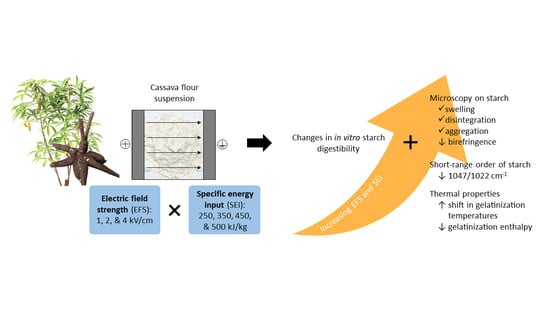
Changes in Starch In Vitro Digestibility and Properties of Cassava Flour Due to Pulsed Electric Field Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEF System
2.3. PEF Treatment of Cassava Flour Suspension
2.4. In Vitro Digestibility
2.4.1. Preparation of Digestion Solutions
2.4.2. In Vitro Digestion Procedure
2.4.3. Measurement of Hydrolyzed Starch
2.4.4. Kinetic Modelling of In Vitro Starch Digestibility at the Small Intestinal Phase
2.5. Polarized Light Microscopy
2.6. Fourier-Transform Infrared Spectroscopy—Attenuated Total Reflection (FTIR-ATR) Analysis
2.7. Differential Scanning Calorimetry (DSC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in PEF Processing Parameters and Physical Properties of Cassava Flour Suspension upon PEF Treatment
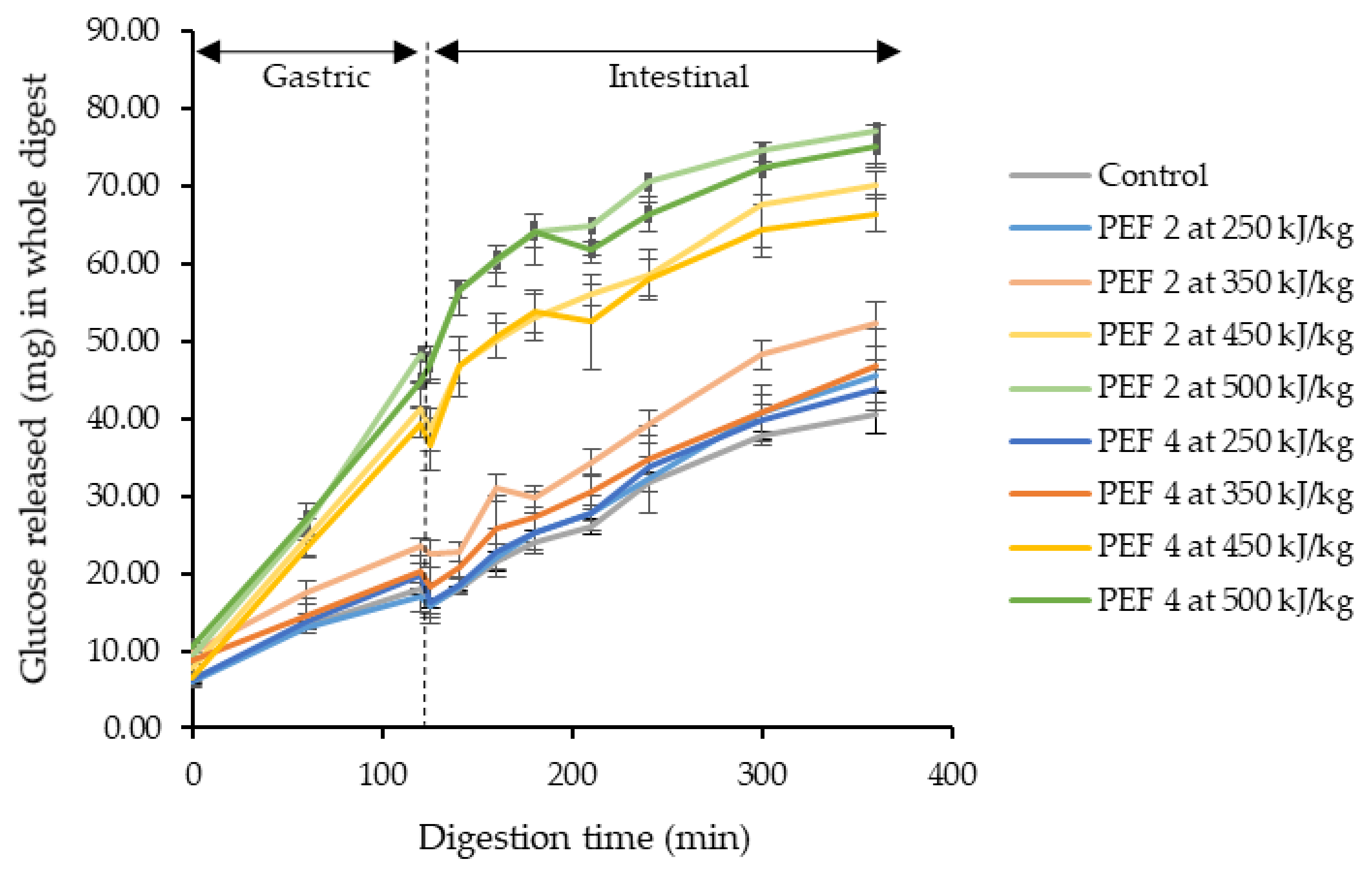
3.2. Effect of PEF on In Vitro Digestibility of Cassava Starches in PEF Treated Cassava Flours
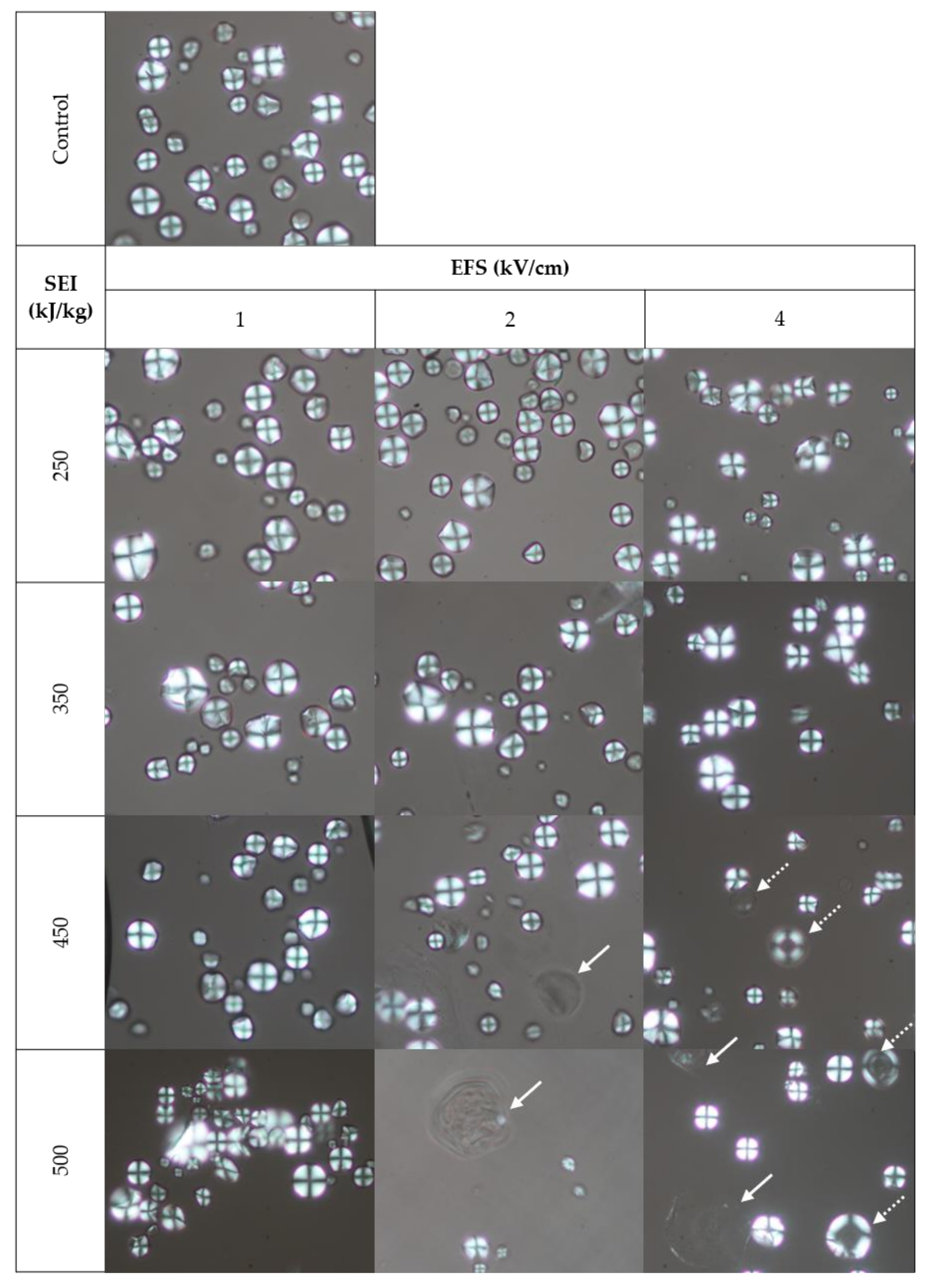
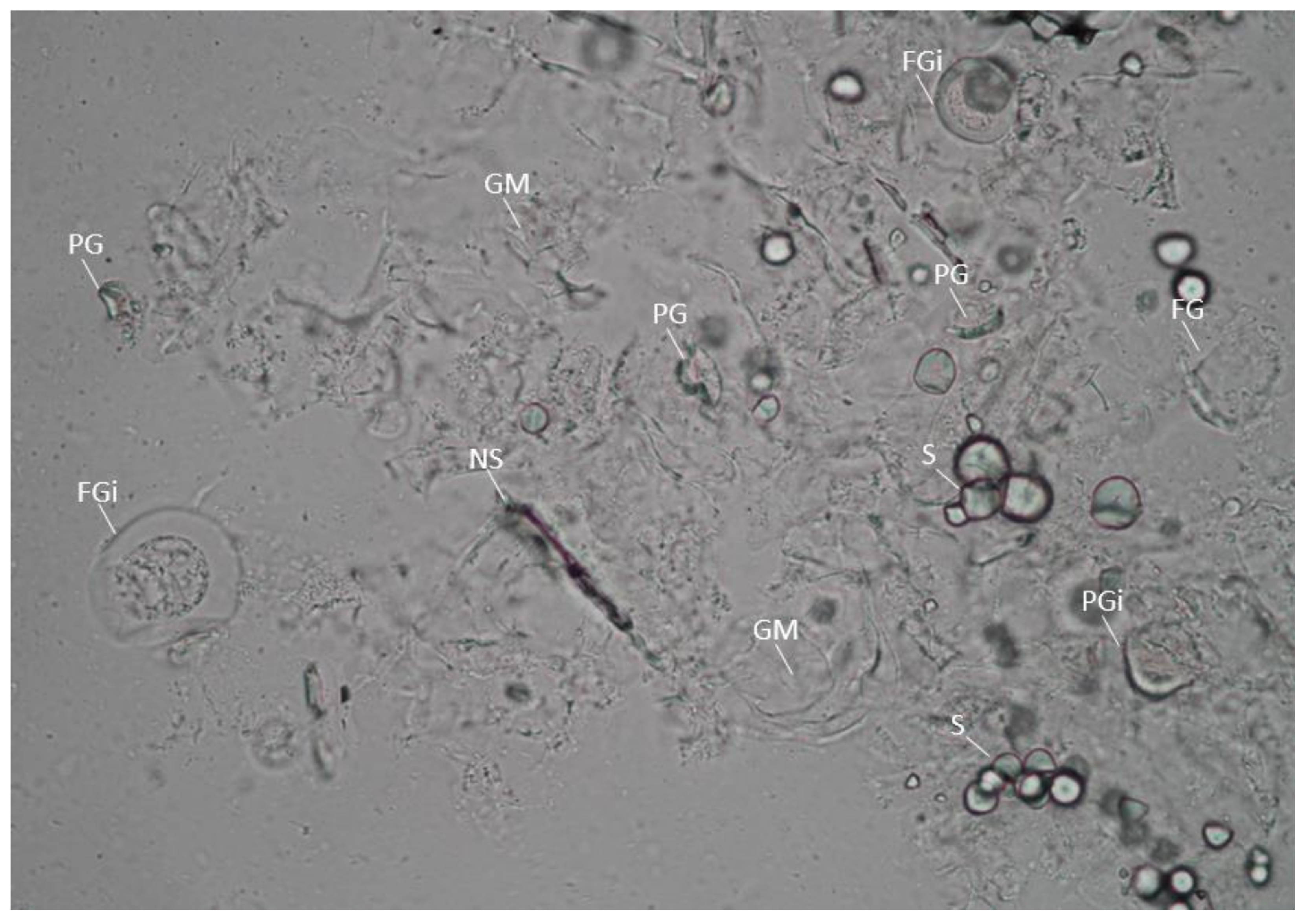
3.3. Effect of PEF on the Morphology and Birefringence of Cassava Starches in Cassava Flours
3.4. Effect of PEF on Short-Range Microstructural Order of Cassava Starches in Cassava Flours
3.5. Effect of PEF on Gelatinization Temperatures and Enthalpy of Cassava Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, L.M.G.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Starch Extraction and Modification by Pulsed Electric Fields. Food Rev. Int. 2021, 1–22. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Zeng, F.; Gao, Q.-Y.; Han, Z.; Zeng, X.-A.; Yu, S.-J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef]
- Salehi, F. Physico-chemical properties of fruit and vegetable juices as affected by pulsed electric field: A review. Int. J. Food Prop. 2020, 23, 1036–1050. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toepfl, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22. [Google Scholar] [CrossRef]
