Maturity Grading and Identification of Camellia oleifera Fruit Based on Unsupervised Image Clustering
Abstract
1. Introduction
- (1)
- Using an unsupervised image clustering method to grade and identification the maturity of Camellia oleifera fruits;
- (2)
- Conjoint analysis was performed for the physical and quality properties of Camellia oleifera fruits with different maturity;
- (3)
- The Gradient-weighted Class Activation Mapping (Grad-CAM) visualization analysis reveals the critical regions for Camellia oleifera fruit maturity identification.
2. Materials and Methods
2.1. Fruit Sample Preparation
2.2. Dataset Details
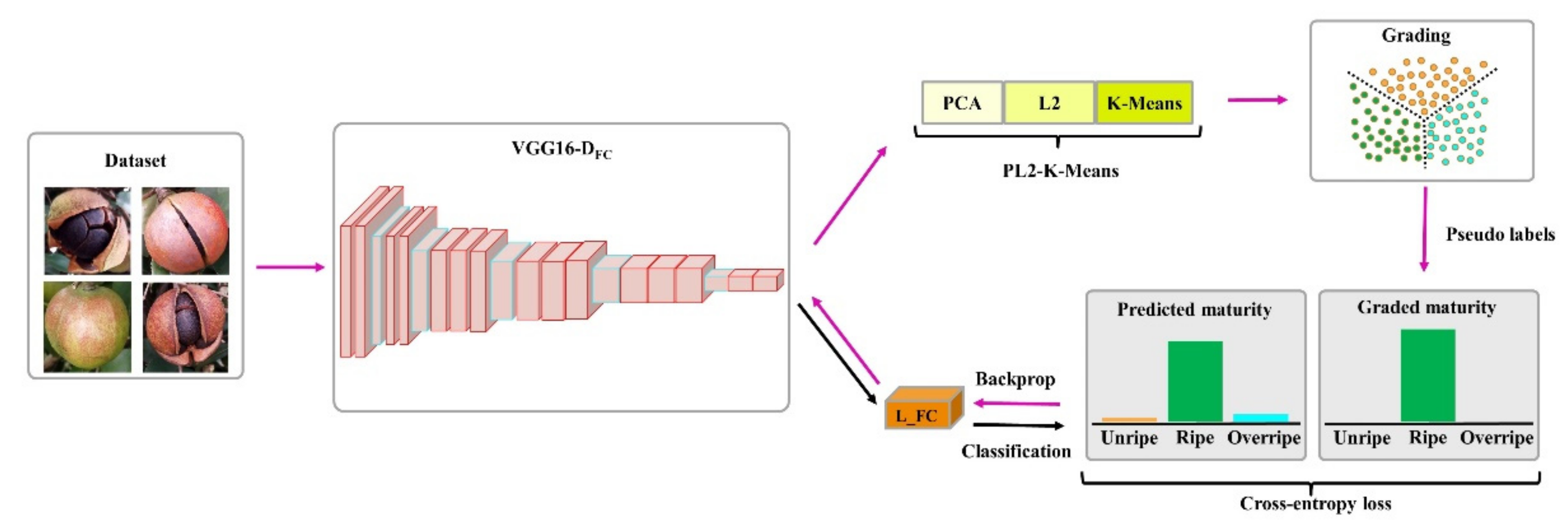
2.3. Proposed Method of Fruit Maturity Grading and Identification
2.3.1. Grading and Identification of Fruit Maturity
