Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Culture
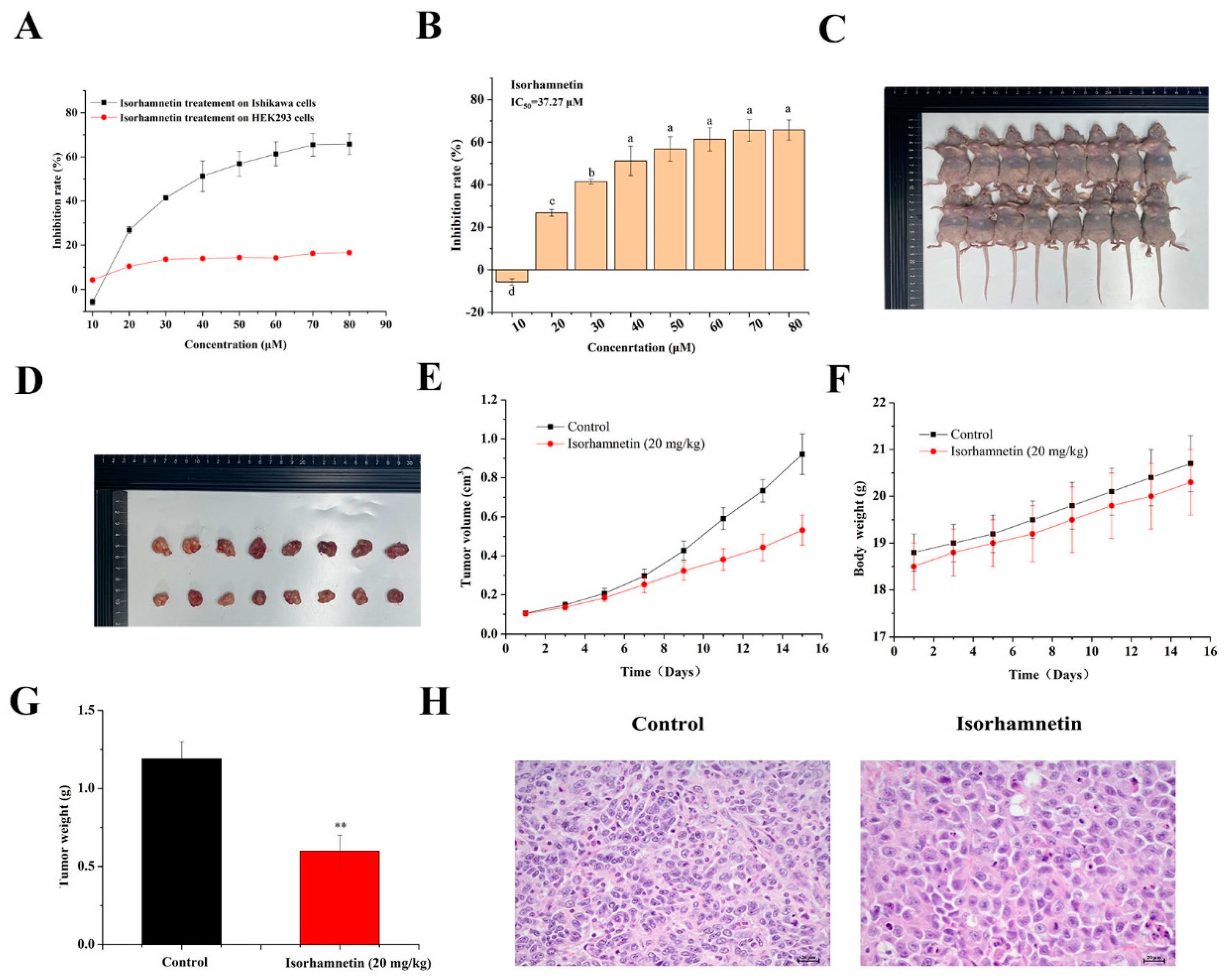
2.3. Cell Inhibition Assay
2.4. Tumor-Bearing Mice Assay
2.5. H&E Staining Test
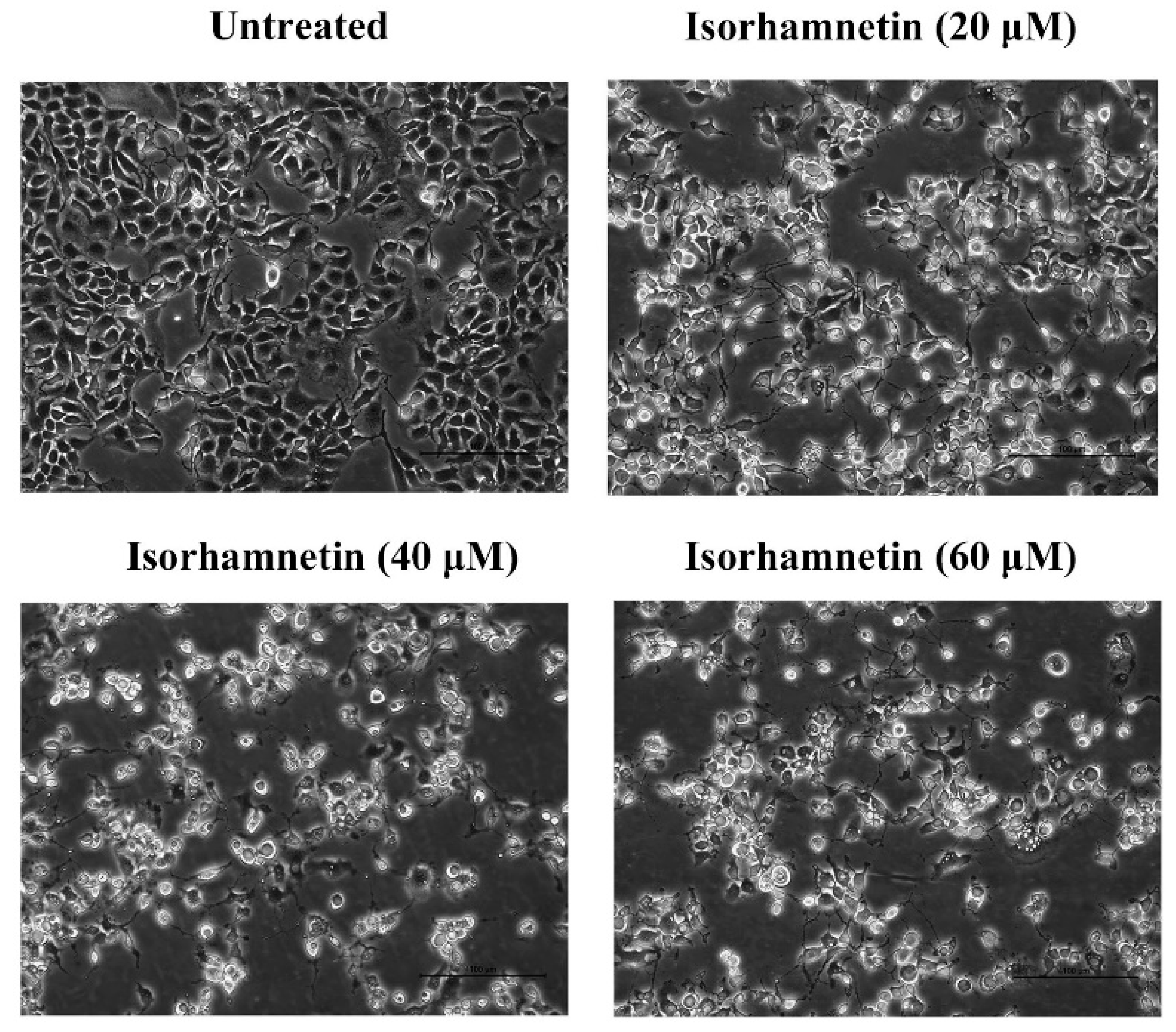
2.6. Cell Morphology Observation
2.7. Cell Cycle Assay
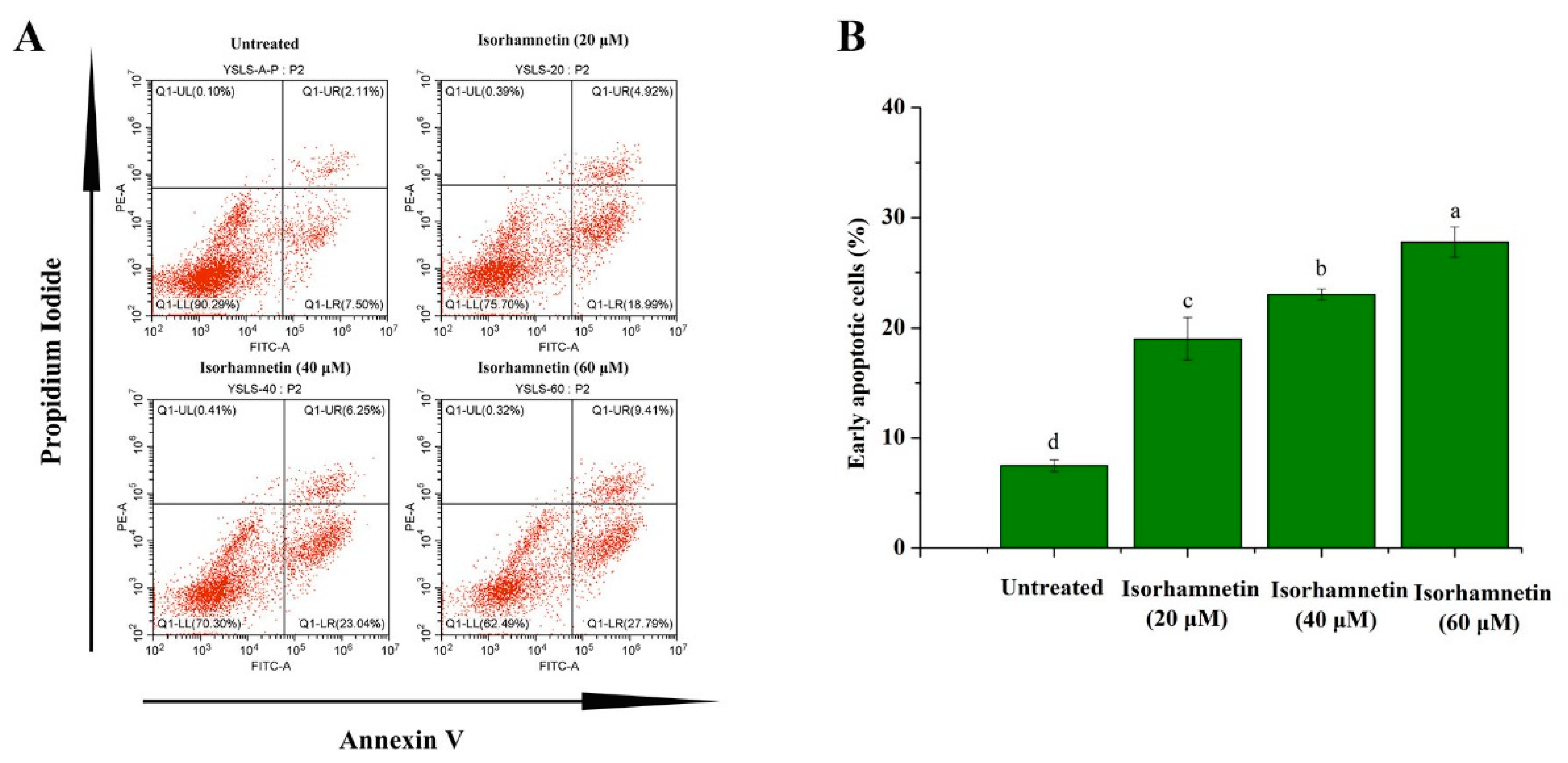
2.8. Apoptosis Assay
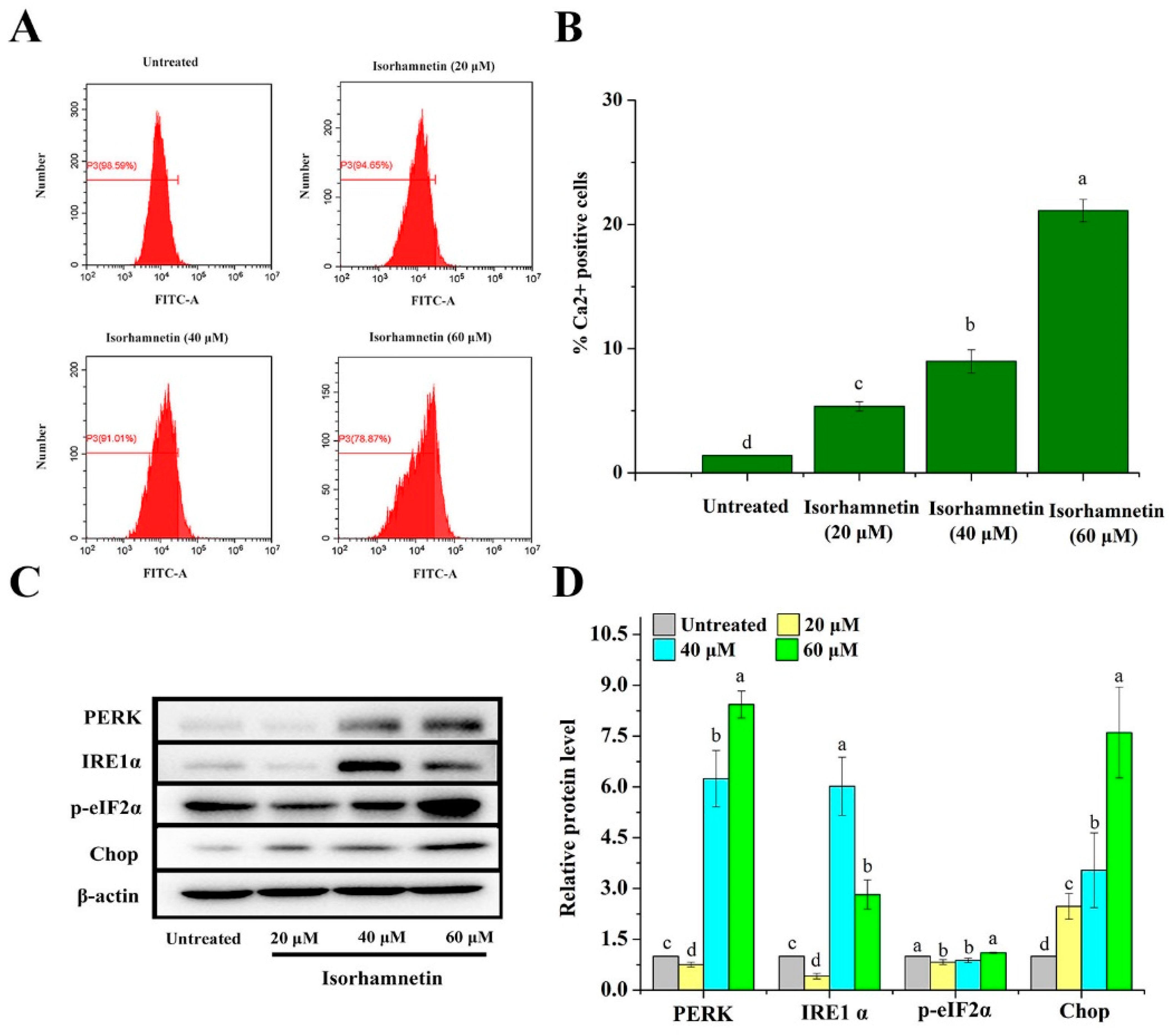
2.9. Cellular Calcium Ion Permeation Assay
2.10. Reactive Oxygen Assay
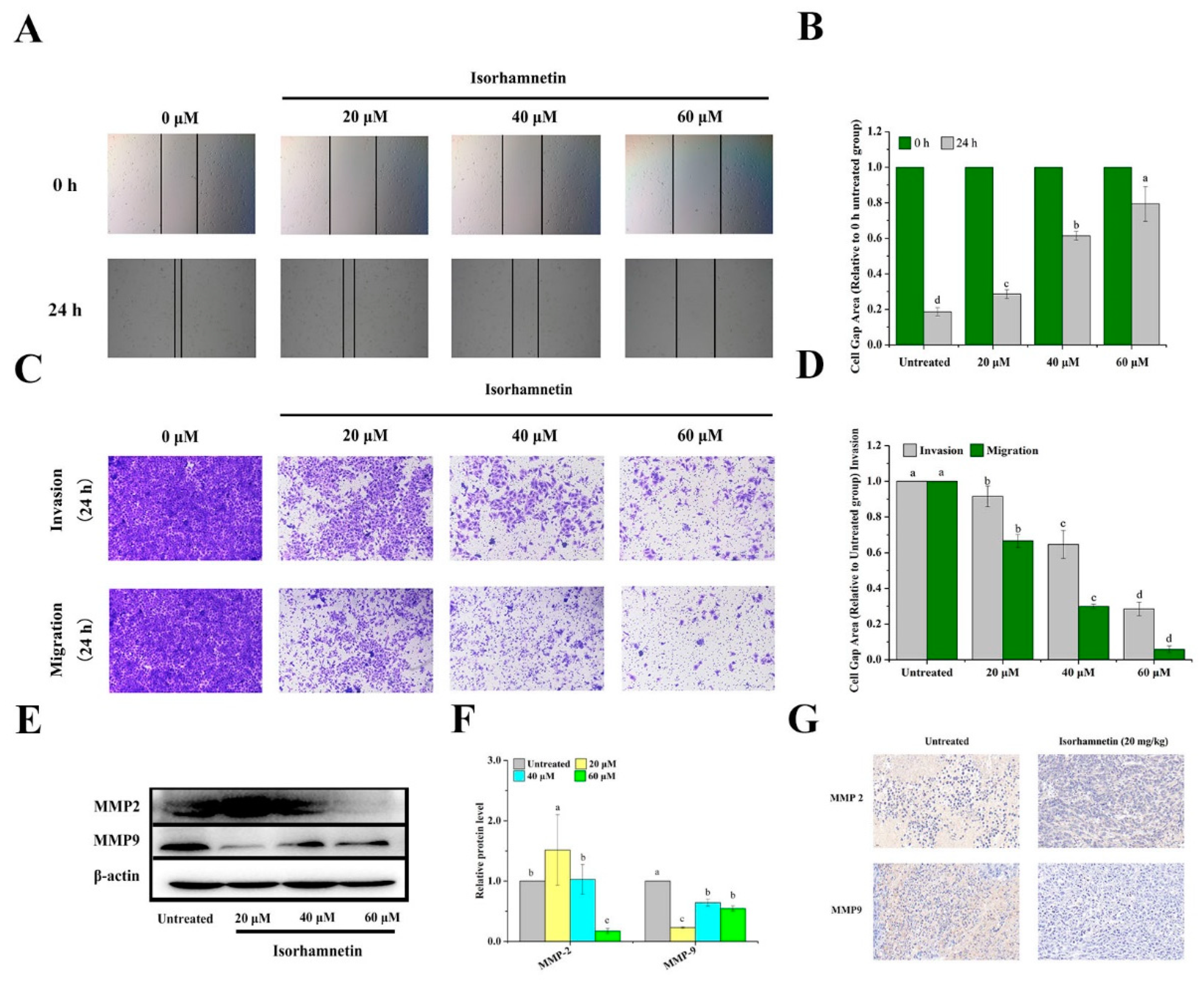
2.11. Cell Scratch Heal Test
2.12. Cell Migration and Invasion Assay
2.13. Cell Protein Extraction and Western Blotting Experiments
2.14. Immunohistochemistry
2.15. Data Analysis
3. Results and Discussion
3.1. Consequence of Isorhamnetin on the Proliferation of Ishikawa Cells
3.2. Effect of Isorhamnetin on the Growth of Tumors in Ishikawa Tumor-Bearing Mice
3.3. Effect of Isorhamnetin on the Morphology of Ishikawa Cells
