Innovative Process for Dried Caper (Capparis spinosa L.) Powder Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Production Processes
- (1)
- BrS (Rt)—fresh buds were placed in brine (18% NaCl) at Room Temperature (~25 °C) for 3 days;
- (2)
- BrS (60 °C)—fresh buds were placed in brine (18% NaCl) at 60 °C for 6 h;
- (3)
- DrS (10d)—fresh buds were mixed with 40% of dry NaCl for 10 days;
- (4)
- DrS (40d)—fresh buds mixed with 40% of dry NaCl for 10 days, and then the brine that had formed was discarded and 25% of dry NaCl was added and mixed for 30 days.
2.3. Drying Procedures
2.4. Moisture Content
2.5. Chlorophylls and Carotenoids
2.6. Total Phenolics and Flavonoids
2.7. Antioxidant Capacity
2.8. FTIR Analysis
2.9. Volatile Analysis Aroma Compounds Analysis
2.10. Sensory Analysis
2.11. Consumer Acceptability Test
2.12. Statistical Analysis
3. Results and Discussion
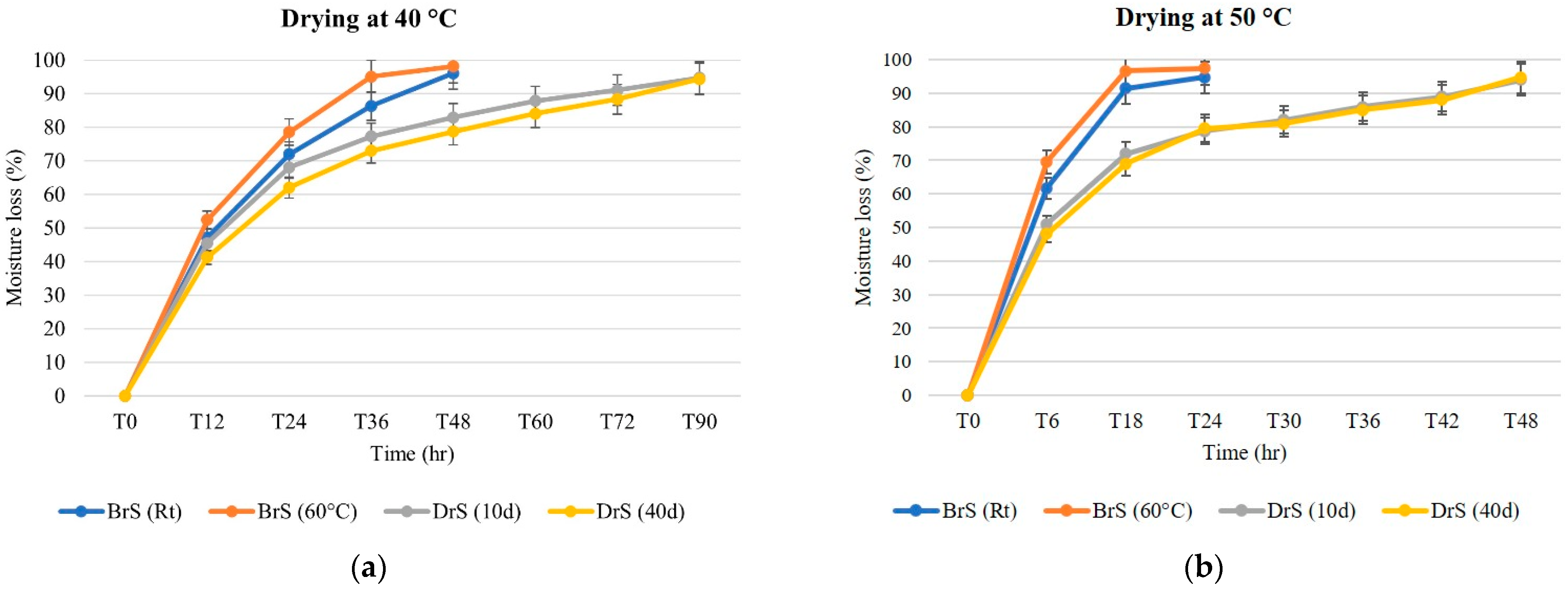
3.1. Drying Kinetics
3.2. Chemical Data
3.2.1. Chlorophylls and Carotenoids
3.2.2. Polyphenols, Flavonoids, and Antioxidant Capacity
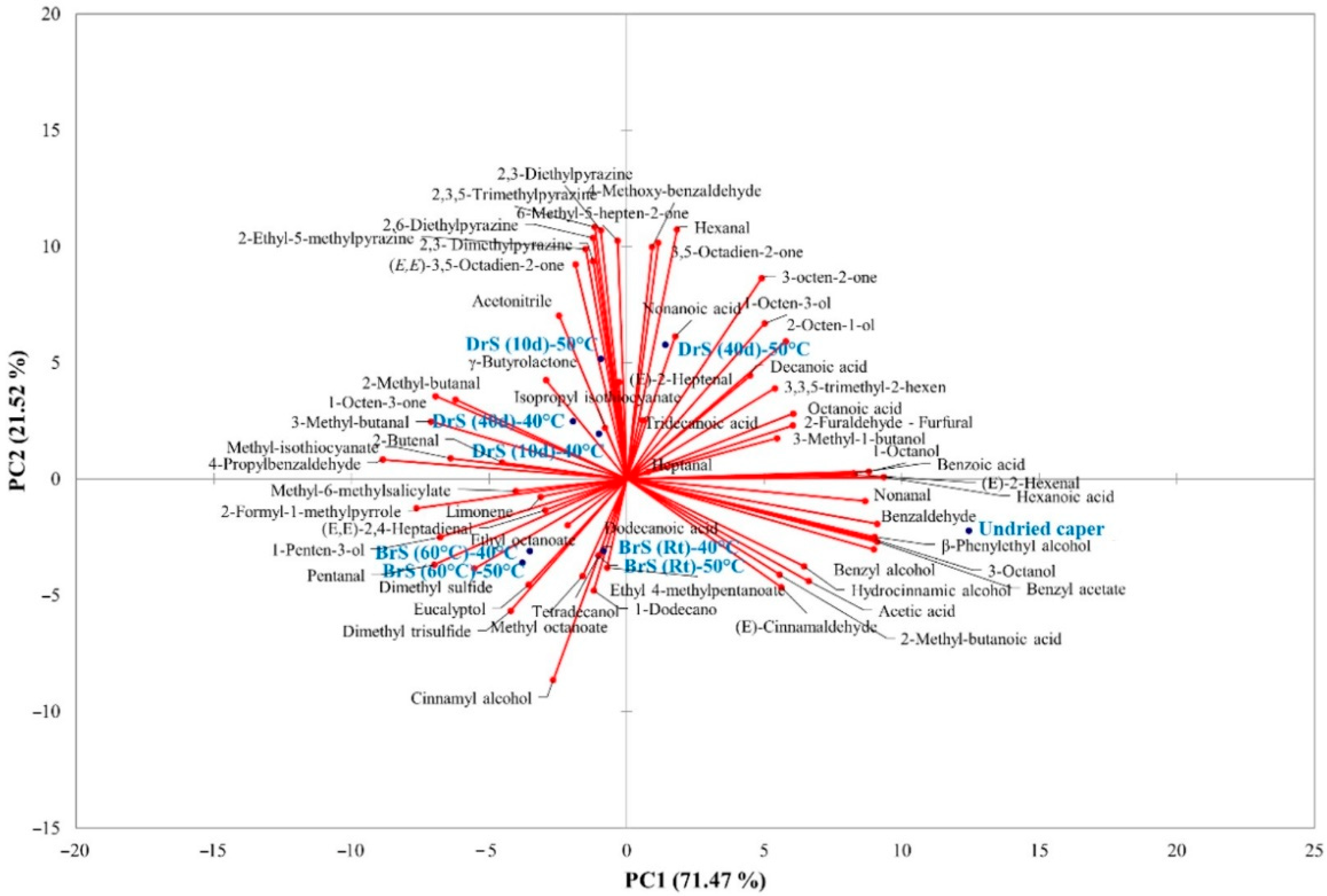
3.2.3. Aroma Compounds
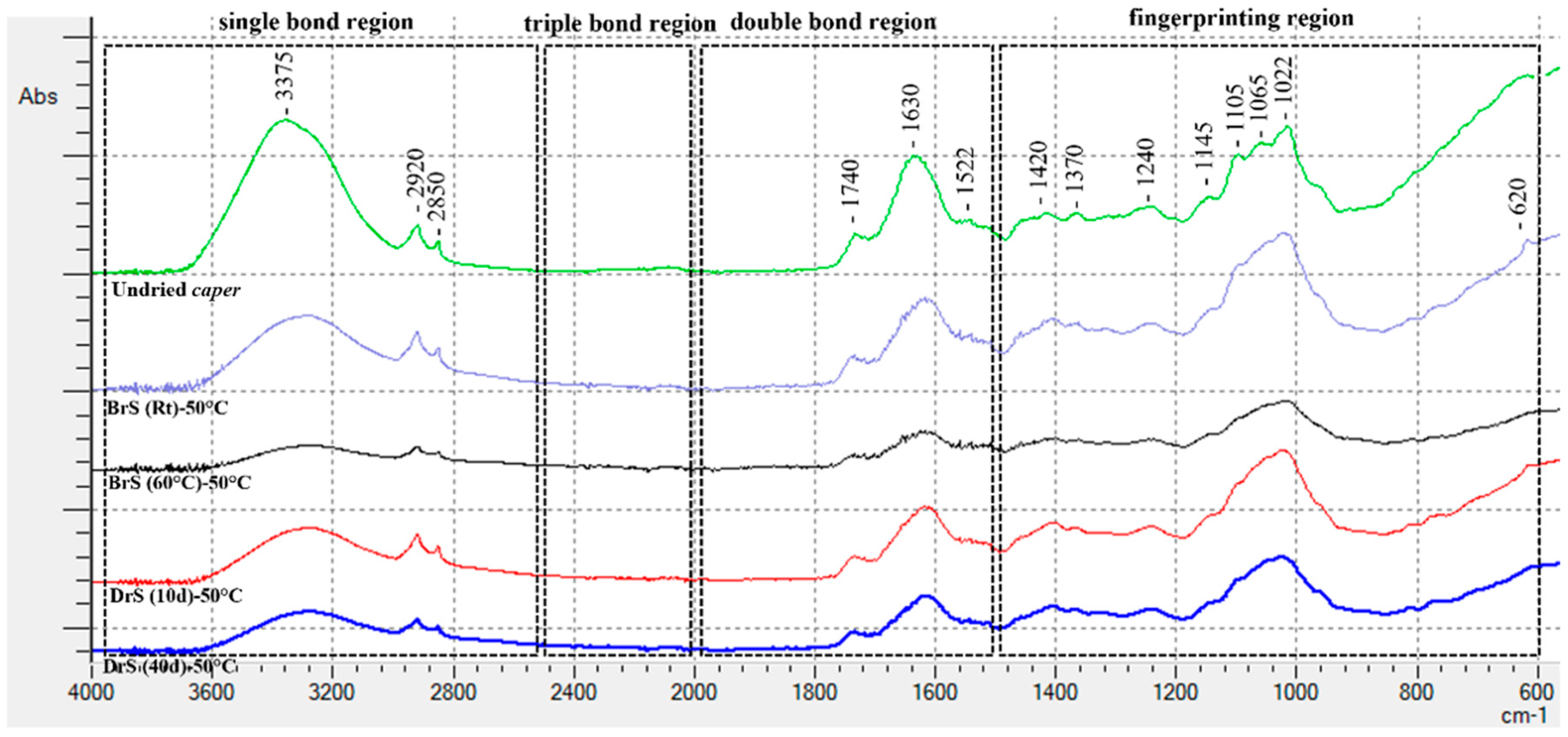
3.2.4. FTIR
3.3. Sensory Analysis
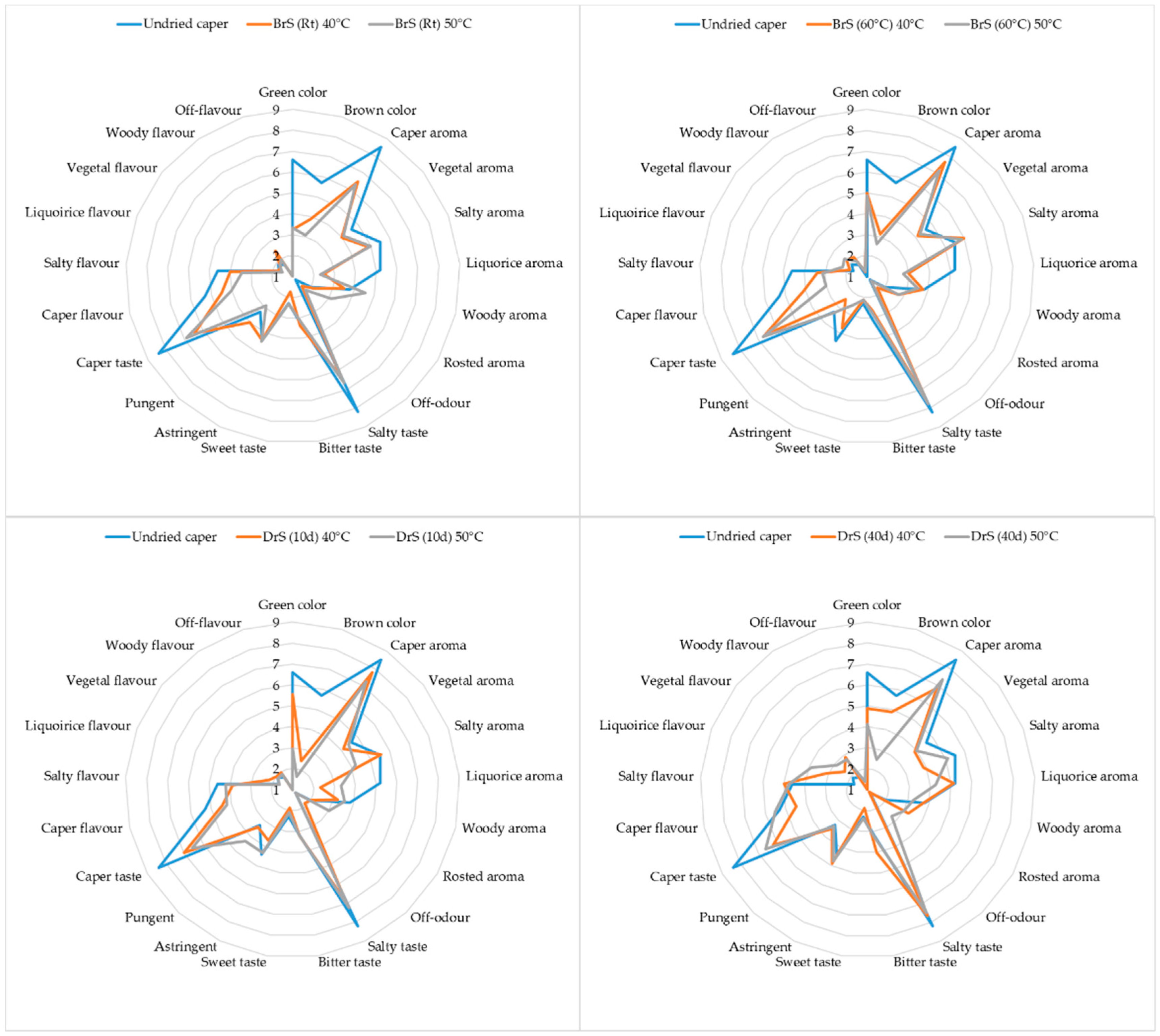
3.3.1. Descriptive Sensory Data
