The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Pesticide Treatment
2.3. Household Processing
2.3.1. Washing (W)
2.3.2. Peeling: Separation of Peel (P) and Pulp (PU)
2.3.3. Frozen Grated Peels (GP)
2.3.4. Fruit Juice (FJ)
2.3.5. Homemade Jam (HJ)
2.4. Pesticide Residue Analysis
2.5. Processing Factor
3. Results and Discussion
3.1. Reliability of the Analytical Method
3.2. Effect of Household Food Processing on Residue Content of Orange
3.2.1. Washing (W)
3.2.2. Peeling: Separation of Peel and Pulp
3.2.3. Processing in to Fruit Juice (FJ)
3.2.4. Frozen Storage of Grated Peels (GP)
3.2.5. Homemade Jam Processing (HJ)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anonymous. Yaş Meyve Ve Sebze Sektörü Türkiye Geneli Değerlendirme Raporu. Akdeniz İhracatçılar Birliği. 2018. Available online: http://www.akib.org.tr/files/downloads/ArastirmaRaporlari/YSM/ocak-2018.pdf (accessed on 18 June 2022).
- Acoglu, B.; Yolci Omeroglu, P. Effectiveness of Different Type of Washing Agents on Reduction of Pesticide Residues in Orange (Citrus sinensis). LWT 2021, 147, 111690. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, B.; Zhao, Q.; Wang, C.; Gong, Y.; Zhang, Y.; Chen, W. Effect of Commercial Processing on Pesticide Residues in Orange Products. Eur. Food Res. Technol. 2012, 234, 449–456. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EU) No 396/2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin, and Amending Council Directive 91/414/EEC; European Commission (EC): Luxembourg, 2005; pp. 1–16. [Google Scholar]
- Scholz, R.; Herrmann, M.; Michalski, B. Compilation of Processing Factors and Evaluation of Quality Controlled Data of Food Processing Studies. J. Verbrauch. Leb. 2017, 12, 3–14. [Google Scholar] [CrossRef]
- European Commission (EC). Information Note on Article 20 of Regulation (EC) No 396/2005 as Regards Processing Factors, Processed and Composite Food and Feed. (SANTE/10704/2021). Available online: https://food.ec.europa.eu/system/files/2022-02/pesticides_mrl_guidelines_proc_imp_sante-2021-10704.pdf (accessed on 6 October 2022).
- FAO. Submission and Evaluation of Pesticide Residues Data for the Estimation of Maximum Residue Levels in Food and Feed, Plant Production and Protection Paper 225, Third Edition. 2016. Available online: https://www.fao.org/3/i5452e/I5452E.pdf (accessed on 6 October 2022).
- Dordevic, T.; Durovic-Pejcev, R. Food Processing as a Means for Pesticide Residue Dissipation. Pestic. Phytomed. 2017, 31, 89–105. [Google Scholar] [CrossRef]
- Chung, S.W.C. How Effective Are Common Household Preparations on Removing Pesticide Residues from Fruit and Vegetables? A Review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- El-Sayed, E.; Hassan, H.; Abd El-Raouf, A.; Salman, S.N. Investigation of the Effects of Household Processing on the Reduction Rate of Chlorpyrifos, Metalaxyl and Diazinon Residues in Orange Fruit Investigation of the Effects of Household Processing on the Reduction Rate of Chlorpyrifos, Metalaxyl and Diazino. Hell. Plant Prot. J. 2021, 14, 64–75. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Yu, H.; Cheng, Y.; Xie, Y.; Yao, W.; Guo, Y.; Qian, H. Chemical Food Contaminants during Food Processing: Sources and Control. Crit. Rev. Food Sci. Nutr. 2021, 61, 1545–1555. [Google Scholar] [CrossRef]
- Kusvuran, E.; Yildirim, D.; Mavruk, F.; Ceyhan, M. Removal of Chloropyrifos Ethyl, Tetradifon and Chlorothalonil Pesticide Residues from Citrus by Using Ozone. J. Hazard. Mater. 2012, 241–242, 287–300. [Google Scholar] [CrossRef]
- Cámara, M.A.; Cermeño, S.; Martínez, G.; Oliva, J. Removal Residues of Pesticides in Apricot, Peach and Orange Processed and Dietary Exposure Assessment. Food Chem. 2020, 325, 126936. [Google Scholar] [CrossRef]
- FAO and WHO Reports. Pesticide Residues in Food—Joint FAO/WHO Meeting on Pesticide Residues. Rome. 2022. Available online: https://www.fao.org/pest-and-pesticide-management/guidelines-standards/faowho-joint-meeting-on-pesticide-residues-jmpr/reports/en (accessed on 6 October 2022).