3.4. Isorhamnetin Blocks the G2/M Phase in Ishikawa Cells
3.5. Isorhamnetin Promotes Apoptosis in Ishikawa Cells
3.6. Effect of Isorhamnetin on Calcium Leakage from the Endoplasmic Reticulum of Ishikawa Cells
3.7. Effect of Isorhamnetin on Mitochondrial Apoptosis and Death Receptor Signaling Pathways in Ishikawa Cells
3.8. Effect of Isorhamnetin on Immunohistochemical Assessment In Vivo
3.9. Reactive Oxygen Species Levels of Ishikawa Cells Treated by Isorhamnetin
3.10. Effect of Isorhamnetin on Metastasis of Ishikawa Cells and Ishikawa Xenografts in Mice
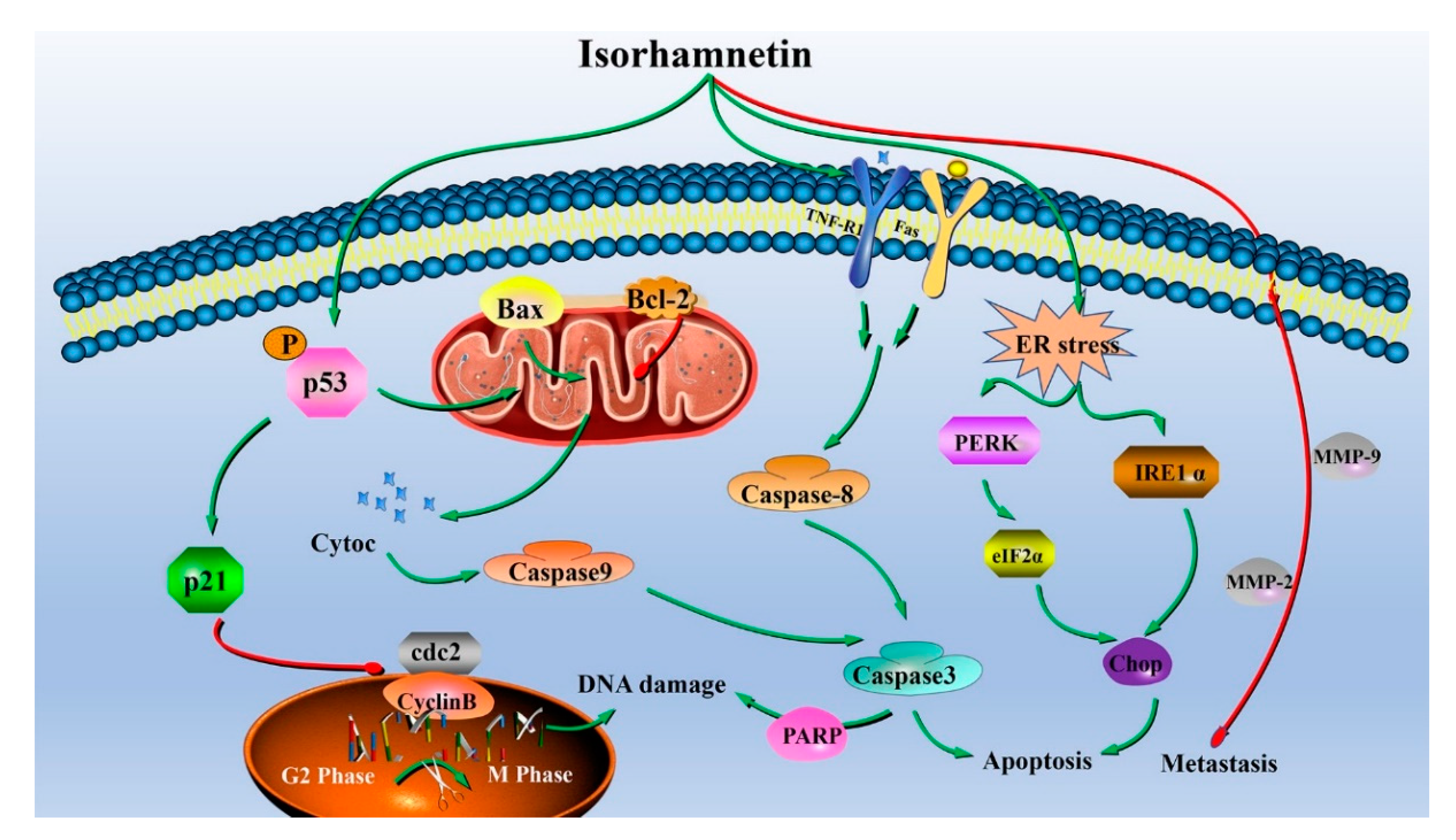
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Njoku, K.; Abiola, J.; Russell, J.; Crosbie, E.J. Endometrial cancer prevention in high-risk women. Best Pr. Res. Clin. Obs. Gynaecol. 2020, 65, 66–78. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Arsad, A.; Yong, C.; Teo, D.B.S. Differential diagnosis of brain lesions in a metastatic endometrial carcinosarcoma patient. Ecancermedicalscience 2021, 15, 1182. [Google Scholar] [CrossRef]