3.3.2. Sensory Acceptability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Air-Dried Food Market Size, Share & Trends Analysis Report by Product (Coffee Beans, Fruits & Vegetables, Meat, Herbs), by Application (Household, Commercial), by Form, by Region, and Segment Forecasts, 2020–2027; Grand View Research Inc. Publisher: San Francisco, CA, USA, 2020; Available online: https://www.marketresearch.com/Grand-View-Research-v4060/Air-dried-Food-Size-Share-30774080/ (accessed on 30 July 2022).
- Murcia, M.A.; Jiménez-Monreal, A.M.; García-Diz, L.; Carmona, M.; Maggi, L.; Martínez-Tomé, M. Antioxidant activity of minimally processed (in modified atmospheres), dehydrated and ready-to-eat vegetables. Food Chem. Toxicol. 2009, 47, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.C.; Petit, J.; Zimmer, D.; Djantou, E.B.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Roratto, T.B.; Monteiro, R.L.; Carciofi, B.A.; Laurindo, J.B. An innovative hybrid-solar-vacuum dryer to produce high-quality dried fruits and vegetables. LWT-Food Sci. Technol. 2021, 140, 110777. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Recent developments in key processing techniques for oriental spices/herbs and condiments: A review. Food Rev. Int. 2020, 38, 1791–1811. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A. Influence of drying technologies on the aroma of Sicilian red garlic. LWT-Food Sci. Technol. 