- OECD. OECD Guideline for the Testing of Chemicals. Magnitude of the Pesticide Residues in Processed Commodities. 2008. Available online: http://www.oecd.org/env/ehs/pesticidesbiocides/publicationsonpesticideresidues.htm (accessed on 19 June 2022).
- European Commission (EC). Commission Directive 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing Directive 79/700/EEC. OJEC 2002, 187, 30–45. [Google Scholar]
- Hassan, H.; Elsayed, E.; El-Raouf, A.E.R.A.; Salman, S.N. Method Validation and Evaluation of Household Processing on Reduction of Pesticide Residues in Tomato. J. Consum. Prot. Food Saf. 2019, 14, 31–39. [Google Scholar] [CrossRef]
- Heshmati, A.; Hamidi, M.; Nili-Ahmadabadi, A. Effect of Storage, Washing, and Cooking on the Stability of Five Pesticides in Edible Fungi of Agaricus Bisporus: A Degradation Kinetic Study. Food Sci. Nutr. 2019, 7, 3993–4000. [Google Scholar] [CrossRef]
- Ruengprapavut, S.; Sophonnithiprasert, T.; Pongpoungphet, N. The Effectiveness of Chemical Solutions on the Removal of Carbaryl Residues from Cucumber and Chili Presoaked in Carbaryl Using the HPLC Technique. Food Chem. 2020, 309, 125659. [Google Scholar] [CrossRef]
- AOAC. Official Method 2007.01: Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. J. AOAC Int. 2007, 90, 485–520. [Google Scholar]
- FAO. Pesticide Residue in Food; FAO Plant Protection Paper, No.163; FAO: Rome, Italy, 2000. [Google Scholar]
- Bian, Y.; Wang, J.; Liu, F.; Mao, B.; Huang, H.; Xu, J.; Li, X.; Guo, Y. Residue Behavior and Removal of Iprodione in Garlic, Green Garlic, and Garlic Shoot. J. Sci. Food Agric. 2020, 100, 4705–4713. [Google Scholar] [CrossRef]
- Magnusson, B.; Ornemak, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation, 2nd ed.; EUURACHEM: London, UK, 2014; Available online: www.eurachem.org (accessed on 6 October 2022).
- SANTE. European Commision (EC) Director General for Food and Health Safety. Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed, Document No: SANTE 11312/2021. 2021. Available online: https://food.ec.europa.eu/system/files/2022-02/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 1 June 2022).
- Ellison, S.L.R.; Williams, A. (Eds.) Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem/CITAC: Teddington, UK, 2012; ISBN 978-0-948926-30-3. Available online: www.eurachem.org (accessed on 4 October 2020).
- Magnusson, B.; Näykki, T.; Hovind, H.; Krysell, M.; Sahlin, E. Handbook for Calculation of Measurement Uncertainty in Environmental Laboratories; 2017. Nordtest Report TR 537 (ed. 4). Available online: www.nordtest.info (accessed on 4 June 2022).