- Kitson, S.J.; Evans, D.G.; Crosbie, E.J. Identifying high-risk women for endometrial cancer prevention strategies: Proposal of an endometrial cancer risk prediction model. Cancer Prev. Res. 2017, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi. 1985, 37, 1103–1111. [Google Scholar] [PubMed]
- Zhang, X.X.; Liao, B.Y.; Guan, Z.J.; Thakur, K.; Khan, M.R.; Busquets, R.; Zhang, J.G.; Wei, Z.J. Interaction between Gelatin and Mulberry Leaf Polysaccharides in Miscible System: Physicochemical Characteristics and Rheological Behavior. Foods 2022, 11, 1571. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Kou, X.; Wang, Y.; Yu, Y.; Zhen, N.; Jiang, J.; Zhaxi, P.; Xue, Z. Synergistic hypolipidemic effects and mechanisms of phytochemicals: A Review. Foods 2022, 11, 2774. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits-review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.M.; Bakhiet, M.; Ehemann, V. Flow cytometry detection of sperm DNA fragmentation and apoptotic markers in the semen of infertile males. Int. J. Reprod. Med. 2021, 2021, 9531775. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ni, Z.J.; Elam, E.; Zhang, F.; Thakur, K.; Wang, S.; Zhang, J.G.; Wei, Z.J. Juglone, a novel activator of ferroptosis, induces cell death in endometrial carcinoma Ishikawa cells. Food Funct. 2021, 12, 4947–4959. [Google Scholar] [CrossRef]
- Dai, G.; Chen, X.; He, Y. The gut microbiota activates AhR through the tryptophan metabolite kyn to mediate renal cell carcinoma metastasis. Front. Nutr. 2021, 8, 712327. [Google Scholar] [CrossRef]
- Wamidh, H.T.; Mallak, J.A.; Sumaiah, A.N.; Rawand, E.A.; Arkan, H.Y.; Anfal, A.D.; Samar, T.; Asma, I.M. Anticancer effect of spices used in Mediterranean diet: Preventive and therapeutic potentials. Front. Nutr. 2022, 9, 905658. [Google Scholar]
