Characterization and Bioactive Potential of Carotenoid Lutein from Gordonia rubripertncta GH-1 Isolated from Traditional Pixian Douban
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Culture Conditions, and Identification
2.2. Preparation and Purification of the Pigment
2.2.1. Preparation of Crude Pigment
2.2.2. Purification by Silica Gel Column Chromatography
2.3. Characterization of Pigment Structure
2.3.1. UV and Infrared Spectra of Pigment
2.3.2. Nuclear Magnetic Resonance (NMR) Analysis
2.3.3. Mass Spectrometry Analysis UV and Infrared Spectra of Pigment
2.4. Bioactivities of Carotenoid Lutein
2.4.1. Assay of Antioxidant Activities
2.4.2. Inhibit Zebrafish Eye Cells Apoptosis Activity
2.5. Statistical Analysis
3. Results and Discussion
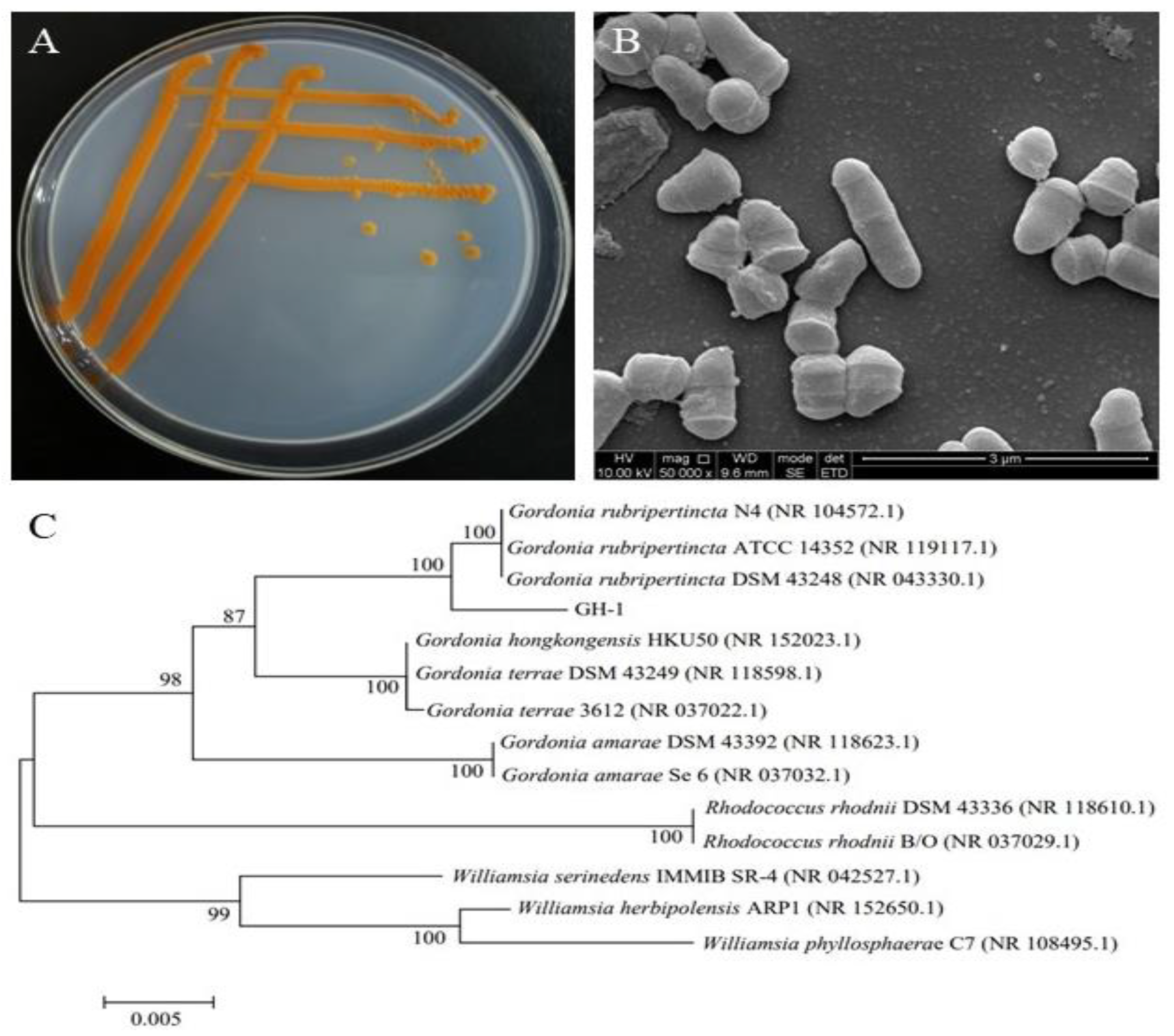
3.1. Identification of GH-1 Strain
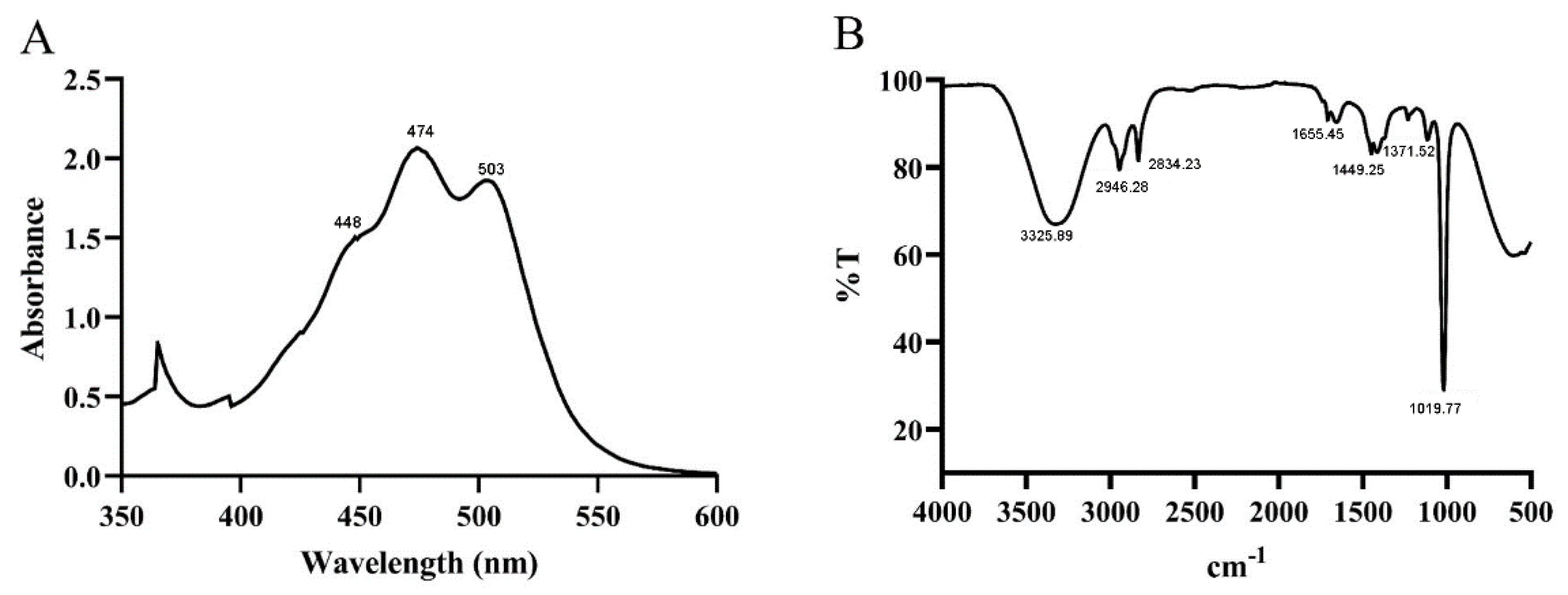
3.2. UV and FT-IR Spectral Analysis of Purified Carotenoid
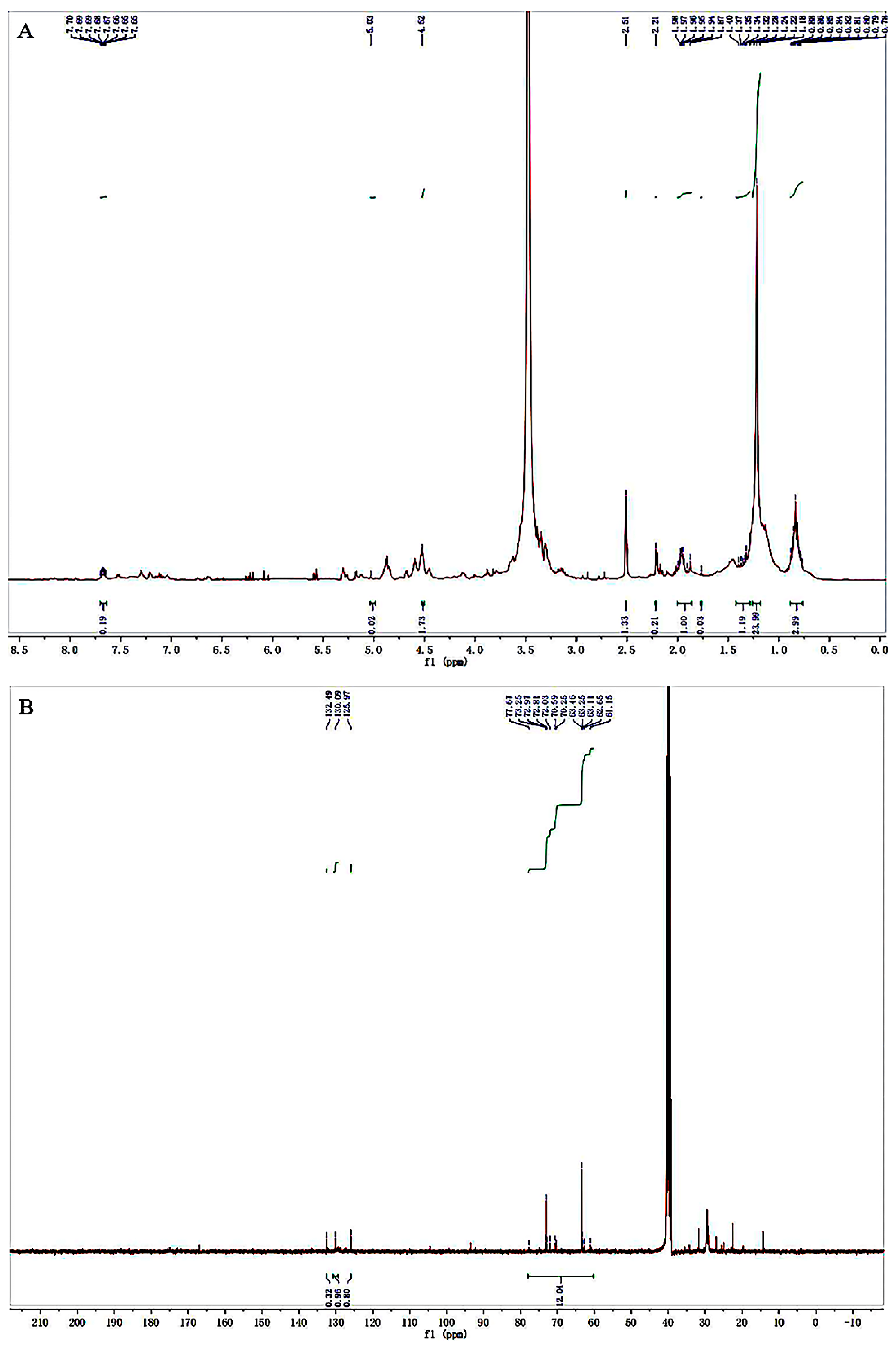
3.3. NMR Spectroscopy Analysis of Purified Carotenoid
3.4. Mass Spectrometry Analysis of Purified Carotenoid
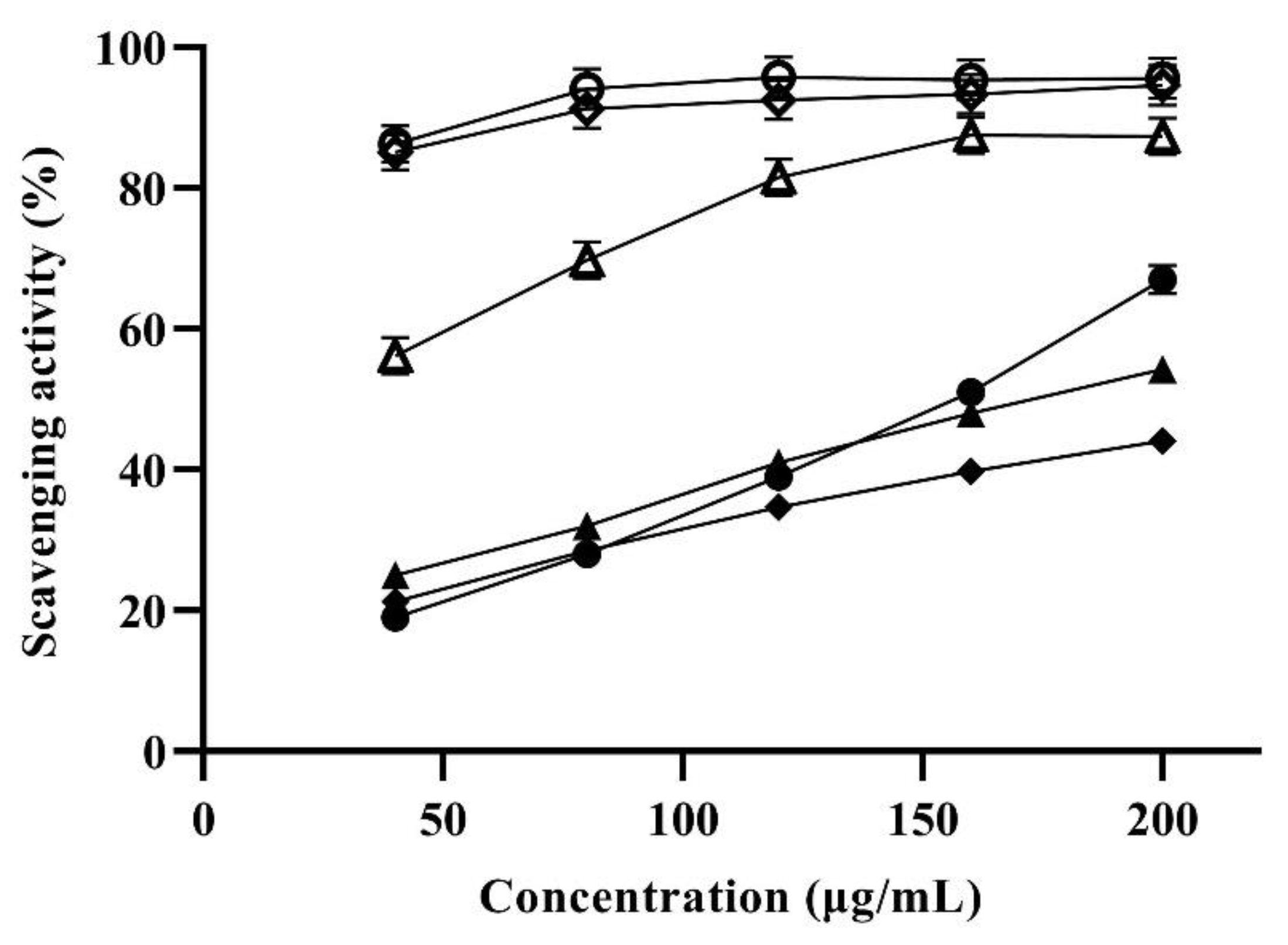
3.5. Antioxidant Activity of Lutein
3.6. Inhibitory Effect of Lutein on Ocular Apoptotic Cells in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biot. 2019, 103, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.E. The role of orange gene in carotenoid accumulation: Manipulating chromoplasts toward a colored future. Front. Plant Sci. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Paixao, S.M.; Silva, T.P.; Roseiro, J.C.; Alves, L. Influence of culture conditions towards optimal carotenoid production by Gordonia alkanivorans strain 1B. Bioprocess Biosyst. Eng. 2018, 41, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods. 2020, 67, 103867. [Google Scholar] [CrossRef]
- Rao, M.P.N.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montaez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixao, S.M.; Alves, L. Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production. RSC Adv. 2016, 6, 58055–58063. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Wan, A.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Loh, W.L.C.; Huang, K.C.; Ng, H.S.; Lan, J.C.W. Exploring the fermentation characteristics of a newly isolated marine bacteria strain, Gordonia terrae TWRH01 for carotenoids production. J. Biosci. Bioeng. 2020, 130, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dufosse, L.; Mabon, P.; Binet, A. Assessment of the coloring strength of Brevibacterium linens strains: Spectrocolorimetry versus total carotenoid extraction/quantification. J. Dairy Sci. 2001, 84, 354–360. [Google Scholar] [CrossRef]
- Li, Z.H.; Rui, J.P.; Li, X.Z.; Li, J.B.; Dong, L.; Huang, Q.L.; Chen, F.S. Bacterial community succession and metabolite changes during doubanjiang-meju fermentation, a Chinese traditional fermented broad bean (Vicia faba L.) paste. Food Chem. 2017, 218, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Yang, L.Z.; Yang, G.H.; Chi, Y.L.; Sun, Q.; He, Q. Insight into the fermentation of Chinese horse bean-chili-paste. Food Rev. Int. 2020, 37, 683–705. [Google Scholar] [CrossRef]
- Giuffrida, D.; Sutthiwong, N.; Dugo, P.; Donato, P.; Cacciola, F.; Girard-Valenciennes, E.; Dufosse, L. Characterisation of the C50 carotenoids produced by strains of the cheese-ripening bacterium Arthrobacter arilaitensis. Int. Dairy J. 2016, 55, 10–16. [Google Scholar] [CrossRef]
- Giuffrida, D.; Monnet, C.; Laurent, F.; Cacciola, F.; Oteri, M.; Le, P.M.; Dufosse, L. Carotenoids from the ripening bacterium Brevibacterium linens impart color to the rind of the French cheese, Fourme de Montbrison (PDO). Nat. Prod. Res. 2020, 34, 10–15. [Google Scholar] [CrossRef]
- Lvarez-Barrios, A.; Lvarez, L.; García, M.; Artime, E.; González-Iglesias, H. Antioxidant defenses in the human eye: A focus on metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.; Jahan, S.; Bin Emran, T.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Sawa, M.; Shunto, T.; Nishiyama, I.; Yokoyama, A.; Shigeta, R.; Miura, S.; Kawasaki, R. Effects of lutein supplementation in Japanese patients with unilateral age-related macular degeneration: The sakai lutein study. Sci. Rep. 2020, 10, 5958. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Ho, Y.J.; Ciou, S.C.; Gong, Z. Innovative disease model: Zebrafish as an in vivo platform for intestinal disorder and tumors. Biomedicines 2017, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Chuang, L.T.; Yu, P.C.; Chen, C.N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Chaudhuri, S.; Dutta, D. Studies on the stability of a carotenoid produced by a novel isolate using low cost agro-industrial residue and its application in different model systems. LWT-Food Sci. Technol. 2015, 63, 780–790. [Google Scholar] [CrossRef]
- Setiyono, E.; Heriyanto; Pringgenies, D.; Shioi, Y.; Kanesaki, Y.; Awai, K.; Brotosudarmo, T.H.P. Sulfur-containing carotenoids from a marine coral symbiont Erythrobacter flavus strain KJ5. Mar. Drugs 2019, 17, 349. [Google Scholar] [CrossRef]
- Kikukawa, H.; Okaya, T.; Maoka, T.; Miyazaki, M.; Murofushi, K.; Kato, T.; Hara, K.Y. Carotenoid nostoxanthin production by Sphingomonas sp. SG73 isolated from deep sea sediment. Mar. Drugs 2021, 19, 274. [Google Scholar] [CrossRef]
- Bijttebier, S.; D’Hondt, E.; Noten, B.; Hermans, N.; Apers, S.; Voorspoels, S. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. J. Chromatogr. A 2014, 1332, 46–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Sun, Q.; Zhang, S.M.; Sun, X.Y.; Li, C.Y.; Tang, J. Characterization and antioxidant activity of released exopolysaccharide from a potential probiotic Leuconostoc mesenteroides LM187. J. Microbiol. Biotechnol. 