2019, 104, 180–185. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Han, X.; Ni, Y.; Zhao, D.; Hao, J. Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT-Food Sci. Technol. 2019, 107, 236–242. [Google Scholar] [CrossRef]
- Majumder, P.; Sinha, A.; Gupta, R.; Sablani, S.S. Drying of Selected Major Spices: Characteristics and Influencing Parameters, Drying Technologies, Quality Retention and Energy Saving, and Mathematical Models. Food Bioprocess Technol. 2021, 14, 1028–1054. [Google Scholar] [CrossRef]
- Condurso, C.; Mazzaglia, A.; Tripodi, G.; Cincotta, F.; Dima, G.; Lanza, M.C.; Verzera, A. Sensory analysis and head-space aroma volatiles for the characterization of capers from different geographic origin. J. Essent. Oil Res. 2016, 28, 185–192. [Google Scholar] [CrossRef]
- Dziki, D. Recent Trends in Pretreatment of Food before Freeze-Drying. Processes 2020, 8, 1661. [Google Scholar] [CrossRef]
- Mandal, S.; Dahuja, A.; Kar, A.; Santha, I.M. In vitro kinetics of soybean lipoxygenase with combinatorial fatty substrates and its functional significance in off flavour development. Food Chem. 2014, 146, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 2020/624 of 30 April 2020, Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/624/oj (accessed on 30 July 2022).
