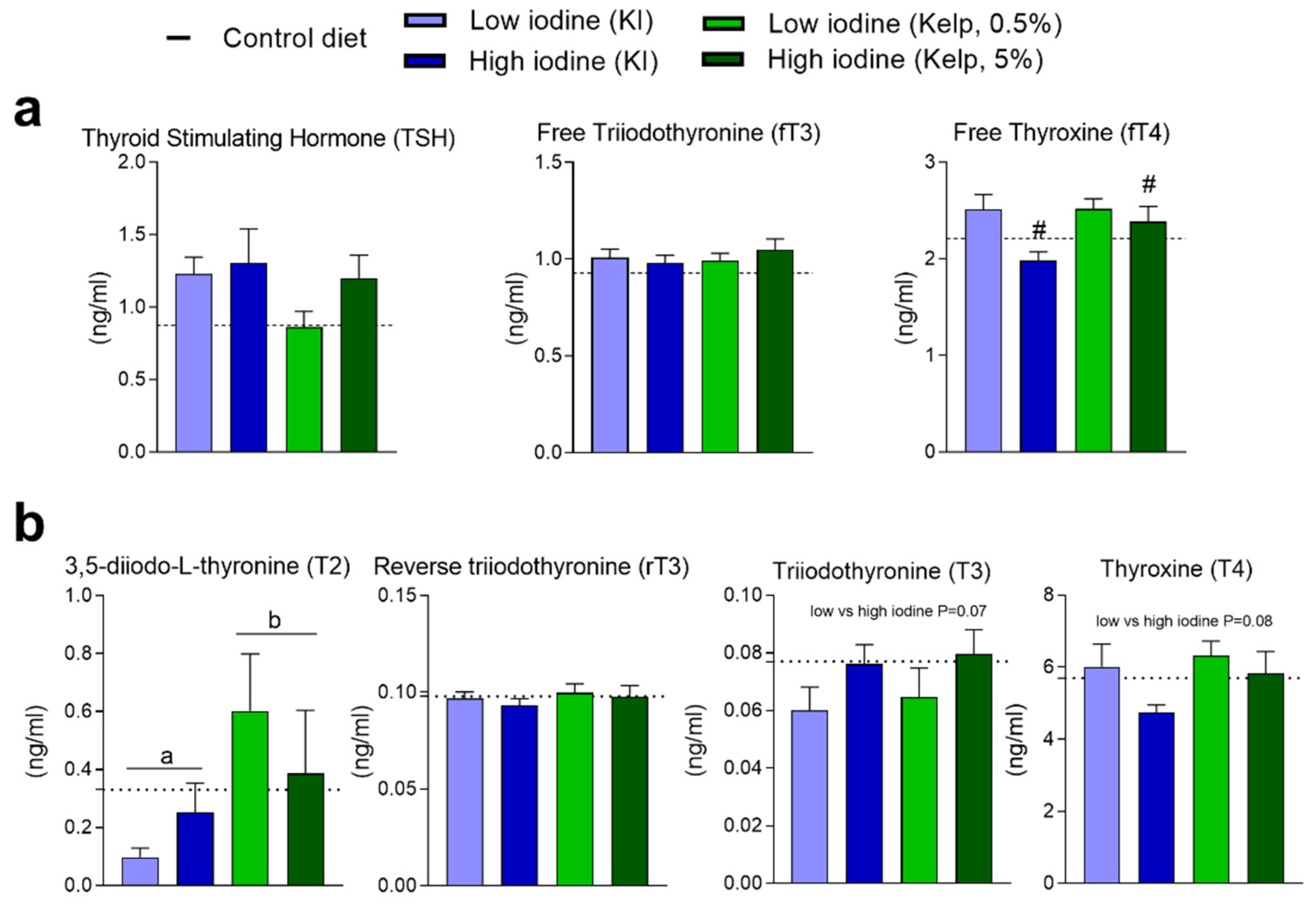
3.5. Thyroid-Stimulating Hormone (TSH), Free Triiodothyronine (fT3), Free Thyroxine (fT4), and Other Thyroid-Related Hormones
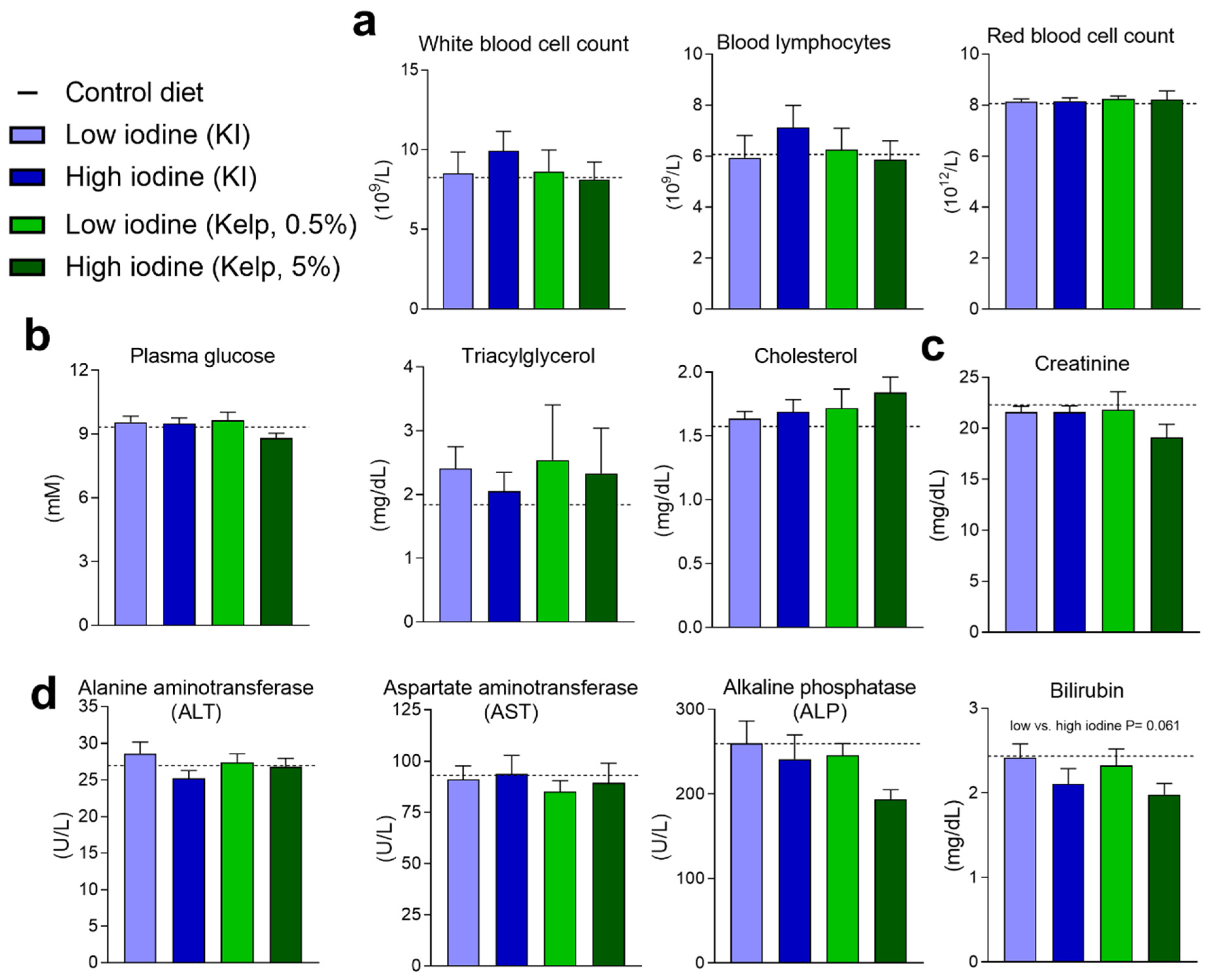
3.6. Blood Haematology and Plasma Markers for Kidney Function and Liver Damage