2.3.2. Determination of Physical Properties
2.3.3. Determination of Quality Properties
2.3.4. Conjoint Analysis of Maturity with Physical and Quality Properties
2.4. Maturity Identification Performance Evaluation Index
2.5. Model Visualization
3. Results and Discussion
3.1. Implementation Details
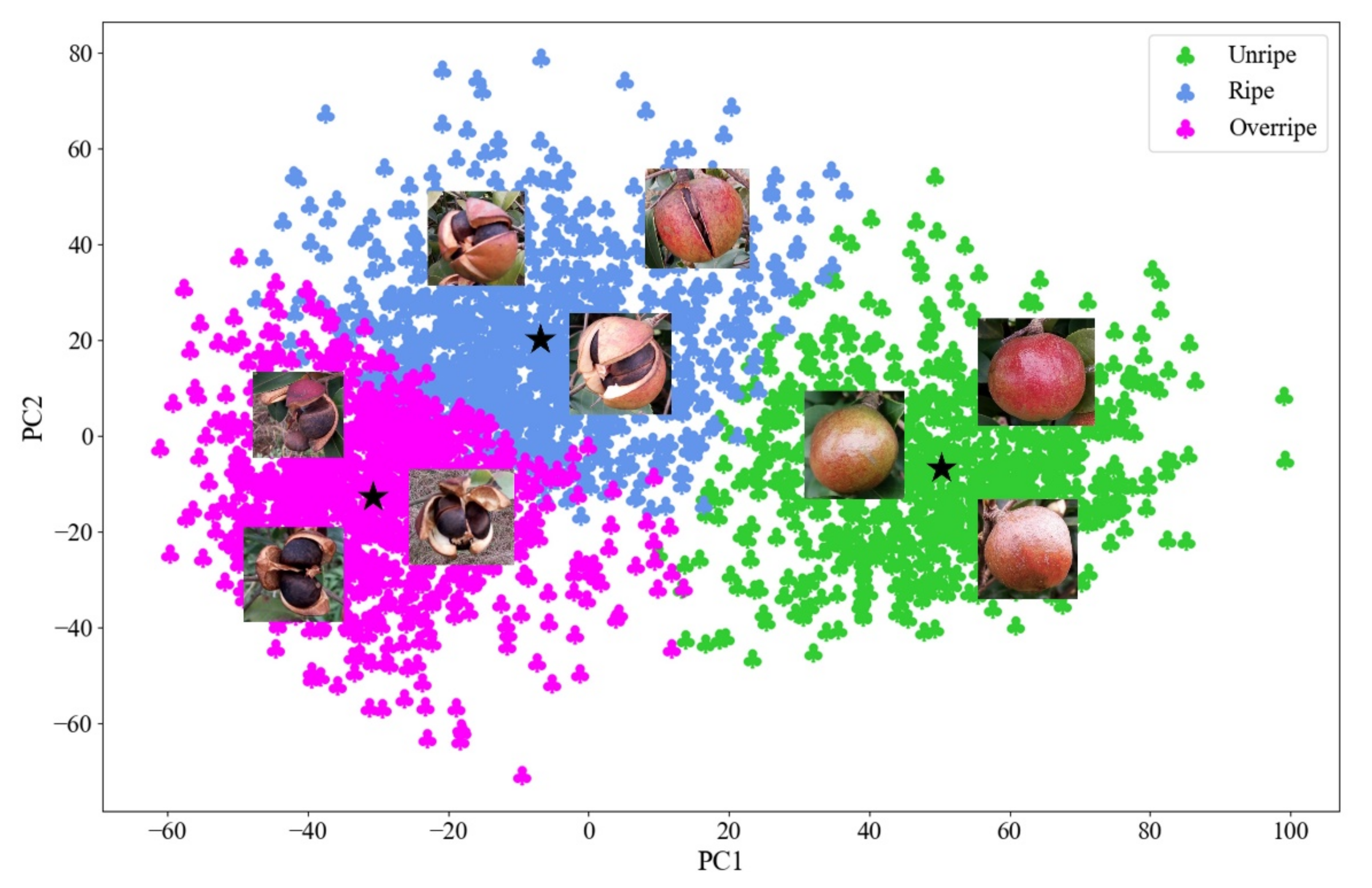
3.2. Fruit Maturity Grading Results
3.3. Conjoint Analysis Results
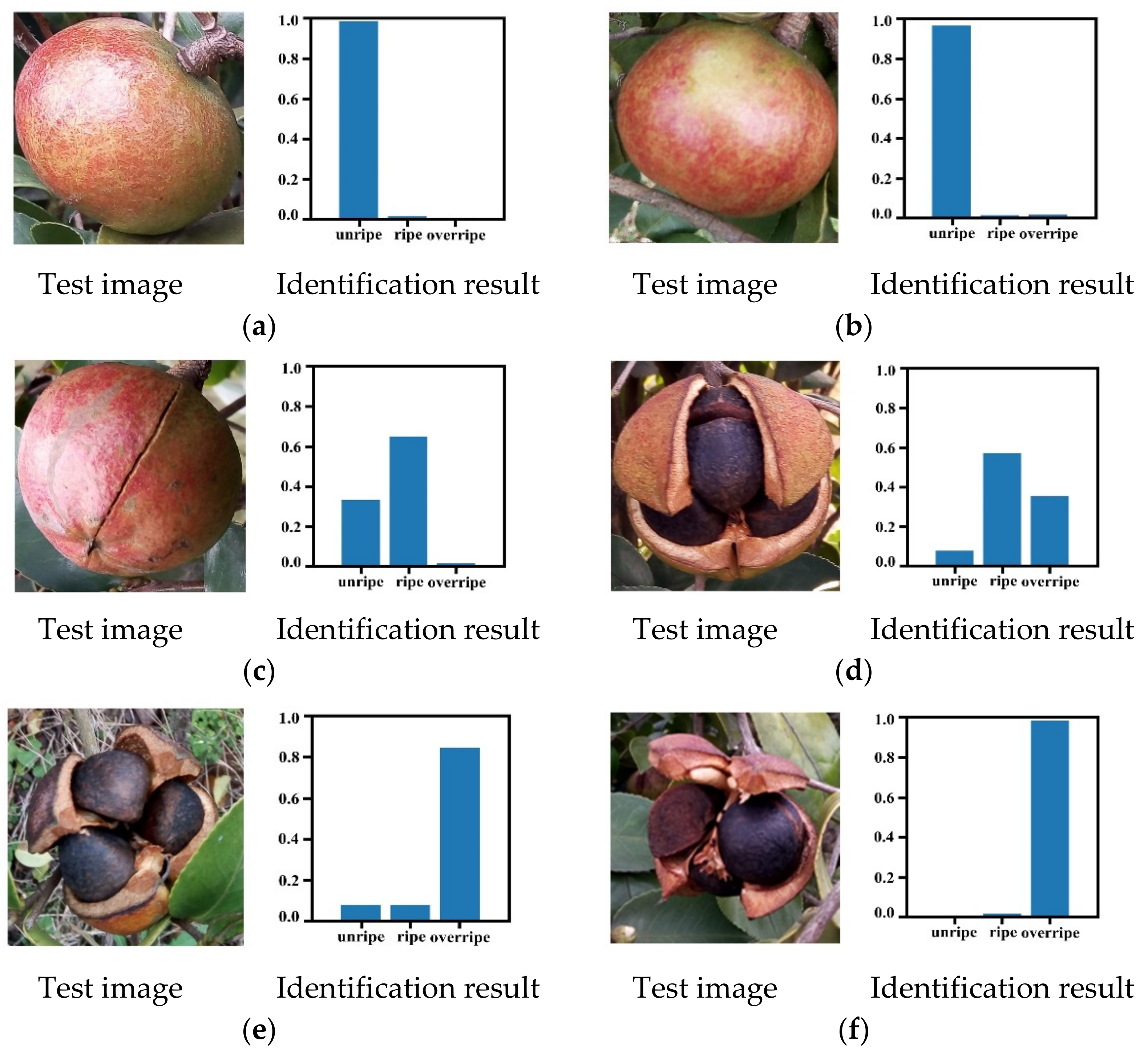
3.4. Maturity Identification Performance Evaluation
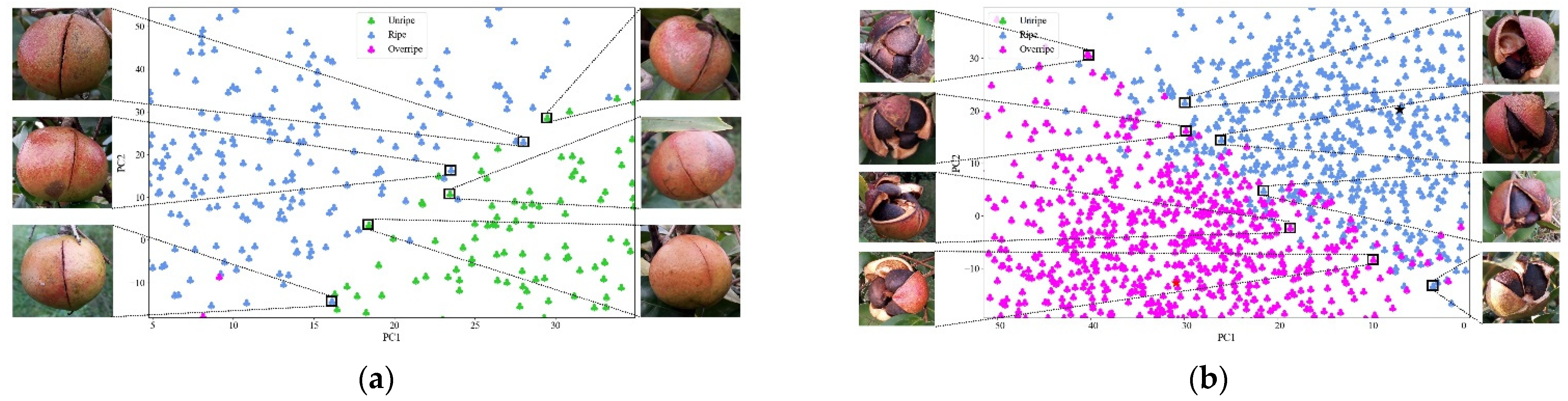
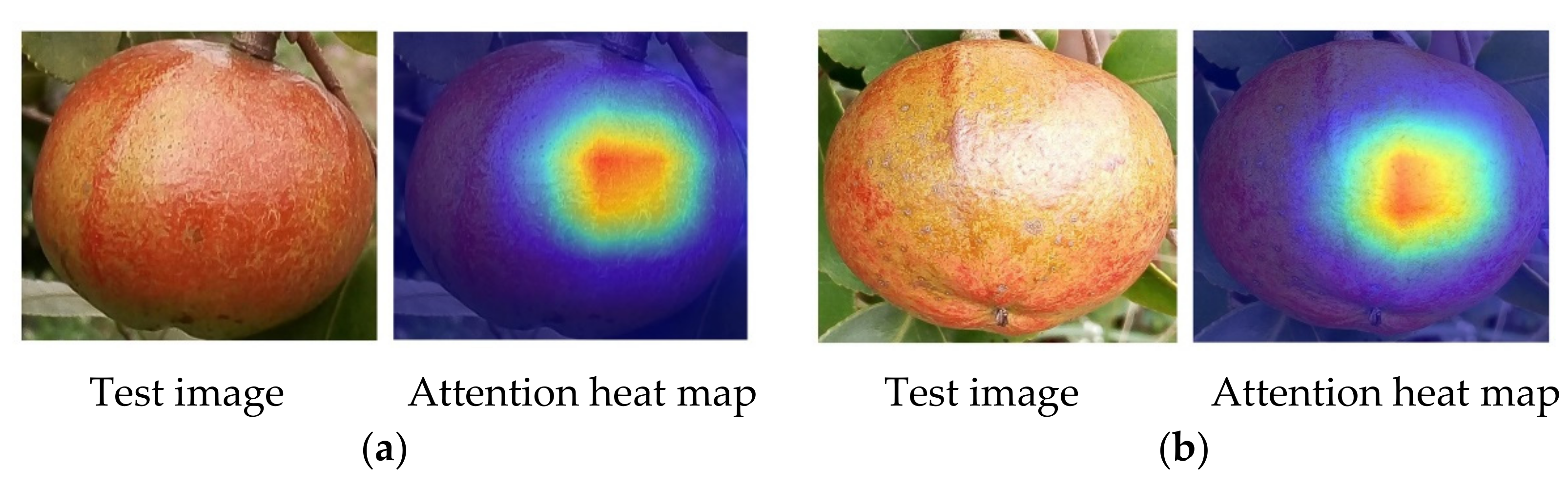
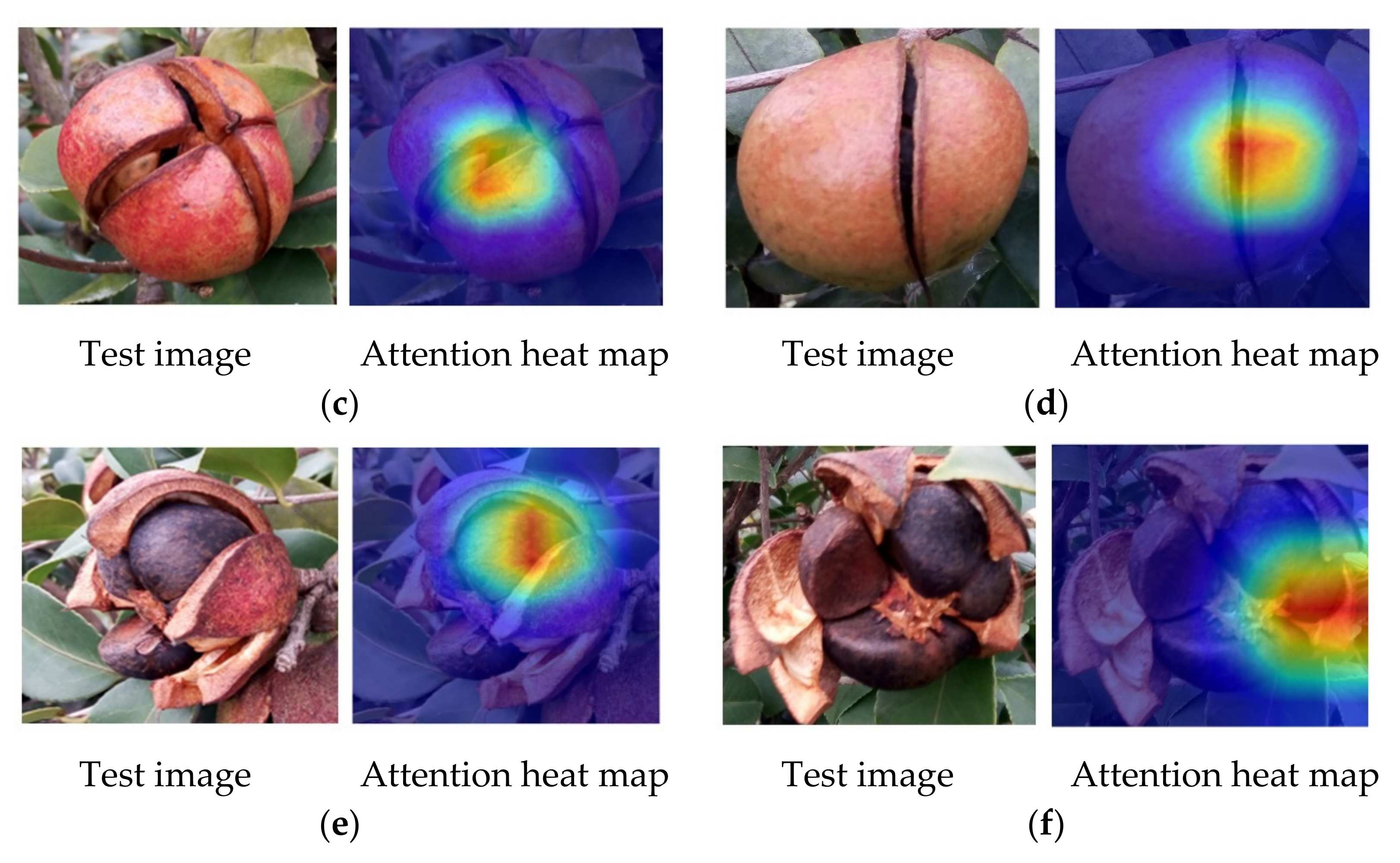
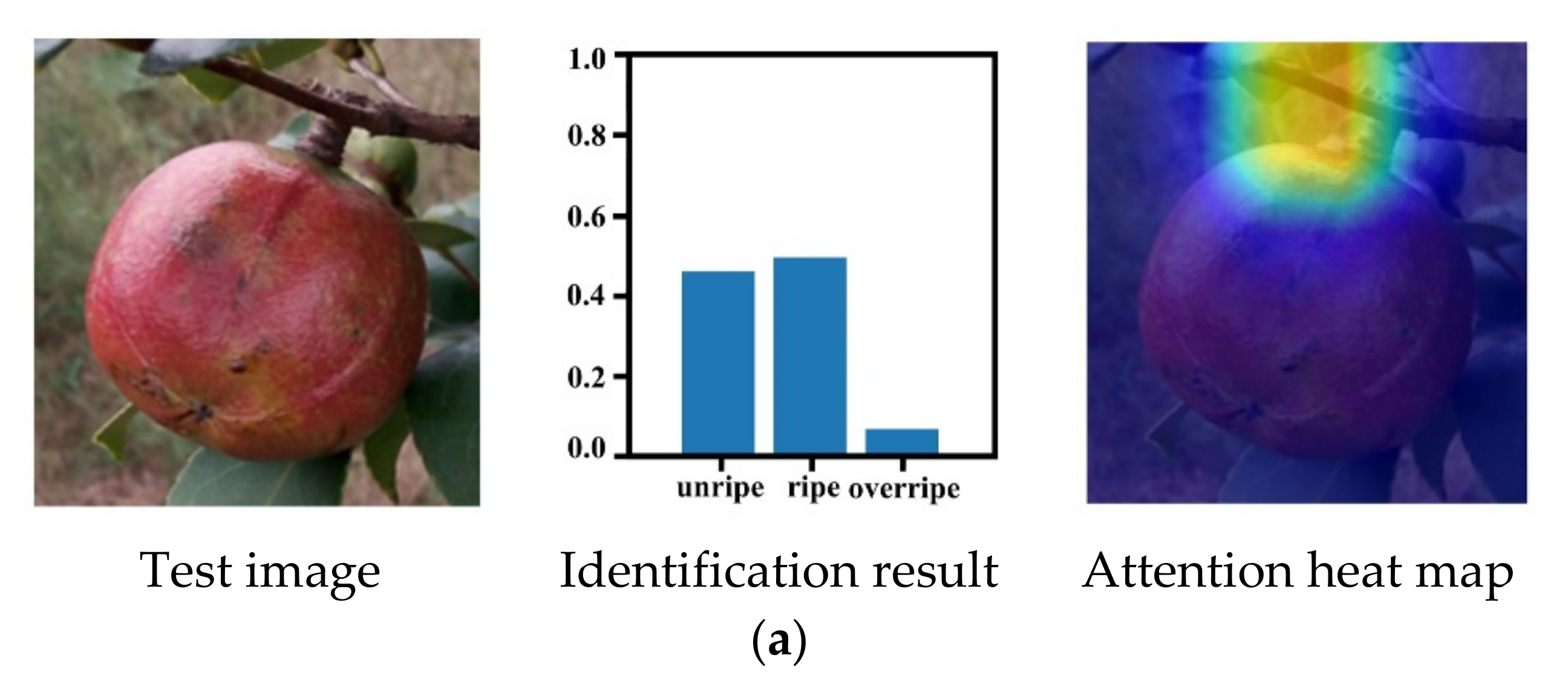
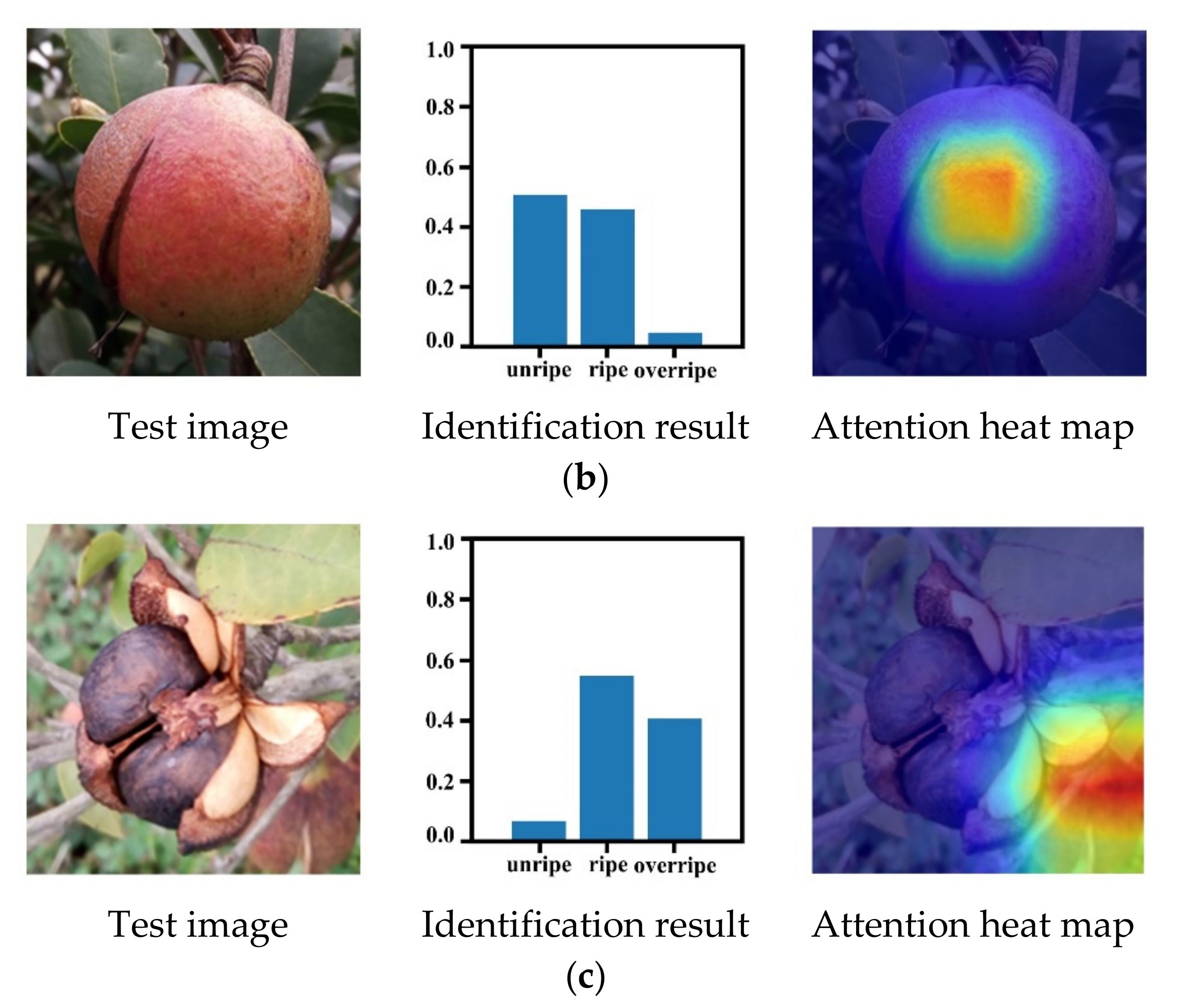