- AOAC. Method 14.003. In Official Methods of Analysis of the Association of the Official Analytical Chemists, 13th ed.; Howitz, W., Ed.; Association of the Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Tayiroğlu, B.; İncedayı, B. Nutritional potential characterization and bioactive properties of caper products. J. Food Process. Preserv. 2021, 45, e14670. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research. Advances in Agricultural Biotechnology; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 2, pp. 9–12. [Google Scholar]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Abi-Khattar, A.M.; Rajha, H.N.; Abdel-Massih, R.M.; Habchi, R.; Maroun, R.G.; Debs, E.; Louka, N. “Intensification of Vaporization by Decompression to the Vacuum” (IVDV), a novel technology applied as a pretreatment to improve polyphenols extraction from olive leaves. Food Chem. 2021, 342, 128236. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Characterization and ageing monitoring of Marsala dessert wines by a rapid FTIR-ATR method coupled with multivariate analysis. Eur. Food Res. Technol. 2018, 244, 1073–1081. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- Aksay, O.; Selli, S.; Kelebek, H. LC-DAD-ESI-MS/MS-based assessment of the bioactive compounds in fresh and fermented caper (Capparis spinosa) buds and berries. Food Chem. 2021, 337, 127959. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Karakurt, Y.; Unlu, H.; Unlu, H.; Padem, H. The influence of foliar and soil fertilization of humic acid on yield and quality of pepper. Acta Agric. Scand. B—Soil Plant Sci. 2009, 59, 233–237. [Google Scholar] [CrossRef]
- İnanç, A.L. Chlorophyll: Structural Properties, Health Benefits and Its Occurrence in Virgin Olive Oils. Acad. Food J. 2011, 9, 26–32. [Google Scholar]
- Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Wang, C.K.; Novellino, E.; Mahomoodally, M.F.; Mollica, A. Impact of different geographical locations on varying profile of bioactives and associated functionalities of caper (Capparis spinosa L.). Food Chem. Toxicol. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Mansour, R.B.; Jilani, I.B.H.; Bouaziz, M.; Gargouri, B.; Elloumi, N.; Attia, H.; Ghrabi-Gammar, Z.; Lassoued, S. Phenolic contents and antioxidant activity of ethanolic extract of Capparis spinosa. Cytotechnology 2016, 68, 135–142. [Google Scholar] [CrossRef]
- Tlili, N.; Mejri, H.; Anouer, F.; Saadaoui, E.; Khaldi, A.; Nasri, N. Phenolic profile and antioxidant activity of Capparis spinosa seeds harvested from different wild habitats. Ind. Crop. Prod. 2015, 76, 930–935. [Google Scholar] [CrossRef]
- Durmaz, E.; Sumnu, G.; Sahin, S. Microwave-assisted extraction of phenolic compounds from caper. Sep. Sci. Technol. 2015, 50, 1986–1992. [Google Scholar] [CrossRef]
- Ozbek Yazici, S.; Ozmen, İ. Ultrasound assisted extraction of phenolic compounds from Capparis Ovata var canescens fruit using deep eutectic solvents. J. Food Process. Preserv. 2022, 46, e16286. [Google Scholar] [CrossRef]
- Romeo, V.; Ziino, M.; Giuffrida, D.; Condurso, C.; Verzera, A. Flavour profile of capers (Capparis spinosa L.) from the Eolian Archipelago by HS-SPME/GC–MS. Food Chem. 2007, 101, 1272–1278. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of Aroma-Active Compounds, Phenolics, and Antioxidant Properties in Fresh and Fermented Capers (Capparis spinosa) by GC-MS-Olfactometry and LC-DAD-ESI-MS/MS. J. Food Sci. 2019, 84, 2449–2457. [Google Scholar] [CrossRef]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT-Food Sci. Technol. 2020, 118, 108718. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Fung, M.F.K.; Senterman, M.K.; Mikhael, N.Z.; Lacelle, S.; Wong, P.T. Pressure-tuning fourier transform infrared spectroscopic study of carcinogenesis in human endometrium. Biospectroscopy 1996, 2, 155–165. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Chegel, V.I.; Gridina, N.Y.; Repnytska, O.P.; Shirshov, Y.M.; Tryndiak, V.P.; Todor, I.M.; Solyanik, G.I. Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study. Biopolymers 2002, 67, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Meijer, G.W.; Lähteenmäki, L.; Stadler, R.H.; Weiss, J. Issues surrounding consumer trust and acceptance of existing and emerging food processing technologies. Crit. Rev. Food Sci. 2021, 61, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Golder, P.N.; Mitra, D.; Moorman, C. What is quality? An integrative framework of processes and states. J. Mark. 2012, 76, 1–23. [Google Scholar] [CrossRef]