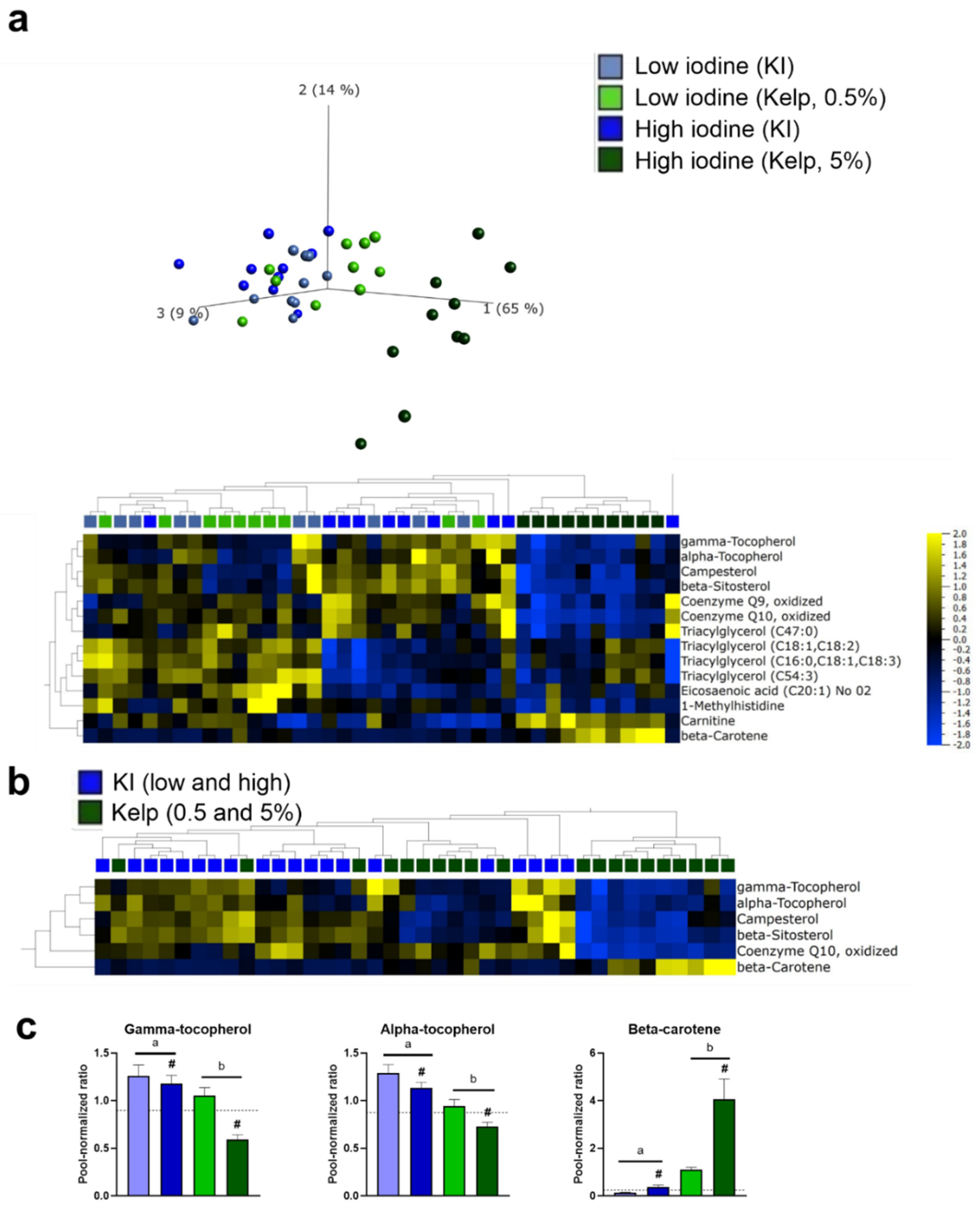
3.7. Rats Fed a High Iodine Diet Based on Kelp Have a Distinct Hepatic Metabolite Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kapil, U. Health consequences of iodine deficiency. Sultan Qaboos Univ. Med. J. 2007, 7, 267–272. [Google Scholar] [PubMed]
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers. Available online: https://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en/ (accessed on 10 December 2020).