- Bonnechere, A.; Hanot, V.; Bragard, C.; Bedoret, T.; Loco, J.V. Effect of Household and Industrial Processing on the Levels of Pesticide Residues and Degradation Products in Melons. Food Addit. Contam. Part A 2012, 29, 1058–1066. [Google Scholar] [CrossRef]
- Polat, B.; Tiryaki, O. Assessing Washing Methods for Reduction of Pesticide Residues in Capia Pepper with LC-MS/MS. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2019, 1–10. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T.K.; Hong, S.M.; Se, E.K.; Cho, N.J.; Kyung, K.S. Effect of Household Processing on Pesticide Residues in Field-Sprayed Tomatoes. Food Sci. Biotechnol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Romeh, A.A.; Mekky, T.M.; Ramadan, R.A.; Hendawi, M.Y. Dissipation of Profenofos, Imidacloprid and Penconazole in Tomato Fruits and Products. Bull. Environ. Contam. Toxicol. 2009, 83, 812–817. [Google Scholar] [CrossRef]
- Vass, A.; Korpics, E.; Dernovics, M. Follow-up of the Fate of Imazalil from Post-Harvest Lemon Surface Treatment to a Baking Experiment. Food Addit. Contam. Part A 2015, 32, 1875–1884. [Google Scholar] [CrossRef]