| BrS (Rt) 40 °C | BrS (Rt) 50 °C | 40 °C vs. 50 °C | BrS (60 °C) 40 °C | BrS (60 °C) 50 °C | 40 °C vs. 50 °C | DrS (10d) 40 °C | DrS (10d) 50 °C | 40 °C vs. 50 °C | DrS (40d) 40 °C | DrS (40d) 50 °C | 40 °C vs. 50 °C | Undried Caper | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll a e | 28.21 ± 1.25 a | 22.15 ± 3.56 A | * | 22.53 ± 1.06 a | 18.62 ± 2.39 B | * | 22.27 ± 3.43 a | 22.36 ± 1.4 A | ns | 11.49 ± 0.67 b | 11.80 ± 0.92 C | ns | 25.85 ± 1.98 a,A |
| Chlorophyll b e | 3.98 ± 0.73 | 3.21 ± 0.54 B | ns | 4.36 ± 0.56 | 4.56 ± 0.12 A | ns | 4.56 ± 0.29 | 4.67 ± 0.36 A | ns | 4.45 ± 0.12 | 2.33 ± 0.34 C | * | 4.01 ± 0.45 A |
| Chlorophylls e | 32.19 ± 1.83 a | 25.36 ± 0.97 A | * | 26.90 ± 3.23 a | 23.17 ± 1.72 A | ns | 26.83 ± 3.85 a | 27.03 ± 1.72 A | ns | 15.94 ± 0.87 b | 14.13 ± 0.75 B | ns | 29.86 ± 1.69 a,A |
| Carotenoids e | 9.44 ± 0.26 a | 5.80 ± 0.42 B | * | 4.11 ± 0.18 b | 9.78 ± 0.78 A | ** | 4.97 ± 0.53 b | 4.99 ± 0.18 B | ns | 1.08 ± 0.02 c | 1.38 ± 0.16 C | ns | 8.75 ± 0.51 a,A |
| Polyphenols f | 3.42 ± 0.06 a | 3.29 ± 0.48 A | ns | 3.40 ± 0.27 a | 3.54 ± 0.12 A | ns | 3.14 ± 0.23 a | 3.06 ± 0.21 A | ns | 2.77 ± 0.23 b | 2.39 ± 0.17 B | ns | 3.49 ± 0.47 a,A |
| Flavonoids g | 87.00 ± 1.95 b | 149.21 ± 4.91 A | ** | 99.98 ± 6.85 a | 117.42 ± 8.84 B | * | 78.08 ± 2.04 c | 73.43 ± 4.86 C | ns | 69.36 ± 3.98 d | 53.66 ± 1.03 C | ns | 71.24 ± 3.24 c,C |
| AC (%) h | 80.66 ± 2.35 b | 77.33 ± 3.38 B | ns | 83.33 ± 4.58 a | 75.55 ± 4.92 B | * | 86.88 ± 3.94 a | 86.66 ± 2.12 A | ns | 81.55 ± 2.37 b | 80.88 ± 2.76 B | ns | 81.77 ± 5.18 b,B |
| LRI | BrS (Rt) 40 °C | BrS (Rt) 50 °C | 40 °C vs. 50 °C | BrS (60 °C) 40 °C | BrS (60 °C) 50 °C | 40 °C vs. 50 °C | DrS (10d) 40 °C | DrS (10d) 50 °C | 40 °C vs. 50 °C | DrS (40d) 40 °C | DrS (40d) 50 °C | 40 °C vs. 50 °C | Undried Caper | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||||||||
| 2-Methyl-butanal | 917 | 3.65 ± 0.15 b | 5.44 ± 0.27 A | * | 2.55 ± 0.11 b | 3.84 ± 0.26 B | * | 2.78 ± 0.17 b | 3.77 ± 0.24 B | * | 6.04 ± 0.37 a | 4.70 ± 0.31 A | * | 0.10 ± 0.02 c,C |
| 3-Methyl-butanal | 920 | 4.21 ± 0.25 b | 6.30 ± 0.28 A | * | 5.26 ± 0.46 b | 7.87 ± 0.81 A | * | 4.48 ± 0.29 b | 4.95 ± 0.31 B | ns | 9.92 ± 0.73 a | 6.52 ± 0.43 A | * | 0.39 ± 0.02 c,C |
| Pentanal | 983 | 1.59 ± 0.08 b | 2.22 ± 0.21 B | * | 4.16 ± 0.29 a | 3.91 ± 0.26 A | ns | 2.04 ± 0.09 b | 1.73 ± 0.09 B | * | 2.76 ± 0.19 b | 1.85 ± 0.84 B | * | 0.86 ± 0.08 c,C |
| 2-Butenal | 1044 | 0.41 ± 0.01 b | 0.34 ± 0.02 A | ns | 0.84 ± 0.05 a | 0.40 ± 0.03 A | * | 0.40 ± 0.02 b | 0.35 ± 0.02 A | ns | 0.89 ± 0.06 a | 0.46 ± 0.05 A | * | 0.26 ± 0.03 c,B |
| Hexanal | 1081 | 1.43 ± 0.09 b | 2.12 ± 0.16 C | * | 1.86 ± 0.09 b | 1.19 ± 0.13 C | * | 3.04 ± 0.27 a | 5.12 ± 0.36 B | * | 3.96 ± 0.27 a | 7.12 ± 0.40 A | * | 2.90 ± 0.21 a,C |
| Heptanal | 1185 | 0.56 ± 0.03 | 0.58 ± 0.04 A | ns | 0.39 ± 0.02 | 0.21 ± 0.03 B | * | 0.42 ± 0.02 | 0.57 ± 0.03 A | ns | 0.35 ± 0.02 | 0.34 ± 0.02 B | ns | 0.41 ± 0.02 B |
| (E)-2-Hexenal | 1221 | 0.22 ± 0.01 c | 0.24 ± 0.02 C | ns | 0.44 ± 0.02 b | 0.31 ± 0.02 C | ns | 0.23 ± 0.01 c | 0.34 ± 0.02 C | ns | 0.41 ± 0.03 b | 0.53 ± 0.03 B | ns | 0.97 ± 0.04 a,A |
| (E)-2-Heptenal | 1326 | 1.03 ± 0.12 | 0.99 ± 0.06 | ns | 1.62 ± 0.11 | 1.05 ± 0.13 | * | 0.79 ± 0.08 | 1.36 ± 0.07 | * | 1.35 ± 0.18 | 1.58 ± 0.12 | ns | 1.18 ± 0.09 |
| Nonanal | 1393 | 0.57 ± 0.34 b | 0.72 ± 0.04 B | * | 0.59 ± 0.03 b | 0.72 ± 0.05 B | ns | 0.73 ± 0.03 b | 0.88 ± 0.06 B | ns | 0.70 ± 0.04 b | 0.65 ± 0.08 B | ns | 1.58 ± 0.13 a,A |