- Leong, S.Y.; Roberts, R.; Hu, Z.; Bremer, P.; Silcock, P.; Toepfl, S.; Oey, I. Texture and in vitro starch digestion kinetics of French fries produced from potatoes (Solanum tuberosum L.) pre-treated with pulsed electric fields. Appl. Food Res. 2022, 2, 100194. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A. Feasibility of using pulsed electric fields to modify biomacromolecules: A review. Trends Food Sci. Technol. 2018, 72, 91–113. [Google Scholar] [CrossRef]
- BeMiller, J.N. Chapter 19—Corn Starch Modification. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 537–549. [Google Scholar]
- Han, Z.; Zeng, X.A.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Effects of pulsed electric fields (PEF) treatment on physicochemical properties of potato starch. Innov. Food Sci. Emerg. Technol. 2009, 10, 481–485. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.-A.; Zhang, B.-S.; Yu, S.-J. Effects of pulsed electric fields (PEF) treatment on the properties of corn starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Q.-Y.; Jiang, W.; Qian, J.-Y.; Zhang, L.; Wu, M.; Rao, S.-Q.; Wu, C.-S. Effect of pulsed electric field on structural properties and digestibility of starches with different crystalline type in solid state. Carbohydr. Polym. 2019, 207, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Abduh, S.B.M.; Leong, S.Y.; Agyei, D.; Oey, I. Understanding the Properties of Starch in Potatoes (Solanum tuberosum var. Agria) after Being Treated with Pulsed Electric Field Processing. Foods 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Alpos, M.; Leong, S.Y.; Liesaputra, V.; Martin, C.E.; Oey, I. Understanding In Vivo Mastication Behaviour and In Vitro Starch and Protein Digestibility of Pulsed Electric Field-Treated Black Beans after Cooking. Foods 2021, 10, 2540. [Google Scholar] [CrossRef]
- Qiu, S.; Abbaspourrad, A.; Padilla-Zakour, O.I. Changes in the Glutinous Rice Grain and Physicochemical Properties of Its Starch upon Moderate Treatment with Pulsed Electric Field. Foods 2021, 10, 395. [Google Scholar] [CrossRef]