2021, 31, 1144–1153. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gomez-Villegas, P.; Ghennai, O.; Bonete, M.J.; León, R.; Kharroub, K. Bioprospecting and characterization of pigmented halophilic archaeal strains from Algerian hypersaline environments with analysis of carotenoids produced by Halorubrum sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid stabilized gold and silver nanoparticles derived from the Actinomycete Gordonia amicalis HS-11 as effective free radical scavengers. Enzyme Microb. Tech. 2016, 95, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; Refaie, A.Z. Purification and characterization of pedioxanthin (carotenoid pigment) produced by Pediococcus pentosaceus N33 strain isolated from pickles. Food Biotechnol. 2019, 33, 217–236. [Google Scholar] [CrossRef]
- Ye, M.; Chen, W.X.; Qiu, T.; Yuan, R.Y.; Ye, Y.W.; Cai, J.M. Structural characterisation and anti-ageing activity of extracellular polysaccharide from a strain of Lachnum sp. Food Chem. 2012, 132, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Parasar, D.P.; Ramakrishnan, E.; Kabilan, S.; Kotoky, J.; Sarma, H.K. Characterization of β-cryptoxanthin and other carotenoid derivatives from Rhodotorula taiwanensis, a novel yeast isolated from traditional starter culture of assam. Chem. Biodivers. 2020, 17, e2000198. [Google Scholar] [CrossRef]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Swetha, S. Bacillus circulans exopolysaccharide: Production, characterization and bioactivities. Int. J. Biol. Macromol. 