- Kaushik, G.; Satya, S.; Naik, S.N. Food Processing a Tool to Pesticide Residue Dissipation—A Review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Yolci Omeroglu, P.; Ambrus, Á.; Boyacioglu, D.; Majzik, E.S. A Case Study to Assess the Sample Preparation Error in Pesticide Residue Analysis. Food Anal. Methods 2015, 8, 474–482. [Google Scholar] [CrossRef]
- Han, Y.; Dong, F.; Xu, J.; Liu, X.; Li, Y.; Kong, Z.; Liang, X.; Liu, N.; Zheng, Y. Residue Change of Pyridaben in Apple Samples during Apple Cider Processing. Food Control 2014, 37, 240–244. [Google Scholar] [CrossRef]
- Andrade, G.C.R.M.; Monteiro, S.H.; Francisco, J.G.; Figueiredo, L.A.; Rocha, A.A.; Tornisielo, V.L. Effects of Types of Washing and Peeling in Relation to Pesticide Residues in Tomatoes. J. Braz. Chem. Soc. 2015, 26, 1994–2002. [Google Scholar] [CrossRef]
- Özel, E.; Tiryaki, O. Elma ve Işlenmiş Ürünlerinde Imidacloprid ve Indoxacarb Kalıntılarının Belirlenmesi. Bitki Koruma Bülteni Plant Prot. Bull. 2019, 59, 23–32. [Google Scholar] [CrossRef]
- Öğüt, S.; Canbay, H.S.; Yilmazer, M.; Üniversitesi, A.M.; Yüksekokulu, A.S. Dondurularak Saklanan Kirazlardaki Pestisit Kalıntı Miktarlarının Zamanla Değişimi Changes in Pesticide Residue Amounts on Frozen Cherries Over Time. Süleyman Demirel Üniversitesi Fen Bilim. Enstitüsü Derg. (Suleyman Demirel Univ. J. Nat. Appl. Sci.) 2014, 18, 72–77. [Google Scholar]