- UNICEF. Sustainable Elimination of Iodine Deficiency. Available online: https://www.unicef.org/publications/files/Sustainable_Elimination_of_Iodine_Deficiency.pdf (accessed on 10 December 2020).
- Delange, F. Iodine deficiency in Europe and its consequences: An update. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (Suppl. 2), S404–S416. [Google Scholar] [CrossRef] [PubMed]
- WHO. Iodine Deficiency in Europe: A Continuing Public Health Problem; Springer: New York, NY, USA, 2007. [Google Scholar]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid. Res. 2011, 4, 14. [Google Scholar] [CrossRef]
- Park, J.-K.; Woo, H.W.; Kim, M.K.; Shin, J.; Lee, Y.-H.; Shin, D.H.; Shin, M.-H.; Choi, B.Y. Dietary iodine, seaweed consumption, and incidence risk of metabolic syndrome among postmenopausal women: A prospective analysis of the Korean Multi-Rural Communities Cohort Study (MRCohort). Eur. J. Nutr. 2020, 60, 135–146. [Google Scholar] [CrossRef]
- Bürgi, H. Iodine excess. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 107–115. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L. Variability of Iodine Content in Common Commercially Available Edible Seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2013, 10, 136–142. [Google Scholar] [CrossRef]
- Nagataki, S. The Average of Dietary Iodine Intake due to the Ingestion of Seaweeds is 1.2 mg/day in Japan. Thyroid 2008, 18, 667–668. [Google Scholar] [CrossRef]
- Fuse, Y.; Shishiba, Y.; Irie, M. Japan’s iodine status—Too high or just right? IDD Newsl. 2015, 9–11. [Google Scholar]
- Aquaron, R.; Delange, F.; Marchal, P.; Lognoné, V.; Ninane, L. Bioavailability of seaweed iodine in human beings. Cell. Mol. Biol. 2002, 48, 563–569. [Google Scholar] [PubMed]
- Combet, E.; Ma, Z.F.; Cousins, F.; Thompson, B.; Lean, M.E.J. Low-level seaweed supplementation improves iodine status in iodine-insufficient women. Br. J. Nutr. 2014, 112, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Committee, E.S. Guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed. EFSA J. 2011, 9, 2438. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.-N.; Liu, J.-Y.; Yan, Y.-Q.; Chen, Z.-P. Type 1 iodothyronine deiodinase activity and mRNA expression in rat thyroid tissue with different iodine intakes. Chin. Med. J. 2006, 119, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Nerhus, I.; Markhus, M.W.; Nilsen, B.M.; Øyen, J.; Maage, A.; Ødegård, E.R.; Midtbø, L.K.; Frantzen, S.; Kögel, T.; Graff, I.E.; et al. Iodine content of six fish species, Norwegian dairy products and hen’s egg. Food Nutr. Res. 2018, 62, 1291. [Google Scholar] [CrossRef]
- Biancarosa, I.; Sele, V.; Belghit, I.; Ørnsrud, R.; Lock, E.-J.; Amlund, H. Replacing fish meal with insect meal in the diet of Atlantic salmon (Salmo salar) does not impact the amount of contaminants in the feed and it lowers accumulation of arsenic in the fillet. Food Addit. Contam. Part A 2019, 36, 1191–1205. [Google Scholar] [CrossRef]
- Hansen, M.; Luong, X.; Sedlak, D.L.; Helbing, C.; Hayes, T. Quantification of 11 thyroid hormones and associated metabolites in blood using isotope-dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5429–5442. [Google Scholar] [CrossRef]