- Duque, S.M.M.; Leong, S.Y.; Agyei, D.; Singh, J.; Larsen, N.; Oey, I. Understanding the impact of Pulsed Electric Fields treatment on the thermal and pasting properties of raw and thermally processed oat flours. Food Res. Int. 2020, 129, 108839. [Google Scholar] [CrossRef]
- Bai, T.-G.; Zhang, L.; Qian, J.-Y.; Jiang, W.; Wu, M.; Rao, S.-Q.; Li, Q.; Zhang, C.; Wu, C. Pulsed electric field pretreatment modifying digestion, texture, structure and flavor of rice. LWT 2021, 138, 110650. [Google Scholar] [CrossRef]
- Alpos, M.; Leong, S.Y.; Liesaputra, V.; Oey, I. Influence of pulsed electric fields (PEF) with calcium addition on the texture profile of cooked black beans (Phaseolus vulgaris) and their particle breakdown during in vivo oral processing. Innov. Food Sci. Emerg. Technol. 2022, 75, 102892. [Google Scholar] [CrossRef]
- Keith, W.D.; Harris, L.J.; Griffiths, M.W. Reduction of bacterial levels in flour by pulsed electric fields. J. Food Process Eng. 1998, 21, 263–269. [Google Scholar] [CrossRef]
- Duque, S.M.M.; Leong, S.Y.; Agyei, D.; Singh, J.; Larsen, N.; Oey, I. Modifications in the physicochemical properties of flour “fractions” after Pulsed Electric Fields treatment of thermally processed oat. Innov. Food Sci. Emerg. Technol. 2020, 64, 102406. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Drought-Tolerant Cassava for Africa, Asia, and Latin America. BioScience 1993, 43, 441–451. [Google Scholar] [CrossRef]
- Aryee, F.N.A.; Oduro, I.; Ellis, W.O.; Afuakwa, J.J. The physicochemical properties of flour samples from the roots of 31 varieties of cassava. Food Control. 2006, 17, 916–922. [Google Scholar] [CrossRef]
- Ciacco, C.F.; D’Appolonia, B.L. Baking Studies with Cassava and Yam Flour. I. Biochemical Composition of Cassava and Yam Flour. Cereal Chem. 1978, 55, 402–411. [Google Scholar]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef]
- Ocloo, F.C.K.; Ayernor, G.S. Production of alcohol from cassava flour hydrolysate. J. Brew. Distill. 2010, 1, 16–21. [Google Scholar] [CrossRef]