- Chauhan, R.; Monga, S.; Kumari, B. Dissipation and Decontamination of Bifenthrin Residues in Tomato (Lycopersicon esculentum Mill). Bull. Environ. Contam. Toxicol. 2012, 89, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Cermeño, S.; Cámara, M.A.; Martínez, G.; Barba, A. Disappearance of Six Pesticides in Fresh and Processed Zucchini, Bioavailability and Health Risk Assessment. Food Chem. 2017, 229, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B.; Munitz, M.S.; Resnik, S.L. Effect of Household Rice Cooking on Pesticide Residues. Food Chem. 2021, 342, 128311. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, U.; Sandhu, K.S. Effect of Handling and Processing on Pesticide Residues in Food—A Review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Jankowska, M.; Kaczynski, P. Behaviour of Selected Pesticide Residues in Blackcurrants (Ribes nigrum) during Technological Processing Monitored by Liquid-Chromatography Tandem Mass Spectrometry. Chem. Pap. 2016, 70, 545–555. [Google Scholar] [CrossRef]
- Hendawi, M.Y.; Romeh, A.A.; Mekky, T.M. Effect of Food Processing on Residue of Imidacloprid in Strawberry Fruits. J. Agric. Sci. Technol. 2013, 15, 951–959. [Google Scholar]
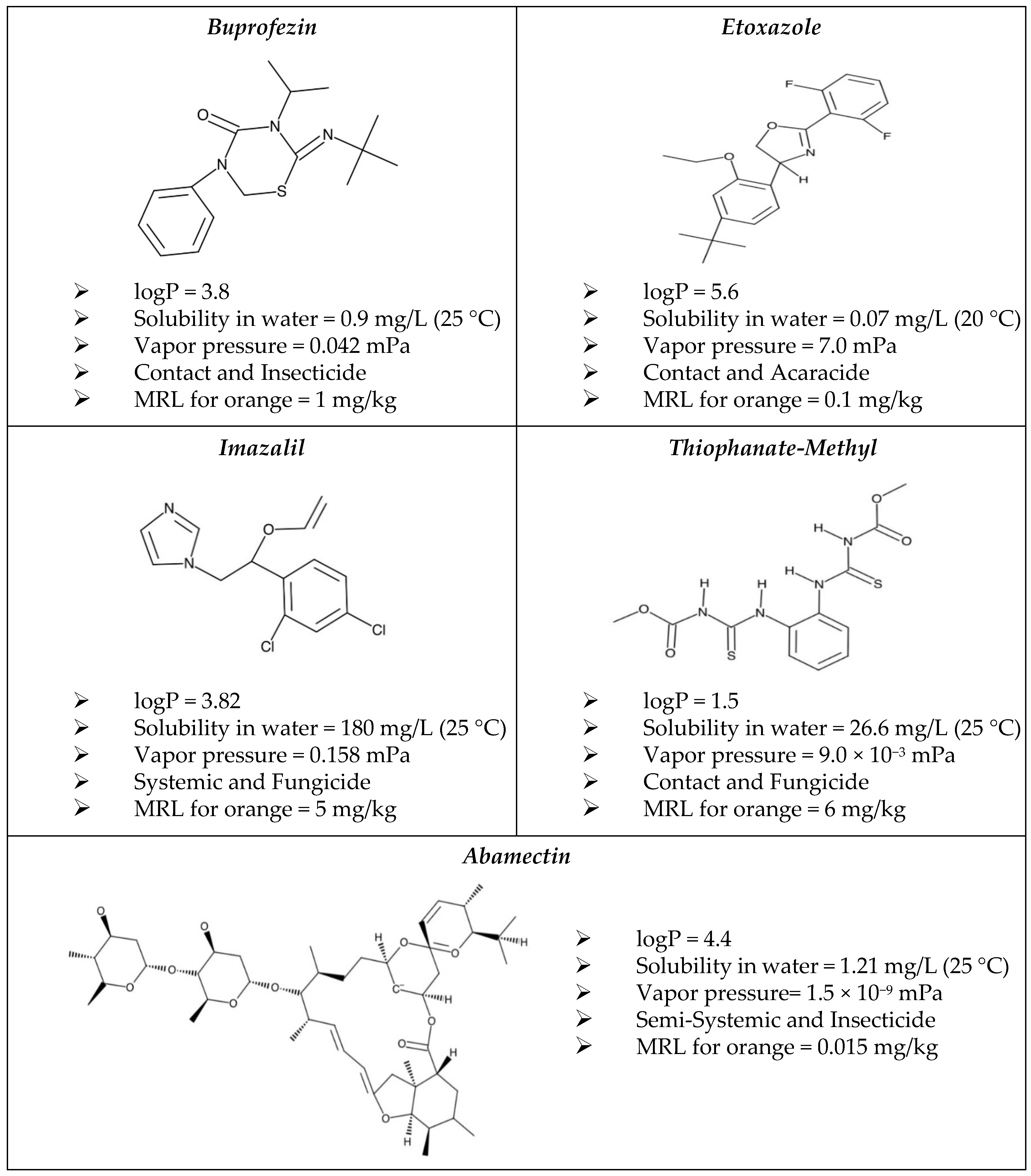
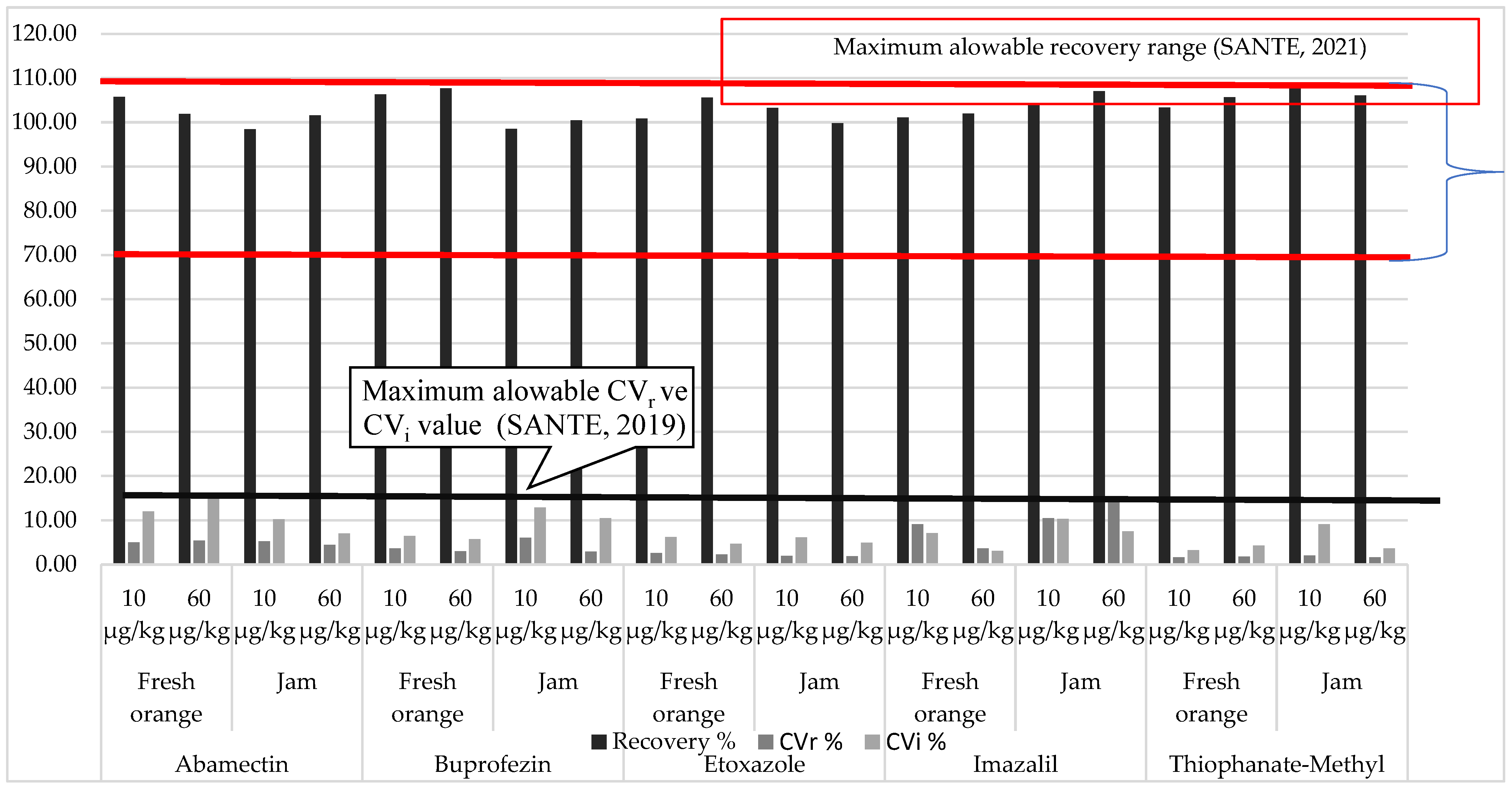