3.5. Maturity Identification Visualization Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azadnia, R.; Kheiralipour, K. Evaluation of hawthorns maturity level by developing an automated machine learning-based algorithm. Ecol. Inform. 2022, 71, 101804. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.; Liu, T.; Zeng, X.; Liang, X.; Wu, X.; Fu, G. Effect of refining degree on the quality changes and lipid oxidation of Camellia (Camellia oleifera) oil during heating. Foods 2022, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, T.; Wang, Y.; Zhou, B.; Yan, L.; Teng, L.; Wang, F.; Chen, L.; He, Y.; Guo, K.; et al. New method for effective identification of adulterated Camellia oil basing on Camellia oleifera-specific DNA. Arab. J. Chem. 2018, 11, 815–826. [Google Scholar] [CrossRef]
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and its By-Products: A Review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Liu, Q.; Liu, Q.; Cao, Z.; Zhang, J.; Kuang, T.; Fang, Y.; Liu, G.; Qian, K.; Fu, J.; et al. Camellia oil (Camellia oleifera Abel.) attenuates CCl4-induced liver fibrosis via suppressing hepatocyte apoptosis in mice. Food Funct. 2020, 11, 4582–4590. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.H.; Chen, S.Y.; Li, Z.Y.; Yen, G.C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free. Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Chen, Z.; Lin, Y.; Wang, S. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. J. Lipids 2016, 2016, 3982486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Wu, S.L.; Ho, C.Y.; Huang, S.M.; Cheng, C.L.; Yen, G.C. Beneficial effects of Camellia Oil (Camellia oleifera Abel.) on ketoprofen-induced gastrointestinal mucosal damage through upregulation of HO-1 and VEGF. J. Agric. Food Chem. 2014, 62, 642–650. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Zhang, L.; Zhang, Y.; Zhou, H.; Wang, Y.; Liu, Y. Photosynthetic carbon and nitrogen metabolism of Camellia oleifera Abel during acclimation to low light conditions. J. Plant Physiol. 2022, 278, 153814. [Google Scholar] [CrossRef]
- Ye, T.; Liu, X.; Liang, X.; Su, S. Comparison of fruit quality differences between two kinds of Camellia oleifera at different maturity. China Oils Fats 2022, 12, 2158. Available online: https://kns.cnki.net/kcms/detail/61.1099.TS.20220518.1932.022.html (accessed on 19 May 2022.).