| (E,E)-2,4-Heptadienal | 1494 | 0.31 ± 0.26 b | 0.28 ± 0.02 B | ns | 0.74 ± 0.05 a | 0.67 ± 0.04 A | ns | 0.34 ± 0.02 b | 0.34 ± 0.02 B | ns | 0.55 ± 0.04 b | 0.54 ± 0.03 A | ns | 0.41 ± 0.05 b,B |
| All | 13.99 | 19.23 | 18.44 | 20.16 | 15.24 | 19.41 | 26.93 | 24.29 | 9.04 | |||||
| Alcohols | ||||||||||||||
| 1-Penten-3-ol | 1157 | 0.64 ± 0.04 b | 0.71 ± 0.04 A | ns | 0.82 ± 0.03 a | 0.90 ± 0.06 A | ns | 0.30 ± 0.02 c | 0.63 ± 0.04 A | ** | 0.50 ± 0.03 b | 0.69 ± 0.07 A | ns | 0.21 ± 0.01 c,B |
| 3-Methyl-1-butanol | 1204 | 0.13 ± 0.02 c | 0.29 ± 0.02 A | * | 0.16 ± 0.02 c | - B | * | 0.23 ± 0.03 b | 0.27 ± 0.02 A | ns | 0.21 ± 0.02 b | 0.16 ± 0.02 A | ns | 0.31 ± 0.02 a,A |
| 3-Octanol | 1388 | - b | - B | ns | - b | - B | ns | - b | - B | ns | - b | - B | ns | 0.30 ± 0.02 a,A |
| 1-Octen-3-ol | 1444 | 1.15 ± 0.16 b | 1.07 ± 0.23 C | ns | 0.72 ± 0.04 c | 0.47 ± 0.03 D | * | 0.55 ± 0.03 c | 2.03 ± 0.14 B | ** | 0.84 ± 0.06 b | 3.92 ± 0.21 A | ** | 2.30 ± 0.17 a,B |
| 1-Octanol | 1551 | 0.18 ± 0.02 a | 0.26 ± 0.02 A | * | 0.26 ± 0.02 a | 0.16 ± 0.02 A | * | 0.21 ± 0.01 a | 0.23 ± 0.04 A | ns | 0.25 ± 0.03 a | 0.31 ± 0.02 A | ns | 0.50 ± 0.06 a,A |
| 2-Octen-1-ol | 1610 | 0.13 ± 0.01 C | 0.14 ± 0.01 C | ns | 0.19 ± 0.01 c | 0.10 ± 0.02 C | * | 0.11 ± 0.01 C | 0.31 ± 0.02 B | * | 0.22 ± 0.01 B | 0.93 ± 0.08 A | * | 0.62 ± 0.04 A,a |
| Tetradecanol | 2173 | 0.20 ± 0.02 | 0.20 ± 0.01 | ns | 0.17 ± 0.02 | 0.17 ± 0.03 | ns | 0.14 ± 0.02 | 0.23 ± 0.03 | * | 0.18 ± 0.02 | 0.16 ± 0.01 | ns | 0.15 ± 0.03 |
| All | 2.44 | 2.66 | 2.32 | 1.80 | 1.54 | 3.69 | 2.21 | 6.16 | 4.38 | |||||
| Esters | ||||||||||||||
| Ethyl 4-methylpentanoate | 1185 | 0.56 ± 0.04 a | 0.04 ± 0.01 A | ** | - b | - B | ns | - b | - B | ns | - b | - B | ns | - b,B |
| Methyl octanoate | 1388 | 1.71 ± 0.23 a | 1.79 ± 0.23 A | ns | 0.04 ± 0.01 b | 0.10 ± 0.01 C | * | 0.13 ± 0.02 b | 0.51 ± 0.04 B | ** | 0.11 ± 0.02 b | 0.08 ± 0.01 C | ns | - c,D |
| Ethyl octanoate | 1435 | 0.47 ± 0.03 a | 0.53 ± 0.06 A | ns | - c | - C | ns | 0.18 ± 0.03 b | 0.25 ± 0.03 B | ns | 0.19 ± 0.03 b | - C | ** | - c,C |
| All | 2.74 | 2.36 | 0.04 | 0.10 | 0.31 | 0.76 | 0.30 | 0.08 | - | |||||
| Ketones | ||||||||||||||
| 1-Octen-3-one | 1303 | 0.23 ± 0.02 a | 0.21 ± 0.02 A | ns | 0.33 ± 0.02 a | 0.26 ± 0.03 A | ns | 0.18 ± 0.02 a | 0.32 ± 0.03 A | * | 0.28 ± 0.01 a | 0.28 ± 0.02 A | ns | 0.11 ± 0.01 b,B |
| 6-Methyl-5-hepten-2-one | 1337 | 0.60 ± 0.03 | 0.60 ± 0.03 | ns | 0.64 ± 0.03 | 0.70 ± 0.04 | ns | 0.72 ± 0.04 | 0.80 ± 0.04 | ns | 0.81 ± 0.04 | 0.81 ± 0.03 | ns | 0.67 ± 0.04 |
| 3-octen-2-one | 1409 | 0.08 ± 0.01 c | - C | * | 0.03 ± 0.01 c | 0.07 ± 0.01 B | ns | 0.15 ± 0.02 b | 0.27 ± 0.02 A | * | 0.20 ± 0.01 b | 0.57 ± 0.03 A | * | 0.33 ± 0.02 a,A |
| (E,Z)-3,5-Octadien-2-one | 1521 | 0.39 ± 0.02 b | 0.40 ± 0.03 C | ns | 0.68 ± 0.04 a | 1.03 ± 0.10 B | * | 0.76 ± 0.06 a | 1.61 ± 0.12 A | * | 1.00 ± 0.05 a | 1.29 ± 0.09 A | ns | 0.55 ± 0.03 b,C |
| (E,E)-3,5-Octadien-2-one | 1569 | 1.14 ± 0.15 b | 0.49 ± 0.03 C | ** | 1.13 ± 0.17 b | 1.36 ± 0.21 B | ns | 1.35 ± 0.16 b | 2.91 ± 0.19 A | * | 2.65 ± 0.13 a | 3.00 ± 0.11 A | ns | 1.69 ± 0.07 b,B |
| All | 2.43 | 1.70 | 2.81 | 3.42 | 3.16 | 5.91 | 4.93 | 5.95 | 3.35 | |||||
| Terpenes | ||||||||||||||
| Limonene | 1197 | 0.22 ± 0.01 c | 0.63 ± 0.03 A | * | 0.85 ± 0.03 a | 0.21 ± 0.01 B | ** | 0.87 ± 0.04 a | 0.30 ± 0.02 B | * | 0.54 ± 0.02 b | 0.48 ± 0.03 A | ns | 0.24 ± 0.03 c,B |
| Eucalyptol | 1208 | 0.04 ± 0.01 b | 0.13 ± 0.01 | * | 0.33 ± 0.04 a | 0.07 ± 0.03 | * | 0.12 ± 0.01 b | 0.01 ± 0.01 | ** | 0.08 ± 0.01 b | 0.06 ± 0.01 | ns | 0.03 ± 0.01 b |
| All | 0.26 | 0.77 | 1.18 | 0.27 | 0.75 | 0.31 | 0.62 | 0.55 | 0.28 | |||||
| Acids | ||||||||||||||
| Acetic acid | 1453 | 1.38 ± 0.11 b | 2.43 ± 0.15 A | * | 0.42 ± 0.03 d | 0.26 ± 0.01 C | * | 0.74 ± 0.05 c | 0.54 ± 0.03 B | * | 0.62 ± 0.04 c | 0.80 ± 0.06 B | ns | 2.51 ± 0.14 a,A |
| 2-Methyl-butanoic acid | 1668 | 0.86 ± 0.04 a | 0.81 ± 0.03 A | ns | 0.16 ± 0.02 b | 0.23 ± 0.01 C | * | 0.24 ± 0.02 b | 0.21 ± 0.01 C | ns | 0.37 ± 0.02 b | 0.51 ± 0.04 B | ns | 0.84 ± 0.02 a,A |