- Han, Z.; Yu, Q.; Zeng, X.A.; Luo, D.H.; Yu, S.J.; Zhang, B.S.; Chen, X.D. Studies on the microstructure and thermal properties of pulsed electric fields (PEF)-treated maize starch. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Khrisanapant, P.; Leong, S.Y.; Kebede, B.; Oey, I. Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans. Foods 2021, 10, 1415. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, Secondary Structure, and Self-Assembly of Oat Protein Isolate. J. Agric. Food Chem. 2009, 57, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Niu, L.; Wu, L.; Li, D.; He, H. Preparation of an In Vitro Low-Digestible Rice Starch by Addition of Grass Carp Protein Hydrolysates and Its Possible Mechanisms. Starch—Stärke 2019, 71, 1800159. [Google Scholar] [CrossRef]
- Xia, W.; Wang, F.; Li, J.; Wei, X.; Fu, T.; Cui, L.; Li, T.; Liu, Y. Effect of high speed jet on the physical properties of tapioca starch. Food Hydrocoll. 2015, 49, 35–41. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Chen, L.; Zheng, B. Physicochemical Properties and Digestion of Lotus Seed Starch under High-Pressure Homogenization. Nutrients 2019, 11, 371. [Google Scholar] [CrossRef]
- Krueger, B.R.; Knutson, C.A.; Inglett, G.E.; Walker, C.E. A Differential Scanning Calorimetry Study on the Effect of Annealing on Gelatinization Behavior of Corn Starch. J. Food Sci. 1987, 52, 715–718. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef]
- Rosenblum, J.L.; Irwin, C.L.; Alpers, D.H. Starch and glucose oligosaccharides protect salivary-type amylase activity at acid pH. Am. J. Physiol.-Gastrointest. Liver Physiol. 1988, 254, G775–G780. [Google Scholar] [CrossRef]
- Englyst, K.; Goux, A.; Meynier, A.; Quigley, M.; Englyst, H.; Brack, O.; Vinoy, S. Inter-laboratory validation of the starch digestibility method for determination of rapidly digestible and slowly digestible starch. Food Chem. 2018, 245, 1183–1189. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Q.-Y.; Wu, M.; Jiang, W.; Qian, J.-Y.; Rao, S.-Q.; Zhang, L.; Li, Q.; Zhang, C. Effect of pulsed electric field on properties and multi-scale structure of japonica rice starch. LWT 2019, 116, 108515. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Biliaderis, C.G. Chapter 8—Structural Transitions and Related Physical Properties of Starch. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 293–372. [Google Scholar]