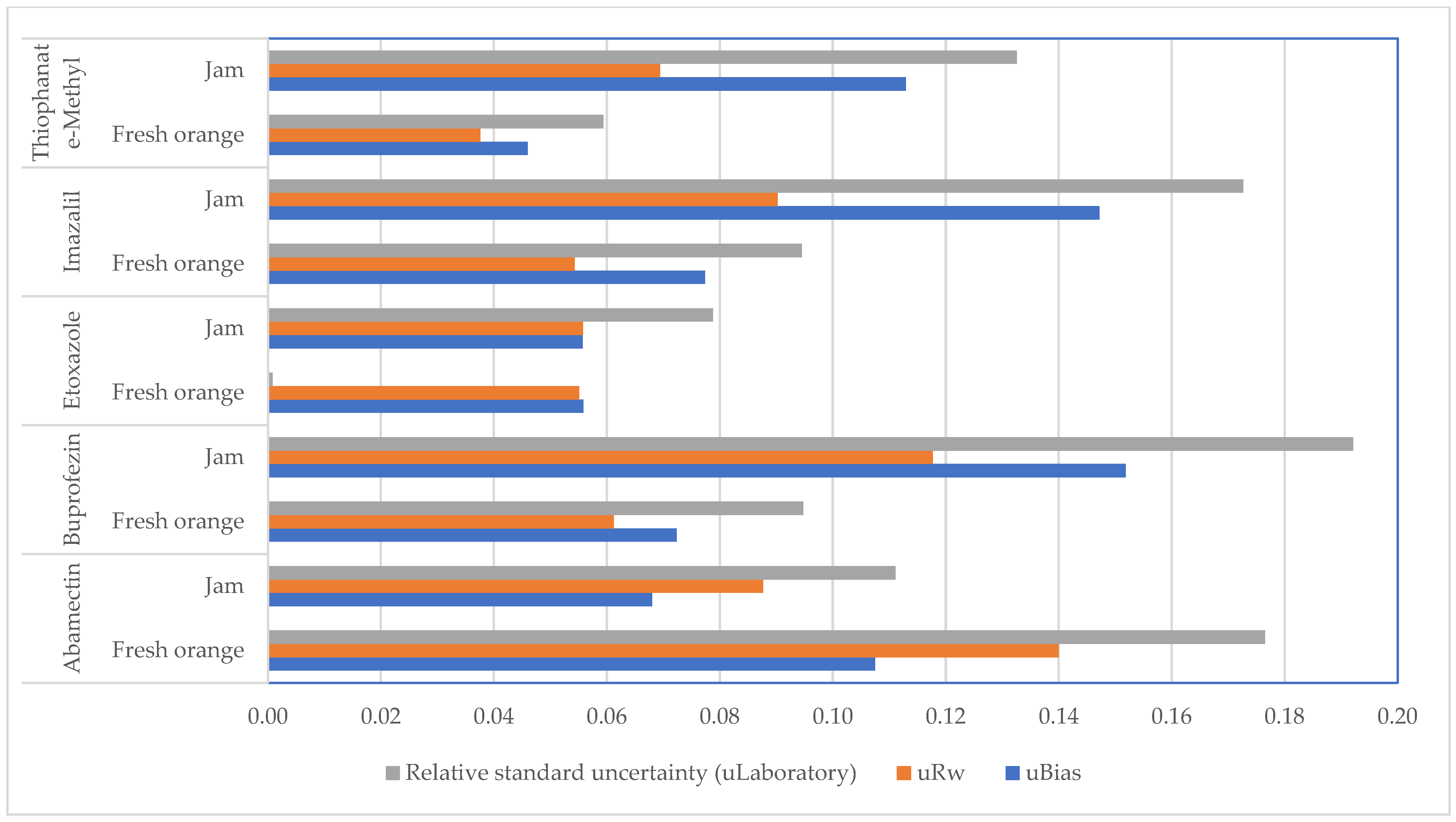
| Abamectin | Buprofezin | Ethoxazole | Imazalil | Thiophanate-Methyl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process | C (mg/kg) | PF | C (mg/kg) | PF | C (mg/kg) | PF | C (mg/kg) | PF | C (mg/kg) | PF |
| Raw agricultural commodity (RAC) | 0.030 ± 0.004 | - | 0.189 ± 0.047 | - | 0.082 ± 0.025 | 2.113 ± 0.475 | 0.110 ± 0.030 | |||
| Washing | 0.020 ± 0.003 | 0.663 ± 0.115 | 0.136 ± 0.023 | 0.720 ± 0.121 | 0.050 ± 0.001 | 0.616 ± 0.011 | 1.643 ± 0.271 | 0.776 ± 0.127 | 0.019 ± 0.003 | 0.173 ± 0.028 |
| Peeling: Separation of peel | 0.080 ± 0.012 | 2.68 ± 0.42 | 0.650 ± 0.091 | 3.43 ± 0.48 | 0.396 ± 0.025 | 4.83 ± 0.307 | 2.963 ± 0.366 | 1.40 ± 0.176 | 0.404 ± 0.041 | 3.67 ± 0.37 |
| Peeling: Separation of pulp | <LOQ | <0.330 | 0.080 ± 0.014 | 0.423 ± 0.076 | <LOQ 1 | <0.120 | 0.453 ± 0.011 | 0.216 ± 0.005 | 0.015 ± 0.005 | 0.146 ± 0.005 |
| Fruit juice | <LOQ | <0.330 | 0.037 ± 0.001 | 0.200 ± 0.001 | <LOQ 1 | <0.120 | 0.580 ± 0.001 | 0.270 ± 0.001 | 0.039 ± 0.316 | 0.363 ± 0.063 |
| Homemade jam | <LOQ | <0.330 | 0.019 ± 0.005 | 0.103 ± 0.028 | <LOQ 1 | <0.120 | 0.105 ± 0.008 | 0.050 ± 0.001 | <LOQ 1 | <0.090 |
| Frozen storage of grated peels (1st month) | 0.090 ± 0.017 | 2.83 ± 0.58 | 0.840 ± 0.036 | 4.60 ± 1.04 | 0.466 ± 0.030 | 6.07 ± 1.92 | 3.866 ± 0.075 | 1.90 ± 0.487 | 0.520 ± 0.051 | 4.88 ± 0.29 |