- Zhu, G.; Liu, H.; Xie, Y.; Liao, Q.; Lin, Y.; Liu, Y.; Liu, Y.; Xiao, H.; Gao, Z.; Hu, S. Postharvest processing and storage methods for Camellia oleifera seeds. Food Rev. Int. 2019, 36, 319–339. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Zhou, J.; Gu, Y.; Tan, X. Comparative study on fruit development and oil synthesis in two cultivars of Camellia oleifera. BMC Plant Biol. 2021, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Song, Q.; Ji, K.; Gong, S.; Wang, L.; Chen, L.; Zhang, J.; Yuan, D. Full-Length transcriptome from Camellia oleifera seed provides insight into the transcript variants involved in oil biosynthesis. J. Agric. Food Chem. 2020, 68, 14670–14683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Liu, M.; Li, J.; Yang, H.; Wang, Z.; Yang, L. Camellia oil trait and DIA quantitative proteomics analyses reveal the impact of harvesting time on the oil content and quality of the late-maturing cultivar C. oleifera ‘Huaxin’. Food Qual. Saf. 2022, 6, fyac055. [Google Scholar] [CrossRef]
- Deng, Q.; Li, J.; Gao, C.; Cheng, J.; Deng, X.; Jiang, D.; Li, L.; Yan, P. New perspective for evaluating the main Camellia oleifera cultivars in China. Sci. Rep. 2020, 10, 20676. [Google Scholar] [CrossRef]
- Iler, A.M.; CaraDonna, P.J.; Forrest, J.R.K.; Post, E. Demographic consequences of phenological shifts in response to climate change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245. [Google Scholar] [CrossRef]
- Xie, H.; Chen, F.; Yin, H.; Peng, G.; You, C.; Qin, P.; Jiang, S.; Guo, X. Characterization and comparison of lipids in Camellia oleifera kernels of XL210 and XL1 based on LC-MS/MS. Reprod. Breed. 2021, 1, 193–203. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Manan, S.; Li, P.; Liu, C.; She, G.; Zhao, S.; Zhao, J. Critical metabolic pathways and SAD/FADs, WRI1s, and DGATs cooperate for high-oleic acid oil production in developing oil tea (Camellia oleifera) seeds. Hortic. Res. 2022, 9, uhac087. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef]
- Song, Q.; Ji, K.; Mo, W.; Wang, L.; Chen, L.; Gao, L.; Gong, W.; Yuan, D. Dynamics of sugars, endogenous hormones, and oil content during the development of Camellia oleifera fruit. Botany 2021, 99, 515–529. [Google Scholar] [CrossRef]
- Liu, X.X.; Luo, X.F.; Luo, K.X.; Liu, Y.L.; Pan, T.; Li, Z.Z.; Duns, G.J.; He, F.L.; Qin, Z.D. Small RNA sequencing reveals dynamic microRNA expression of important nutrient metabolism during development of Camellia oleifera fruit. Int. J. Biol. Sci. 2019, 15, 416–429. [Google Scholar] [CrossRef]
- Wei, T.; Dong, L.; Zhong, S.; Jing, H.; Deng, Z.; Wen, Q.; Li, J. Chemical composition of Camellia chekiangoleosa Hu. seeds during ripening and evaluations of seed oils quality. Ind. Crops Prod. 2022, 177, 114499. [Google Scholar] [CrossRef]
- Sinambela, R.; Mandang, T.; Subrata, I.D.M.; Hermawan, W. Application of an inductive sensor system for identifying ripeness and forecasting harvest time of oil palm. Sci. Hortic. 2020, 265, 109231. [Google Scholar] [CrossRef]
- Misron, N.; Aliteh, N.A.; Harun, N.H.; Tashiro, K.; Sato, T.; Wakiwaka, H. Relative estimation of water content for flat-type inductive-based oil palm fruit maturity sensor. Sensors 2016, 17, 52. [Google Scholar] [CrossRef]
- Saeed, O.M.B.; Sankaran, S.; Shariff, A.R.M.; Shafri, H.Z.M.; Ehsani, R.; Alfatni, M.S.; Hazir, M.H.M. Classification of oil palm fresh fruit bunches based on their maturity using portable four-band sensor system. Comput. Electron. Agric. 2012, 82, 55–60. [Google Scholar] [CrossRef]
- Alamprese, C.; Grassi, S.; Tugnolo, A.; Casiraghi, E. Prediction of olive ripening degree combining image analysis and FT-NIR spectroscopy for virgin olive oil optimisation. Food Control 2021, 123, 107755. [Google Scholar] [CrossRef]
- Greco, M.; Spadafora, N.; Shine, M.; Smith, A.; Muto, A.; Muzzalupo, I.; Chiappetta, A.; Bruno, L.; Muller, C.; Rogers, H.; et al. Identifying volatile and non-volatile organic compounds to discriminate cultivar, growth location, and stage of ripening in olive fruits and oils. J. Sci. Food Agric. 2022, 102, 4500–4513. [Google Scholar] [CrossRef]
- Tugnolo, A.; Giovenzana, V.; Beghi, R.; Grassi, S.; Alamprese, C.; Casson, A.; Casiraghi, E.; Guidetti, R. A diagnostic visible/near infrared tool for a fully automated olive ripeness evaluation in a view of a simplified optical system. Comput. Electron. Agric. 2021, 180, 105887. [Google Scholar] [CrossRef]
- Raj, T.; Hashim, F.H.; Huddin, A.B.; Hussain, A.; Ibrahim, M.F.; Abdul, P.M. Classification of oil palm fresh fruit maturity based on carotene content from Raman spectra. Sci. Rep. 2021, 11, 18315. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Jiang, X.; Zhou, H. Maturity Stage Discrimination of Camellia oleifera fruit using visible and near-infrared hyperspectral imaging. Molecules 2022, 27, 6318. [Google Scholar] [CrossRef]
- Behera, S.K.; Rath, A.K.; Sethy, P.K. Maturity status classification of papaya fruits based on machine learning and transfer learning approach. Inf. Process. Agric. 2021, 8, 244–250. [Google Scholar] [CrossRef]