- Bosch, H.F.M.V.D.; Morshuis, P.H.F.; Smit, J.J. Temperature distribution in fluids treated by pulsed electric fields. In Proceedings of the 2002 IEEE 14th International Conference on Dielectric Liquids. ICDL 2002 (Cat. No.02CH37319), Graz, Austria, 12 July 2002; pp. 67–70. [Google Scholar]
- Maniglia, B.C.; Pataro, G.; Ferrari, G.; Augusto, P.E.D.; Le-Bail, P.; Le-Bail, A. Pulsed electric fields (PEF) treatment to enhance starch 3D printing application: Effect on structure, properties, and functionality of wheat and cassava starches. Innov. Food Sci. Emerg. Technol. 2021, 68, 102602. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Chapter 12—Tapioca/Cassava Starch: Production and Use. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 541–568. [Google Scholar]
- Asaoka, M.; Blanshard, J.M.V.; Rickard, J.E. Seasonal Effects on the Physico-chemical Properties of Starch from Four Cultivars of Cassava. Starch—Stärke 1991, 43, 455–459. [Google Scholar] [CrossRef]
- Defloor, I.; Dehing, I.; Delcour, J.A. Physico-Chemical Properties of Cassava Starch. Starch—Stärke 1998, 50, 58–64. [Google Scholar] [CrossRef]
- Błaszczak, W.; Valverde, S.; Fornal, J. Effect of high pressure on the structure of potato starch. Carbohydr. Polym. 2005, 59, 377–383. [Google Scholar] [CrossRef]
- Katopo, H.; Song, Y.; Jane, J.-L. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002, 47, 233–244. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Whitney, K.; Reuhs, B.L.; Doehlert, D.C.; Simsek, S. Effect of hydrothermal treatment on physicochemical and digestibility properties of oat starch. Food Res. Int. 2013, 52, 17–25. [Google Scholar] [CrossRef]
- Hublin, L. Influence des Caractéristiques Structurales des Amidons Natifs sur Leur Réactivité Chimique. Ph.D. Thesis, University of Nantes, Nantes, France, 1994. [Google Scholar]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