2016, 87, 405–414. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Liu, J.; Wu, L.L.; Cao, Y.R.; Xu, J.; Xue, C.H. Preparation, characterization and antioxidant activity of astaxanthin esters with different molecular structures. J. Sci. Food Agric. 2020, 101, 2576–2583. [Google Scholar] [CrossRef]
- Finger, S.; Godoy, F.A.; Wittwer, G.; Aranda, C.P.; Calderon, R.; Miranda, C.D. Purification and characterization of indochrome type blue pigment produced by Pseudarthrobacter sp. 34LCH1 isolated from Atacama desert. J. Ind. Microbiol. Biot. 2019, 46, 101–111. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Diaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The benefits and risks of certain dietary carotenoids that exhibit both anti-and pro-oxidative mechanisms-a comprehensive review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tei, M.; Longini, M.; Buonocore, G. The multiple facets of lutein: A call for further investigation in the perinatal period. Oxidative Med. Cell. Longev. 2016, 2016, 5381540. [Google Scholar] [CrossRef] [PubMed]
- Saidi, E.A.; Davey, P.G.; Cameron, D.J. The effect of zeaxanthin on the visual acuity of zebrafish. PLoS ONE 2015, 10, e0135211. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chang, C.Y.; Kao, A.; His, B.; Lee, S.H.; Chen, Y.H.; Wang, I.J. Hypoxia-induced retinal neovascularization in zebrafish embryos: A potential model of retinopathy of prematurity. PLoS ONE 2015, 10, e0126750. [Google Scholar] [CrossRef]