| Hexanoic acid | 1851 | 0.75 ± 0.06 b | 0.49 ± 0.02 C | * | 0.12 ± 0.01 c | 0.15 ± 0.01 D | ns | 0.54 ± 0.03 b | 0.38 ± 0.02 C | ns | 0.80 ± 0.05 b | 1.16 ± 0.14 B | * | 2.90 ± 0.16 a,A |
| Octanoic acid | 2064 | 6.82 ± 0.23 a | 7.37 ± 0.26 A | ns | 1.13 ± 0.10 b | 1.10 ± 0.14 C | ns | 6.27 ± 0.27 a | 5.57 ± 0.24 B | ns | 7.59 ± 0.38 a | 5.91 ± 0.32 B | * | 8.93 ± 0.41 a,A |
| Nonanoic acid | 2169 | 0.39 ± 0.02 | 0.40 ± 0.03 B | ns | 0.30 ± 0.02 | 0.29 ± 0.03 B | ns | 0.26 ± 0.02 | 0.36 ± 0.04 B | ns | 0.37 ± 0.02 | 0.84 ± 0.05 A | * | 0.36 ± 0.02 B |
| Decanoic acid | 2275 | 0.28 ± 0.01 a | 0.12 ± 0.01 C | * | 0.10 ± 0.01 b | 0.09 ± 0.01 C | ns | 0.10 ± 0.01 b | 0.19 ± 0.01 B | * | 0.13 ± 0.01 b | 0.34 ± 0.02 A | * | 0.23 ± 0.01 a,B |
| Dodecanoic acid | 2427 | 0.88 ± 0.04 a | 0.24 ± 0.02 B | * | 0.17 ± 0.02 b | 0.13 ± 0.02 C | ns | 0.12 ± 0.01 b | 0.19 ± 0.02 C | * | 0.11 ± 0.01 b | 0.43 ± 0.03 A | ** | 0.14 ± 0.01 b,C |
| Tridecanoic acid | 2553 | 0.69 ± 0.05 a | 0.34 ± 0.02 B | * | 0.98 ± 0.05 a | 0.16 ± 0.01 C | * | 0.32 ± 0.02 b | 0.40 ± 0.03 B | ns | 0.19 ± 0.02 b | 1.26 ± 0.08 A | ** | 0.44 ± 0.03 b,B |
| All | 12.05 | 12.21 | 3.37 | 2.42 | 8.59 | 7.84 | 10.17 | 11.24 | 16.36 | |||||
| Sulfur compounds | ||||||||||||||
| Dimethyl sulfide | 745 | 1.08 ± 0.07 b | 2.71 ± 0.18 B | * | 10.85 ± 1.97 a | 16.01 ± 1.02 A | * | 2.48 ± 0.12 b | 1.41 ± 0.09 B | * | 9.19 ± 0.71 a | 2.07 ± 0.15 B | ** | 0.18 ± 0.01 c,C |
| Isopropyl isothiocyanate | 1186 | - b | - | - b | - | ns | 0.42 ± 0.02 a | - | ** | - b | - | ns | - b | |
| Methyl-isothiocyanate | 1241 | 12.81 ± 0.87 b | 15.47 ± 1.17 B | ns | 28.01 ± 1.39 a | 24.89 ± 1.23 A | ns | 33.30 ± 1.86 a | 28.49 ± 2.26 A | * | 12.91 ± 0.95 b | 9.35 ± 0.74 B | * | 3.06 ± 0.17 c,C |
| Dimethyl trisulfide | 1386 | - b | - B | 0.03 ± 0.01 a | 0.04 ± 0.01 A | ns | - b | - B | ns | - b | - B | - b,B | ||
| All | 13.90 | 18.18 | 38.89 | 40.95 | 36.19 | 29.90 | 22.10 | 11.41 | 3.24 | |||||
| Pyrazines | ||||||||||||||
| 2,3- Dimethylpyrazine | 1348 | - b | - B | ns | - b | - B | ns | 0.53 ± 0.03 a | 0.26 ± 0.02 A | * | 0.32 ± 0.02 a | 0.32 ± 0.04 A | ns | - b,B |
| 2-Ethyl-5-methylpyrazine | 1387 | - b | - B | ns | - b | - B | ns | 0.25 ± 0.01 a | 0.40 ± 0.03 A | * | 0.20 ± 0.03 a | 0.25 ± 0.01 A | ns | - b,B |
| 2,3,5-Trimethylpyrazine | 1405 | - b | - B | ns | - b | - B | ns | 0.12 ± 0.02 a | 0.17 ± 0.02 A | ns | 0.09 ± 0.01 a | 0.11 ± 0.02 A | ns | - b,B |
| 2,6-Diethylpyrazine | 1433 | - b | - C | ns | - b | - C | ns | 0.26 ± 0.03 a | 0.43 ± 0.03 A | * | 0.19 ± 0.02 a | 0.20 ± 0.03 B | ns | - b,C |
| 2,3-Diethylpyrazine | 1459 | - b | - B | ns | - b | - B | ns | 0.12 ± 0.01 a | 0.19 ± 0.02 A | ns | 0.07 ± 0.01 a | 0.12 ± 0.01 A | ns | - b,B |
| All | - | - | - | - | 1.29 | 1.46 | 0.86 | 1.00 | - | |||||
| Furanoic compounds | ||||||||||||||
| Furfural | 1466 | 0.28 ± 0.03 b | 0.22 ± 0.01 B | ns | 0.39 ± 0.05 a | 0.34 ± 0.02 B | ns | 0.19 ± 0.01 b | 0.28 ± 0.02 B | ns | 0.49 ± 0.05 a | 0.59 ± 0.06 A | ns | 0.64 ± 0.05 a,A |
| γ-Butyrolactone | 1632 | 0.33 ± 0.02 b | 1.98 ± 0.14 A | ** | 0.09 ± 0.01 c | 0.08 ± 0.01 C | ns | 0.73 ± 0.06 a | 0.40 ± 0.03 B | * | 0.23 ± 0.03 b | 0.22 ± 0.01 C | ns | - d,D |
| All | 0.61 | 2.20 | 0.47 | 0.41 | 0.91 | 0.68 | 0.72 | 0.81 | 0.64 | |||||
| Aromatic compounds | ||||||||||||||
| Aldehydes | ||||||||||||||
| Benzaldehyde | 1525 | 1.59 ± 0.26 b | 1.68 ± 0.16 B | ns | 1.46 ± 0.15 b | 1.63 ± 0.08 B | ns | 1.51 ± 0.10 b | 1.89 ± 0.09 B | ns | 1.40 ± 0.11 b | 1.72 ± 0.03 B | ns | 4.47 ± 0.21 a,A |
| 4-Propylbenzaldehyde | 1826 | 0.69 ± 0.05 a | 1.07 ± 0.09 A | * | 1.11 ± 0.21 a | 1.15 ± 0.09 A | ns | 0.78 ± 0.06 a | 1.07 ± 0.12 A | * | 0.95 ± 0.05 a | 0.82 ± 0.05 A | ns | 0.10 ± 0.02 b,B |
| 4-Methoxy-benzaldehyde | 2029 | 0.65 ± 0.03 c | 0.84 ± 0.05 B | ns | 0.50 ± 0.04 c | 0.65 ± 0.05 C | ns | 2.07 ± 0.15 a | 1.69 ± 0.04 A | ns | 1.97 ± 0.06 a | 1.75 ± 0.16 A | ns | 1.11 ± 0.10 b,B |
| (E)-Cinnamaldehyde | 2044 | 0.09 ± 0.05 c | 0.16 ± 0.02 C | ns | 0.25 ± 0.01 b | 0.37 ± 0.04 B | * | 0.26 ± 0.03 b | 0.16 ± 0.01 C | ns | 0.17 ± 0.01 b | 0.10 ± 0.01 C | ns | 0.54 ± 0.04 a,A |
| Alcohols | ||||||||||||||