| Sample | EFS (kV/cm) | SEI (kJ/kg) | Pulse Number | Initial Temperature (°C) | Final Temperature (°C) |
|---|---|---|---|---|---|
| PEF 1 | 1.02 | 249.7 ± 0.1 | 14,584 | 13.3 ± 0.6 | 34.3 ± 1.3 |
| 1.05 | 349.3 ± 0.4 | 20,417 | 11.1 ± 0.7 | 37.1 ± 0.7 | |
| 1.05 | 452.0 ± 4.1 | 26,250 | 9.9 ± 0.5 | 39.3 ± 0.9 | |
| 1.05 | 499.2 ± 0.2 | 29,167 | 8.2 ± 0.1 | 38.2 ± 0.4 | |
| PEF 2 | 2.00 | 250.4 ± 2.0 | 3210 | 10.8 ± 0.3 | 34.0 ± 0.1 |
| 2.00 | 348.8 ± 1.7 | 3990 | 11.7 ± 0.9 | 40.5 ± 1.1 | |
| 1.95 | 449.7 ± 0.1 | 4777 | 12.4 ± 0.1 | 46.3 ± 1.3 | |
| 1.95 | 498.6 ± 1.4 | 5148 | 12.7 ± 0.3 | 48.7 ± 0.2 | |
| PEF 4 | 4.03 | 249.9 ± 0.7 | 724 | 11.9 ± 0.6 | 34.1 ± 1.1 |
| 3.99 | 350.7 ± 0.6 | 920 | 12.1 ± 0.2 | 40.8 ± 0.8 | |
| 3.96 | 449.1 ± 2.0 | 1117 | 11.1 ± 0.4 | 46.1 ± 0.9 | |
| 3.95 | 504.2 ± 4.3 | 1203 | 10.3 ± 0.4 | 48.4 ± 0.3 |
| Sample | Electric Field Strength (kV/cm) | Specific Energy Input (kJ/kg) | RDS (%) | SDS (%) | RS (%) | k (×10−2 min−1) | Range of R2 for k estimation |
|---|---|---|---|---|---|---|---|
| Control | - | - | 1.89 ± 0.06 c | 1.45 ± 0.16 ab | 96.66 ± 0.22 ab | 10.76 ± 1.00 ab | 0.95–0.99 |
| PEF 1 | 1.02 | 249.7 ± 0.1 | 1.64 ± 0.14 c | 1.53 ± 0.04 a | 96.83 ± 0.18 a | 11.74 ± 1.51 ab | 0.97–0.98 |
| 1.05 | 349.3 ± 0.4 | 1.88 ± 0.02 bc | 1.51 ± 0.36 ab | 96.61 ± 0.34 ab | 12.52 ± 1.97 ab | 0.94–0.98 | |
| 1.05 | 452.0 ± 4.1 | 1.99 ± 0.14 bc | 1.36 ± 0.06 a | 96.64 ± 0.08 ab | 11.88 ± 0.19 ab | 0.97–0.99 | |
| 1.05 | 499.2 ± 0.2 | 2.19 ± 0.41 bc | 1.65 ± 0.09 a | 96.16 ± 0.32 ab | 12.53 ± 1.29 ab | 0.93–0.97 | |
| PEF 2 | 2.00 | 250.4 ± 2.0 | 1.97 ± 0.08 bc | 1.49 ± 0.35 ab | 96.54 ± 0.43 ab | 12.69 ± 0.41 ab | 0.97–0.98 |
| 2.00 | 348.8 ± 1.7 | 2.35 ± 0.09 b | 1.73 ± 0.10 a | 95.92 ± 0.12 b | 13.07 ± 0.70 a | 0.94–0.97 | |
| 1.95 | 449.7 ± 0.1 | 5.06 ± 0.29 a | 1.29 ± 0.26 ab | 93.65 ± 0.51 cd | 12.27 ± 0.47 ab | 0.92–0.93 | |
| 1.95 | 498.6 ± 1.4 | 6.22 ± 0.35 a | 1.04 ± 0.07 b | 92.75 ± 0.28 e | 11.09 ± 0.15 ab | 0.86–0.90 | |
| PEF 4 | 4.03 | 249.9 ± 0.7 | 1.90 ± 0.09 c | 1.58 ± 0.07 a | 96.52 ± 0.11 ab | 11.86 ± 0.40 ab | 0.97–0.98 |