- Sun, L.; Inaba, Y.; Sogo, Y.; Kunugita, N.; Chida, K.; Moritake, T. Ionizing radiation reduces glutathione levels in the eye: A pilot study. J. Radiat. Res. Appl. Sc. 2022, 15, 106–110. [Google Scholar] [CrossRef]
- Lai, J.Y.; Luo, L.J.; Nguyen, D.D. Multifunctional glutathione-dependent hydrogel eye drops with enhanced drug bioavailability for glaucoma therapy. Chem. Eng. J. 2020, 402, 126190. [Google Scholar] [CrossRef]
- Heruye, S.H.; Maffofou, N.L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.F.; Opere, C.A. Current trends in the pharmacotherapy of cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef]

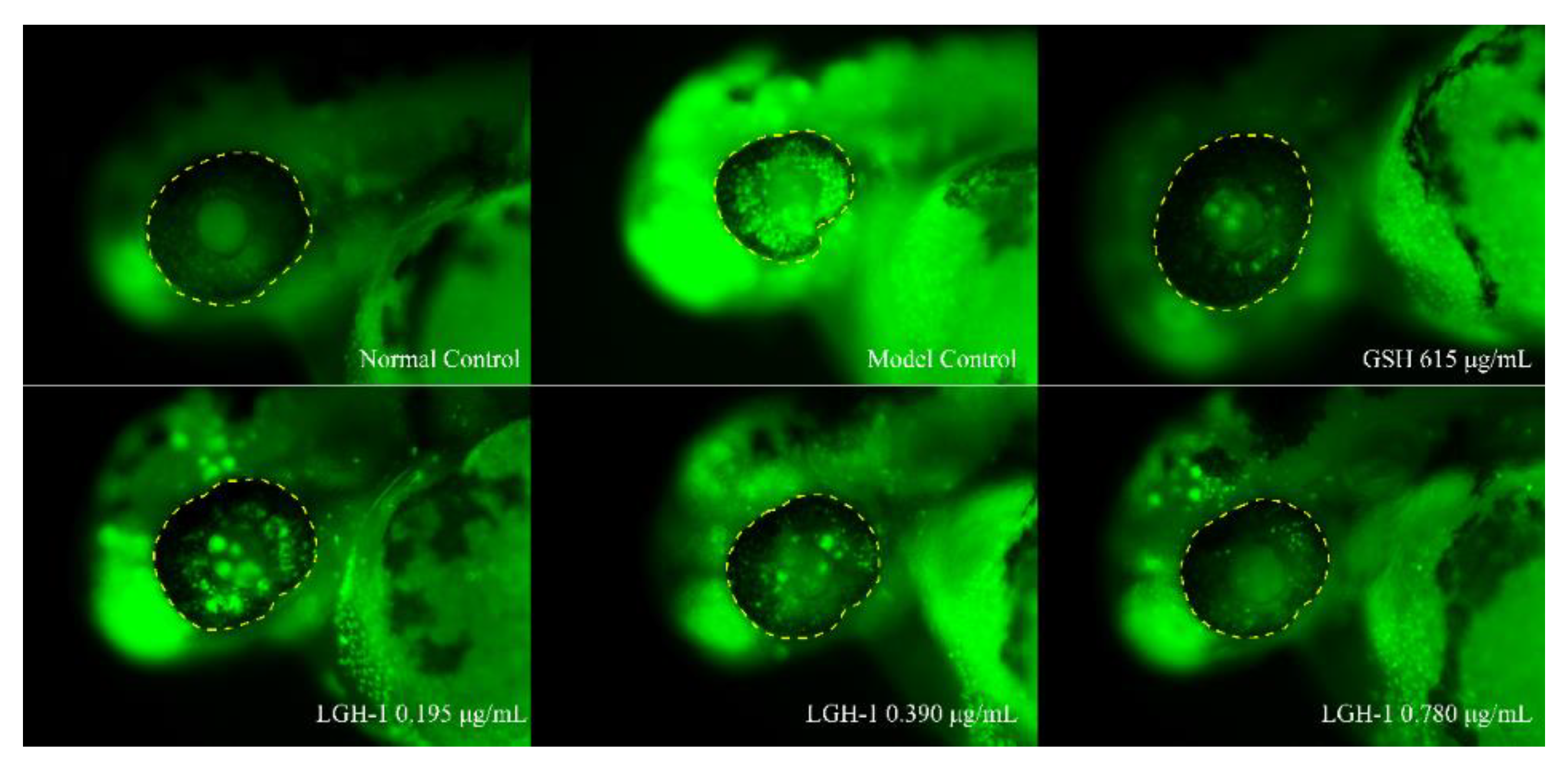
| Group | Concentration (μg/mL) | Mortality (%) | Phenotype |
|---|---|---|---|
| Normal Control | - | 0 | No obvious abnormality No obvious abnormality |
| Model Control | - | 0 | |
| LGH-1 | 0.049 | 0 | The state was similar to that of the model control |
| 0.098 | 0 | ||
| 0.195 | 0 | ||
| 0.390 | 0 | ||
| 0.780 | 0 | ||
| 1.560 | 0 | The state was worse than that of the model control |
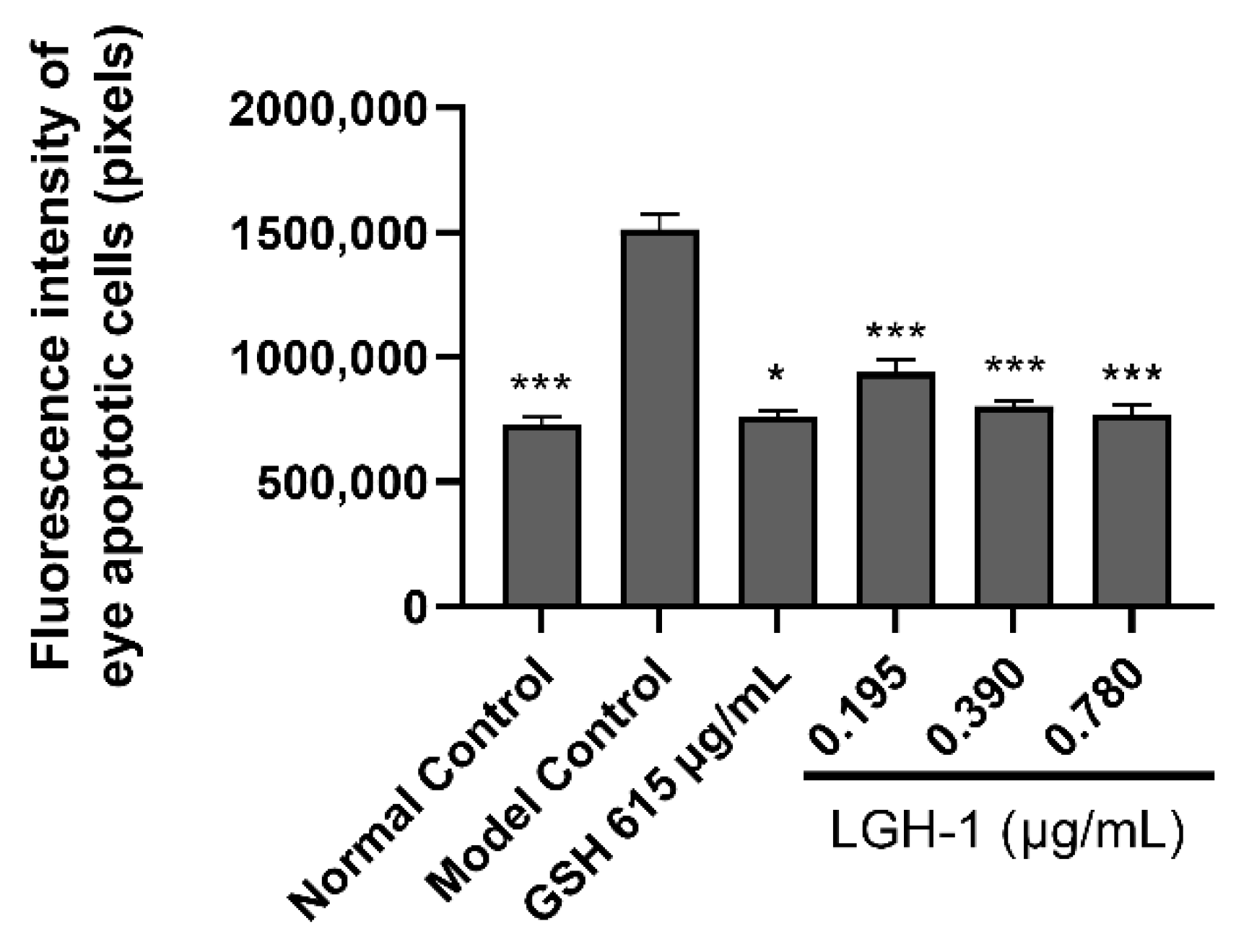
| Group | Concentration (μg/mL) | Fluorescence Intensity of Ocular Apoptotic Cells (Pixel, Mean ± SE) |
|---|---|---|
| Normal Control | - | 733,008 ± 27,451 *** |
| Model Control | - | 1,513,891 ± 58,443 |