| Benzyl alcohol | 1877 | 1.91 ± 0.15 b | 2.10 ± 0.16 B | ns | 1.14 ± 0.23 b | 1.44 ± 0.10 B | ns | 1.63 ± 0.09 b | 0.91 ± 0.04 C | * | 1.31 ± 0.15 b | 1.10 ± 0.06 C | ns | 20.13 ± 1.21 a,A |
| β-Phenylethyl alcohol | 1912 | 0.08 ± 0.01 b | 0.08 ± 0.01 B | ns | 0.05 ± 0.01 b | 0.07 ± 0.01 B | ns | 0.13 ± 0.01 b | 0.07 ± 0.02 B | ns | 0.13 ± 0.02 b | 0.12 ± 0.02 B | ns | 6.59 ± 0.43 a,A |
| Hydrocinnamic alcohol | 2047 | - c | 0.67 ± 0.05 A | * | 0.27 ± 0.02 b | 0.28 ± 0.03 B | ns | 0.31 ± 0.03 b | 0.27 ± 0.03 B | ns | 0.29 ± 0.03 b | 0.11 ± 0.01 C | ns | 0.91 ± 0.04 a,A |
| Cinnamyl alcohol | 2287 | 0.44 ± 0.02 b | 1.30 ± 0.16 A | * | 1.15 ± 0.14 a | 1.86 ± 0.15 A | * | 0.37 ± 0.02 c | 0.28 ± 0.03 C | ns | 0.24 ± 0.02 c | 0.22 ± 0.03 C | ns | 0.65 ± 0.02 b,B |
| Ketones | ||||||||||||||
| Acetophenone | 1663 | 0.42 ± 0.04 a | 0.70 ± 0.04 A | * | 0.25 ± 0.01 b | 0.21 ± 0.03 B | ns | 0.12 ± 0.02 b | 0.15 ± 0.02 B | ns | 0.21 ± 0.01 b | 0.32 ± 0.05 B | ns | 0.13 ± 0.01 b,B |
| Esters | ||||||||||||||
| Benzyl acetate | 1728 | 0.10 ± 0.01 b | 0.14 ± 0.02 B | ns | 0.03 ± 0.01 b | 0.04 ± 0.01 C | ns | 0.08 ± 0.01 b | 0.08 ± 0.01 C | ns | 0.08 ± 0.01 b | 0.06 ± 0.01 C | ns | 0.72 ± 0.04 a,A |
| Methyl-6-methylsalicylate | 1965 | 0.41 ± 0.05 a | 0.81 ± 0.06 A | * | 0.29 ± 0.03 b | 0.38 ± 0.04 B | * | 0.47 ± 0.05 a | 0.43 ± 0.03 B | ns | 0.43 ± 0.05 a | 0.38 ± 0.05 B | ns | 0.19 ± 0.02 c,C |
| Acids | ||||||||||||||
| Benzoic acid | 2401 | - c | - D | ns | - c | 0.26 ± 0.03 B | ** | 0.37 ± 0.02 b | 0.06 ± 0.01 C | ** | - c | 0.40 ± 0.06 B | * | 0.95 ± 0.06 a,A |
| All | 6.38 | 9.53 | 6.50 | 8.34 | 8.12 | 7.07 | 7.18 | 7.09 | 36.49 | |||||
| Nitrogen compounds | ||||||||||||||
| Acetonitrile | 1010 | 0.36 ± 0.04 a | - C | * | 0.02 ± 0.01 b | 0.03 ± 0.01 B | ns | 0.37 ± 0.03 a | 0.52 ± 0.03 A | ns | 0.42 ± 0.03 a | 0.13 ± 0.02 B | * | - c,C |
| 2-Formyl-1-methyl pyrrole | 1621 | 0.54 ± 0.03 a | 0.83 ± 0.04 A | * | 0.62 ± 0.04 a | 0.99 ± 0.05 A | * | 0.45 ± 0.03 a | 0.41 ± 0.02 B | ns | 0.74 ± 0.05 a | 0.71 ± 0.06 A | ns | - b,C |
| All | 0.90 | 0.83 | 0.64 | 1.03 | 0.83 | 0.93 | 1.16 | 0.84 | - |
| BrS (Rt) 40 °C | BrS (Rt) 50 °C | 40 °C vs. 50 °C | BrS (60 °C) 40 °C | BrS (60 °C) 50 °C | 40 °C vs. 50 °C | DrS (10d) 40 °C | DrS (10d) 50 °C | 40 °C vs. 50 °C | DrS (40d) 40 °C | DrS (40d) 50 °C | 40 °C vs. 50 °C | Undried Caper | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | 6.38 ± 0.12 b | 6.75 ± 0.07 C | ns | 7.49 ± 0.15 a | 7.20 ± 0.07 B | ns | 6.88 ± 0.05 b | 6.25 ± 0.06 C | ns | 7.57 ± 0.03 a | 6.71 ± 0.04 B | * | 7.80 ± 0.05 a,A |
| Odor | 4.50 ± 0.09 b | 4.88 ± 0.20 B | ns | 7.14 ± 0.06 a | 6.71 ± 0.13 A | * | 5.88 ± 0.07 b | 5.63 ± 0.08 A | ns | 6.86 ± 0.17 a | 6.96 ± 0.15 A | ns | 6.90 ± 0.16 a,A |
| Taste | 4.25 ± 0.02 b | 5.00 ± 0.16 B | * | 8.00 ± 0.23 a | 8.29 ± 0.25 A | ns | 5.38 ± 0.14 b | 5.25 ± 0.24 B | ns | 7.71 ± 0.18 a | 7.86 ± 0.21 A | ns | 7.40 ± 0.19 a,A |
| Overall acceptability | 5.04 ± 0.18 b | 5.54 ± 0.05 B | ns | 7.54 ± 0.16 a | 7.40 ± 0.08 A | ns | 6.04 ± 0.18 b | 5.71 ± 0.07 B | ns | 7.38 ± 0.13 a | 7.17 ± 0.09 A | ns | 7.37 ± 0.07 a,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cincotta, F.; Merlino, M.; Verzera, A.; Gugliandolo, E.; Condurso, C. Innovative Process for Dried Caper (Capparis spinosa L.) Powder Production. Foods 2022, 11, 3765. https://doi.org/10.3390/foods11233765
Cincotta F, Merlino M, Verzera A, Gugliandolo E, Condurso C. Innovative Process for Dried Caper (Capparis spinosa L.) Powder Production. Foods. 2022; 11(23):3765. https://doi.org/10.3390/foods11233765
Chicago/Turabian StyleCincotta, Fabrizio, Maria Merlino, Antonella Verzera, Enrico Gugliandolo, and Concetta Condurso. 2022. "Innovative Process for Dried Caper (Capparis spinosa L.) Powder Production" Foods 11, no. 23: 3765. https://doi.org/10.3390/foods11233765
APA StyleCincotta, F., Merlino, M., Verzera, A., Gugliandolo, E., & Condurso, C. (2022). Innovative Process for Dried Caper (Capparis spinosa L.) Powder Production. Foods, 11(23), 3765. https://doi.org/10.3390/foods11233765