| 3.99 | 350.7 ± 0.6 | 2.13 ± 0.34 bc | 1.44 ± 0.13 ab | 96.43 ± 0.40 ab | 11.70 ± 0.43 ab | 0.97–0.98 | |
| 3.96 | 449.1 ± 2.0 | 4.84 ± 0.39 a | 1.18 ± 0.22 ab | 93.98 ± 0.24 c | 10.81 ± 0.82 ab | 0.85–0.88 | |
| 3.95 | 504.2 ± 4.3 | 6.05 ± 0.17 a | 1.03 ± 0.32 ab | 92.93 ± 0.19 de | 9.88 ± 1.29 b | 0.69–0.90 | |
| p-value | < 0.01 | < 0.01 | < 0.01 | 0.021 |
| Sample | Electric Field Strength (kV/cm) | Specific Energy Input (kJ/kg) | Abs 1047/1022 * | Abs 1022/995 ns |
|---|---|---|---|---|
| Control | - | - | 0.799 ± 0.055 a | 0.787 ± 0.083 |
| PEF 1 | 1.02 | 249.7 ± 0.1 | 0.778 ± 0.082 a | 0.834 ± 0.047 |
| 1.05 | 349.3 ± 0.4 | 0.787 ± 0.103 a | 0.897 ± 0.158 | |
| 1.05 | 452.0 ± 4.1 | 0.748 ± 0.047 ab | 0.833 ± 0.008 | |
| 1.05 | 499.2 ± 0.2 | 0.730 ± 0.030 abc | 0.857 ± 0.049 | |
| PEF 2 | 2.00 | 250.4 ± 2.0 | 0.759 ± 0.052 a | 0.820 ± 0.045 |
| 2.00 | 348.8 ± 1.7 | 0.750 ± 0.113 ab | 0.820 ± 0.008 | |
| 1.95 | 449.7 ± 0.1 | 0.601 ± 0.039 abcd | 0.847 ± 0.065 | |
| 1.95 | 498.6 ± 1.4 | 0.511 ± 0.065 d | 0.951 ± 0.132 | |
| PEF 4 | 4.03 | 249.9 ± 0.7 | 0.748 ± 0.026 ab | 0.864 ± 0.081 |
| 3.99 | 350.7 ± 0.6 | 0.782 ± 0.016 a | 0.837 ± 0.039 | |
| 3.96 | 449.1 ± 2.0 | 0.545 ± 0.043 cd | 0.802 ± 0.039 | |
| 3.95 | 504.2 ± 4.3 | 0.563 ± 0.082 bcd | 0.912 ± 0.057 |
| Sample | Electric Field Strength (kV/cm) | Specific Energy Input (kJ/kg) | Tonset | Tpeak | Tconclusion | Trange | ∆H (J/g) | Degree of Gelatinization (%) |
|---|---|---|---|---|---|---|---|---|
| Control | - | - | 63.07 ± 0.22 c | 68.04 ± 0.33 ab | 73.43 ± 0.30 b | 9.95 ± 0.63 ab | 3.75 ± 0.19 a | - |
| PEF 1 | 1.02 | 249.7 ± 0.1 | 63.44 ± 0.21 c | 68.26 ± 0.11 b | 73.56 ± 0.17 b | 9.64 ± 0.39 abc | 3.77 ± 0.11 a | −0.47 ± 2.84 d |
| 1.05 | 349.3 ± 0.4 | 63.56 ± 0.36 c | 68.45 ± 0.23 ab | 74.06 ± 0.21 b | 9.79 ± 0.27 abc | 3.87 ± 0.16 a | −3.02 ± 4.19 d | |
| 1.05 | 452.0 ± 4.1 | 63.84 ± 0.45 bc | 68.67 ± 0.60 ab | 73.85 ± 0.31 b | 9.66 ± 0.34 abc | 3.73 ± 0.19 a | 0.65 ± 4.89 d | |
| 1.05 | 499.2 ± 0.2 | 63.48 ± 0.55 c | 68.26 ± 0.56 ab | 73.78 ± 0.69 b | 9.56 ± 0.06 abc | 4.01 ± 0.30 a | −6.68 ± 7.86 d | |