| GSH | 615 | 761,321 ± 23,260 *** |
| LGH-1 | 0.195 | 940,424 ± 51,050 * |
| 0.390 | 804,441 ± 17,855 *** | |
| 0.780 | 768,614 ± 40,160 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, J.; Li, C.; Zheng, M.; He, Z.; Zou, Y.; Xiong, H.; Xu, B.; Xiang, W.; Tang, J. Characterization and Bioactive Potential of Carotenoid Lutein from Gordonia rubripertncta GH-1 Isolated from Traditional Pixian Douban. Foods 2022, 11, 3649. https://doi.org/10.3390/foods11223649
Zhang Q, Wang J, Li C, Zheng M, He Z, Zou Y, Xiong H, Xu B, Xiang W, Tang J. Characterization and Bioactive Potential of Carotenoid Lutein from Gordonia rubripertncta GH-1 Isolated from Traditional Pixian Douban. Foods. 2022; 11(22):3649. https://doi.org/10.3390/foods11223649
Chicago/Turabian StyleZhang, Qing, Jie Wang, Chanyuan Li, Miaoxin Zheng, Zihan He, Yuting Zou, Haibo Xiong, Bitao Xu, Wenliang Xiang, and Jie Tang. 2022. "Characterization and Bioactive Potential of Carotenoid Lutein from Gordonia rubripertncta GH-1 Isolated from Traditional Pixian Douban" Foods 11, no. 22: 3649. https://doi.org/10.3390/foods11223649
APA StyleZhang, Q., Wang, J., Li, C., Zheng, M., He, Z., Zou, Y., Xiong, H., Xu, B., Xiang, W., & Tang, J. (2022). Characterization and Bioactive Potential of Carotenoid Lutein from Gordonia rubripertncta GH-1 Isolated from Traditional Pixian Douban. Foods, 11(22), 3649. https://doi.org/10.3390/foods11223649