- Begum, N.; Hazarika, M.K. Maturity detection of tomatoes using transfer learning. Meas. Food 2022, 7, 100038. [Google Scholar] [CrossRef]
- Alfatni, M.S.M.; Khairunniza-Bejo, S.; Marhaban, M.H.B.; Saaed, O.M.B.; Mustapha, A.; Shariff, A.R.M. Towards a real-time oil palm fruit maturity system using supervised classifiers based on feature analysis. Agriculture 2022, 12, 1461. [Google Scholar] [CrossRef]
- Khan, N.; Kamaruddin, M.A.; Sheikh, U.U.; Yusup, Y.; Bakht, M.P. Oil palm and machine learning: Reviewing one decade of ideas, innovations, applications, and gaps. Agriculture 2021, 11, 832. [Google Scholar] [CrossRef]
- Tan, X.J.; Cheor, W.L.; Yeo, K.S.; Leow, W.Z. Expert systems in oil palm precision agriculture: A decade systematic review. J King Saud Univ.-Comput. 2022, 34, 1569–1594. [Google Scholar] [CrossRef]
- Caladcad, J.A.; Cabahug, S.; Catamco, M.R.; Villaceran, P.E.; Cosgafa, L.; Cabizares, K.N.; Hermosilla, M.; Piedad, E., Jr. Determining Philippine coconut maturity level using machine learning algorithms based on acoustic signal. Comput. Electron. Agric. 2020, 172, 105327. [Google Scholar] [CrossRef]
- Sola-Guirado, R.R.; Bayano-Tejero, S.; Aragón-Rodríguez, F.; Bernardi, B.; Benalia, S.; Castro-García, S. A smart system for the automatic evaluation of green olives visual quality in the field. Comput. Electron. Agric. 2020, 179, 105858. [Google Scholar] [CrossRef]
- Khosravi, H.; Saedi, S.I.; Rezaei, M. Real-time recognition of on-branch olive ripening stages by a deep convolutional neural network. Sci. Hortic. 2021, 287, 110252. [Google Scholar] [CrossRef]
- Parvathi, S.; Tamil Selvi, S. Detection of maturity stages of coconuts in complex background using Faster R-CNN model. Biosyst. Eng. 2021, 202, 119–132. [Google Scholar] [CrossRef]
- Mubin, N.A.; Nadarajoo, E.; Shafri, H.Z.M.; Hamedianfar, A. Young and mature oil palm tree detection and counting using convolutional neural network deep learning method. Int. J. Remote Sens. 2019, 40, 7500–7515. [Google Scholar] [CrossRef]
- Wu, B.; Ruan, C.; Han, P.; Ruan, D.; Xiong, C.; Ding, J.; Liu, S. Comparative transcriptomic analysis of high- and low-oil Camellia oleifera reveals a coordinated mechanism for the regulation of upstream and downstream multigenes for high oleic acid accumulation. 3 Biotech 2019, 9, 257. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, J.; Wang, G.; Liang, J. GATCluster: Self-supervised gaussian-attention network for image clustering. In Proceedings of the European Conference on Computer Vision, Glasgow, UK, 23–28 August 2020; pp. 735–751. [Google Scholar] [CrossRef]
- Mehdi, N.; Paolo, F. Unsupervised learning of visual representations by solving jigsaw puzzles. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 8–16 October 2016; pp. 69–84. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Wang, K.; Zhang, M.; Li, Z. Surface defect detection of fresh-cut cauliflowers based on convolutional neural network with transfer learning. Foods 2022, 11, 2915. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Su, Y.; Xie, B.; Gu, Q.; Zheng, K. Quantitative extraction and analysis of pear fruit spot phenotypes based on image recognition. Comput. Electron. Agric. 2021, 190, 106474. [Google Scholar] [CrossRef]
- Al-Dakheel, A.J.; Hussain, M.I.; Abdulrahman, A.; Abdullah, A. Long term assessment of salinity impact on fruit yield in eighteen date palm varieties. Agric. Water Manag. 2022, 269, 107683. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Sun, J.; Geng, J.; Fan, R.; Kang, Z. Distinguishing different varieties of oolong tea by fluorescence hyperspectral technology combined with chemometrics. Foods 2022, 11, 2344. [Google Scholar] [CrossRef]
- Aytekin, C.; Ni, X.; Cricri, F.; Aksu, E. Clustering and Unsupervised Anomaly Detectionwith l2 Normalized Deep Auto-Encoder Representations. In Proceedings of the 2018 International Joint Conference on Neural Networks (IJCNN), Rio de Janeiro, Brazil, 8–13 July 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Jegou, H.; Perronnin, F.; Douze, M.; Sanchez, J.; Perez, P.; Schmid, C. Aggregating local image descriptors into compact codes. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 34, 1704–1716. [Google Scholar] [CrossRef]
- Wang, C.; Xu, R.; Xu, S.; Meng, W.; Zhang, X. CNDesc: Cross normalization for local descriptors learning. IEEE Trans Multimed. 2022, in press. [Google Scholar] [CrossRef]
- Jain, M.; Kaur, G.; Saxena, V. A K-Means clustering and SVM based hybrid concept drift detection technique for network anomaly detection. Expert Syst. Appl. 2022, 193, 116510. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, Q. PSO and K-means-based semantic segmentation toward agricultural products. Future Gener. Comput. Syst. 2022, 126, 82–87. [Google Scholar] [CrossRef]