| PEF 2 | 2.00 | 250.4 ± 2.0 | 63.79 ± 0.32 bc | 68.47 ± 0.11 b | 73.94 ± 0.21 b | 9.36 ± 0.46 bc | 3.70 ± 0.12 a | 1.55 ± 3.15 d |
| 2.00 | 348.8 ± 1.7 | 63.14 ± 0.40 c | 67.83 ± 0.46 ab | 73.41 ± 0.48 b | 9.38 ± 0.24 bc | 3.91 ± 0.18 a | −4.07 ± 4.58 d | |
| 1.95 | 449.7 ± 0.1 | 63.91 ± 0.28 bc | 68.21 ± 0.12 b | 74.44 ± 0.26 ab | 8.62 ± 0.49 c | 2.94 ± 0.11 b | 21.63 ± 2.82 c | |
| 1.95 | 498.6 ± 1.4 | 64.90 ± 0.48 ab | 69.84 ± 0.77 ab | 76.09 ± 0.74 ab | 9.89 ± 0.62 abc | 2.23 ± 0.03 c | 40.67 ± 0.57 b | |
| PEF 4 | 4.03 | 249.9 ± 0.7 | 63.96 ± 0.55 bc | 68.76 ± 0.58 ab | 74.24 ± 0.36 b | 9.60 ± 0.19 abc | 4.03 ± 0.17 a | −7.44 ± 4.34 d |
| 3.99 | 350.7 ± 0.6 | 63.80 ± 0.28 bc | 68.44 ± 0.12 b | 74.01 ± 0.15 ab | 9.30 ± 0.37 bc | 3.87 ± 0.06 a | −3.14 ± 1.39 d | |
| 3.96 | 449.1 ± 2.0 | 64.75 ± 0.25 ab | 69.19 ± 0.16 a | 74.93 ± 0.76 ab | 8.88 ± 0.43 bc | 2.89 ± 0.15 b | 22.95 ± 3.83 c | |
| 3.95 | 504.2 ± 4.3 | 65.56 ± 0.42 a | 70.91 ± 0.70 ab | 77.91 ± 0.69 a | 10.72 ± 0.78 a | 1.40 ± 0.22 d | 62.88 ± 5.64 a | |
| p-value | <0.001 | 0.002 | <0.001 | 0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, L.A.; Kebede, B.; Leong, S.Y.; Oey, I. Changes in Starch In Vitro Digestibility and Properties of Cassava Flour Due to Pulsed Electric Field Processing. Foods 2022, 11, 3714. https://doi.org/10.3390/foods11223714
Conde LA, Kebede B, Leong SY, Oey I. Changes in Starch In Vitro Digestibility and Properties of Cassava Flour Due to Pulsed Electric Field Processing. Foods. 2022; 11(22):3714. https://doi.org/10.3390/foods11223714
Chicago/Turabian StyleConde, Ladie Anne, Biniam Kebede, Sze Ying Leong, and Indrawati Oey. 2022. "Changes in Starch In Vitro Digestibility and Properties of Cassava Flour Due to Pulsed Electric Field Processing" Foods 11, no. 22: 3714. https://doi.org/10.3390/foods11223714
APA StyleConde, L. A., Kebede, B., Leong, S. Y., & Oey, I. (2022). Changes in Starch In Vitro Digestibility and Properties of Cassava Flour Due to Pulsed Electric Field Processing. Foods, 11(22), 3714. https://doi.org/10.3390/foods11223714