- Ma, N.L.; Hansen, M.; Therkildsen, O.R.; Christensen, T.K.; Tjørnløv, R.S.; Garbus, S.-E.; Lyngs, P.; Peng, W.; Lam, S.S.; Krogh, A.K.H.; et al. Body mass, mercury exposure, biochemistry and untargeted metabolomics of incubating common eiders (Somateria mollissima) in three Baltic colonies. Environ. Int. 2020, 142, 105866. [Google Scholar] [CrossRef]
- Hylling, O.; Carstens, A.B.; Kot, W.; Hansen, M.; Neve, H.; Franz, C.M.A.P.; Johansen, A.; Ellegaard-Jensen, L.; Hansen, L.H. Two novel bacteriophage genera from a groundwater reservoir highlight subsurface environments as underexplored biotopes in bacteriophage ecology. Sci. Rep. 2020, 10, 11879. [Google Scholar] [CrossRef]
- Yoshida, M.; Mukama, A.; Hosomi, R.; Fukunaga, K.; Nishiyama, T. Serum and Tissue Iodine Concentrations in Rats Fed Diets Supplemented with Kombu Powder or Potassium Iodide. J. Nutr. Sci. Vitaminol. 2014, 60, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Blikra, M.J.; Henjum, S.; Aakre, I. Iodine from brown algae in human nutrition, with an emphasis on bioaccessibility, bioavailability, chemistry, and effects of processing: A systematic review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Small, M.D.; Bezman, A.; Longarini, A.E.; Fennell, A.; Zamcheck, N. Absorption of Potassium Iodide from Gastro-Intestinal Tract. Exp. Biol. Med. 1961, 106, 450–452. [Google Scholar] [CrossRef]
- Gao, J.; Lin, X.; Liu, X.; Yang, Q.; Zhang, Z.; Jiang, Q.; Bian, J. Effect of Combined Excess Iodine and Low-Protein Diet on Thyroid Hormones and Ultrastructure in Wistar Rats. Biol. Trace Element Res. 2013, 155, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Calil-Silveira, J.; Serrano-Nascimento, C.; Kopp, P.A.; Nunes, M.T. Iodide excess regulates its own efflux: A possible involvement of pendrin. Am. J. Physiol. Physiol. 2016, 310, C576–C582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.K.R.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed Supplements Normalise Metabolic, Cardiovascular and Liver Responses in High-Carbohydrate, High-Fat Fed Rats. Mar. Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [PubMed]
- Phaneuf, D.; Côté, I.; Dumas, P.; Ferron, L.A.; LeBlanc, A. Evaluation of the Contamination of Marine Algae (Seaweed) from the St. Lawrence River and Likely to Be Consumed by Humans. Environ. Res. 1999, 80, S175–S182. [Google Scholar] [CrossRef]
- Mise, N.; Ohtsu, M.; Ikegami, A.; Mizuno, A.; Cui, X.; Kobayashi, Y.; Nakagi, Y.; Nohara, K.; Yoshida, T.; Kayama, F. Hijiki seaweed consumption elevates levels of inorganic arsenic intake in Japanese children and pregnant women. Food Addit. Contam. A 2019, 36, 84–95. [Google Scholar] [CrossRef]
- Schmidt, S. Matching Rodents to People: A Humanized Mouse Model of iAs Methylation. Environ. Health Perspect. 2020, 128, 104003. [Google Scholar] [CrossRef]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium Exposure and Liver Disease among US Adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2012. [Google Scholar]