| Frozen storage of grated peels (2nd month) | 0.069 ± 0.011 | 2.32 ± 0.39 | 0.686 ± 0.025 | 3.62 ± 0.13 | 0.436 ± 0.005 | 5.33 ± 0.08 | 3.753 ± 0.254 | 1.78 ± 0.121 | 0.486 ± 0.127 | 3.53 ± 0.77 |
| Frozen storage of grated peels (3rd month) | 0.032 ± 0.011 | 1.11 ± 0.43 | 0.533 ± 0.020 | 2.81 ± 0.11 | 0.393 ± 0.152 | 4.80 ± 0.19 | 2.846 ± 0.06 | 1.35 ± 0.02 | 0.390 ± 0.085 | 3.26 ± 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omeroglu, P.Y.; Acoglu Celik, B.; Koc Alibasoglu, E. The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods 2022, 11, 3918. https://doi.org/10.3390/foods11233918
Omeroglu PY, Acoglu Celik B, Koc Alibasoglu E. The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods. 2022; 11(23):3918. https://doi.org/10.3390/foods11233918
Chicago/Turabian StyleOmeroglu, Perihan Yolci, Busra Acoglu Celik, and Elif Koc Alibasoglu. 2022. "The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis)" Foods 11, no. 23: 3918. https://doi.org/10.3390/foods11233918
APA StyleOmeroglu, P. Y., Acoglu Celik, B., & Koc Alibasoglu, E. (2022). The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods, 11(23), 3918. https://doi.org/10.3390/foods11233918