- Fredes, C.; Valero, C.; Diezma, B.; Mora, M.; Naranjo-Torres, J.; Wilson, M.; Delgadillo, G. A model based on clusters of similar color and NIR to estimate oil content of single olives. Foods 2021, 10, 609. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlation with increased levels of membrane permeability and lipid peroxidation and increased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Septiarini, A.; Sunyoto, A.; Hamdani, H.; Kasim, A.-A.; Utaminingrum, F.; Hatta, H.-R. Machine vision for the maturity classification of oil palm fresh fruit bunches based on color and texture features. Sci. Hortic. 2021, 286, 110245. [Google Scholar] [CrossRef]
- Osako, Y.; Yamane, H.; Lin, S.-Y.; Chen, P.-A.; Tao, R. Cultivar discrimination of litchi fruit images using deep learning. Sci. Hortic. 2020, 269, 109360. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Sun, Z.; Zheng, Y.; Su, S.; Chen, F. Identification of oil tea (Camellia oleifera C.Abel) cultivars using EfficientNet-B4 CNN model with attention mechanism. Forests 2021, 13, 1. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual explanations from deep networks via gradient-based localization. Int. J. Comput. Vis. 2019, 128, 336–359. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, H.; Zhou, H.; Xu, L.; Ju, H.; Wang, Y. Maturity detection of Camellia oleifera by hyperspectral imaging. Food Sci. 2022, 43, 324–331. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, H.; Liu, Z.; Liu, L. Changes of phenotypic characters and oil content of Camellia oleifera during fruit development. China Oils Fats 2021, 46, 102–107. [Google Scholar] [CrossRef]
- Hongzhe, J.; Wei, W.; Bo, W.; Xuan, C.; Xin, Z. Design and experimental research of shelling and sorting machine for fresh Camellia oleifera fruit based on physical properties. In Proceedings of the 2018 ASABE Annual International Meeting, St. Joseph, MI, USA, 19 July–1 August 2018; p. 1800815. [Google Scholar] [CrossRef]
- Ma, L.; Zhong, H.; Chen, Y.; Peng, S.; Zhu, N.; Chen, L.; Wang, R. The effect of post—Harvest treatment of Camellia Fruit on quality of Camellia Seed. J. Chin. Cereals Oils Assoc. 2014, 12, 73–76. Available online: http://www.cnki.net/kcms/detail/11.2864.TS.20141210.1100.001.html (accessed on 10 December 2014).
- Yue, X.-Q.; Shang, Z.-Y.; Yang, J.-Y.; Huang, L.; Wang, Y.-Q. A smart data-driven rapid method to recognize the strawberry maturity. Inf. Process. Agric. 2020, 7, 575–584. [Google Scholar] [CrossRef]
- Mazen, F.M.A.; Nashat, A.A. Ripeness classification of bananas using an artificial neural network. Arab. J. Sci. Eng. 2019, 44, 6901–6910. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Y.; Zheng, Y.; Su, S.; Chen, F. Bilinear attention network for image-based fine-grained recognition of oil tea (Camellia oleifera Abel.) cultivars. Agronomy 2022, 12, 1846. [Google Scholar] [CrossRef]



| Dataset Category | Camellia oleifera Fruit Images | Camellia oleifera Fruit Samples |
|---|---|---|
| TN | 2628 | 160 |
| Unripe_TS | 50 | 50 |
| Ripe_TS | 60 | 60 |
| Overripe_TS | 50 | 50 |
| Physical Property | Maturity Stage | ||
|---|---|---|---|
| Unripe | Ripe | Overripe | |
| Transverse diameter (mm) | 46.05 ± 1.54 a | 47.31 ± 1.65 a | 49.32 ± 1.57 a |
| Vertical diameter (mm) | 38.78 ± 1.33 a | 40.10 ± 1.95 a | 40.64 ± 1.71 a |
| Dry seed weight (g) | 8.06 ± 0.41 c | 12.44 ± 1.02 a | 11.05 ± 0.38 b |
| Moisture content (%) | 69.55 ± 3.74 a | 58.79 ± 2.13 b | 48.15 ± 0.62 c |
| Quality Property | Maturity Stage | ||
|---|---|---|---|
| Unripe | Ripe | Overripe | |
| Seed oil content (%) | 39.12 ± 0.99 c | 46.05 ± 0.49 a | 44.42 ± 0.53 b |
| Seed-soluble protein content (%) | 5.21 ± 0.22 c | 5.61 ± 0.16 b | 6.11 ± 0.07 a |
| Seed-soluble sugar content (%) | 25.69 ± 1.16 a | 19.28 ± 3.85 b | 13.75 ± 0.93 c |
| Seed starch content (%) | 3.31 ± 0.55 a | 2.30 ± 0.24 b | 1.54 ± 0.82 c |
| Maturity | Evaluation Index | |||
|---|---|---|---|---|
| Prec (%) | Rec (%) | F1score (%) | OAcc (%) | |
| Unripe | 94.12 | 96.00 | 95.05 | 91.25 |
| Ripe | 89.66 | 86.67 | 88.14 | |
| Overripe | 90.20 | 92.00 | 91.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Shen, D.; Wang, R.; Zheng, Y.; Su, S.; Chen, F. Maturity Grading and Identification of Camellia oleifera Fruit Based on Unsupervised Image Clustering. Foods 2022, 11, 3800. https://doi.org/10.3390/foods11233800
Zhu X, Shen D, Wang R, Zheng Y, Su S, Chen F. Maturity Grading and Identification of Camellia oleifera Fruit Based on Unsupervised Image Clustering. Foods. 2022; 11(23):3800. https://doi.org/10.3390/foods11233800
Chicago/Turabian StyleZhu, Xueyan, Deyu Shen, Ruipeng Wang, Yili Zheng, Shuchai Su, and Fengjun Chen. 2022. "Maturity Grading and Identification of Camellia oleifera Fruit Based on Unsupervised Image Clustering" Foods 11, no. 23: 3800. https://doi.org/10.3390/foods11233800
APA StyleZhu, X., Shen, D., Wang, R., Zheng, Y., Su, S., & Chen, F. (2022). Maturity Grading and Identification of Camellia oleifera Fruit Based on Unsupervised Image Clustering. Foods, 11(23), 3800. https://doi.org/10.3390/foods11233800