- Xia, Y.; Qu, W.; Zhao, L.-N.; Han, H.; Yang, X.-F.; Sun, X.-F.; Hao, L.-P.; Xu, J. Iodine Excess Induces Hepatic Steatosis through Disturbance of Thyroid Hormone Metabolism Involving Oxidative Stress in BaLB/c Mice. Biol. Trace Element Res. 2013, 154, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Shila, S.; Kumaran, S.; Panneerselvam, C. Protective role of ascorbic acid and a-tocopherol on arsenic-induced microsomal dysfunctions. Hum. Exp. Toxicol. 2003, 22, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hansen, V.; Aldahan, A.; Possnert, G.; Lind, O.C.; Lujaniene, G. A review on speciation of iodine-129 in the environmental and biological samples. Anal. Chim. Acta 2009, 632, 181–196. [Google Scholar] [CrossRef]
- Galinier, A.; Carrière, A.; Fernandez, Y.; Bessac, A.; Caspar-Bauguil, S.; Periquet, B.; Comtat, M.; Thouvenot, J.; Casteilla, L. Biological validation of coenzyme Q redox state by HPLC-EC measurement: Relationship between coenzyme Q redox state and coenzyme Q content in rat tissues. FEBS Lett. 2004, 578, 53–57. [Google Scholar] [CrossRef]
- Tang, P.H.; Miles, M.V.; Degrauw, A.; Hershey, A.; Pesce, A. HPLC Analysis of Reduced and Oxidized Coenzyme Q10 in Human Plasma. Clin. Chem. 2001, 47, 256–265. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamashita, S. Plasma ratio of ubiquinol and ubiquinone as a marker of oxidative stress. Mol. Asp. Med. 1997, 18, 79–84. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar] [CrossRef]

| Diet | AIN-93G Control Diet | Low Iodine (KI) | High Iodine (KI) | Low Iodine (Kelp, 0.5%) | High Iodine (Kelp, 5%) |
|---|---|---|---|---|---|
| Iodine (mg/kg diet) | 0.15 ± 0.01 | 14 ± 1.5 | 160 ± 12 | 17 ± 1 | 200 ± 12 |
| Inorganic As (µg/kg diet) | <LOQ | <LOQ | <LOQ | <LOQ | 18.8 ± 0.9 |
| Total As (mg/kg diet) | <LOQ | <LOQ | <LOQ | 0.27 ± 0.015 | 3 ± 0.3 |
| Cd (mg/kg diet) | <LOQ | <LOQ | <LOQ | 0.006 ± 0.0005 | 0.027 ± 0.015 |
| Cu (mg/kg diet) | 8.7 ± 0.4 | 9.1 ± 0.1 | 9.2 ± 0.3 | 9.4 ± 0.5 | 9.6 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fjære, E.; Poulsen, R.; Duinker, A.; Liaset, B.; Hansen, M.; Madsen, L.; Myrmel, L.S. Iodine Bioavailability and Accumulation of Arsenic and Cadmium in Rats Fed Sugar Kelp (Saccharina latissima). Foods 2022, 11, 3943. https://doi.org/10.3390/foods11243943
Fjære E, Poulsen R, Duinker A, Liaset B, Hansen M, Madsen L, Myrmel LS. Iodine Bioavailability and Accumulation of Arsenic and Cadmium in Rats Fed Sugar Kelp (Saccharina latissima). Foods. 2022; 11(24):3943. https://doi.org/10.3390/foods11243943
Chicago/Turabian StyleFjære, Even, Rikke Poulsen, Arne Duinker, Bjørn Liaset, Martin Hansen, Lise Madsen, and Lene Secher Myrmel. 2022. "Iodine Bioavailability and Accumulation of Arsenic and Cadmium in Rats Fed Sugar Kelp (Saccharina latissima)" Foods 11, no. 24: 3943. https://doi.org/10.3390/foods11243943
APA StyleFjære, E., Poulsen, R., Duinker, A., Liaset, B., Hansen, M., Madsen, L., & Myrmel, L. S. (2022). Iodine Bioavailability and Accumulation of Arsenic and Cadmium in Rats Fed Sugar Kelp (Saccharina latissima). Foods, 11(24), 3943. https://doi.org/10.3390/foods11243943