- Song, Z.; Tian, X.; Shi, Q. Fas, Caspase-8, and Caspase-9 pathway-mediated bile acid-induced fetal cardiomyocyte apoptosis in intrahepatic cholestasis pregnant rat models. J. Obstet. Gynaecol. Res. 2021, 47, 2298–2306. [Google Scholar] [CrossRef]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing Caspase-3 and Caspase-9 substrates in and out of apoptosis with deep substrate profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef]
- Chan, Y.P.; Chuang, C.H.; Lee, I.; Yang, N.C. Lycopene in combination with sorafenib additively inhibits tumor metastasis in mice xenografted with lewis lung carcinoma cells. Front. Nutr. 2022, 9, 886988. [Google Scholar] [CrossRef]
- Cirillo, N.; Wu, C.; Prime, S.S. Heterogeneity of cancer stem cells in tumorigenesis, metastasis, and resistance to antineoplastic treatment of head and neck tumours. Cells 2021, 10, 3068. [Google Scholar] [CrossRef]
- Han, W.Y.; Li, Q.S.; Zhang, Q.; Xu, F. Epithelial-mesenchymal transition and breast cancer stem cells in breast cancer progression. ABCR 2022, 11, 141–151. [Google Scholar] [CrossRef]
- Ma, X.; Ge, A.; Han, J.; Kang, J.; Zhang, Y.; Liu, X.H.; Xing, L.; Liu, X.C.; Dong, L. Meta-analysis of downregulated E-cadherin as a diagnostic biomarker for cervical cancer. Arch. Gynecol. Obstet. 2022, 1–11. [Google Scholar] [CrossRef]
- Liang, Y.C.; Zhong, Q.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Khan, M.R.; Rosa, B.; Zhang, J.G.; Wei, Z.J. Apigenin inhibits migration and induces apoptosis of human endometrial carcinoma Ishikawa cells via PI3K-AKT-GSK-3β pathway and endoplasmic reticulum stress. J. Funct. Foods 2022, 94, 105116. [Google Scholar] [CrossRef]
- Ma, R.H.; Ni, Z.J.; Zhang, F.; Zhang, Y.Y.; Liu, M.M.; Thakur, K.; Zhang, J.G.; Wang, S.Y.; Wei, Z.J. 6-Shogaol mediated ROS production and apoptosis via endoplasmic reticulum and mitochondrial pathways in human endometrial carcinoma Ishikawa cells. J. Funct. Foods 2020, 74, 104178. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.Y.; Sun, Y.S.; Ma, R.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Asparanin A from asparagus officinalis l. induces G0/G1 cell cycle arrest and apoptosis in human endometrial carcinoma ishikawa cells via mitochondrial and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2020, 68, 213–224. [Google Scholar] [CrossRef]
- Sun, Y.S.; Thakur, K.; Hu, F.; Cespedes-Acuña, C.L.; Zhang, J.G.; Wei, Z.J. Icariside II suppresses cervical cancer cell migration through JNK modulated matrix metalloproteinase-2/9 inhibition in vitro and in vivo. Biomed. Pharmacother. 2020, 125, 110013. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Cespedes-Acuña, C.L.; Jiang, L.; Wei, Z.J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer HeLa cells. Food Chem. Toxicol. 2020, 146, 111843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Cespedes-Acuña, C.L.; Wang, S.Y.; Zhang, J.G.; Wei, Z.J. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial-dependent signaling pathway. Food Chem. Toxicol. 2021, 148, 111941. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Q.; Qin, H.X.; Jiang, B.; Lu, W.F.; Hao, J.G.; Cao, W.M.; Du, L.; Chen, W.; Zhao, X.Z.; Guo, H.Q. KLF2 inhibits cancer cell migration and invasion by regulating ferroptosis through GPX4 in clear cell renal cell carcinoma. Cancer Lett. 2021, 522, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.S.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Icariside II inhibits tumorigenesis via inhibiting AKT/Cyclin E/CDK 2 pathway and activating mitochondria-dependent pathway. Pharmacol. Res. 2020, 152, 104616. [Google Scholar] [CrossRef]
- Liu, J.Y.; Fu, W.Q.; Zheng, X.J.; Li, W.; Ren, L.W.; Wang, J.H.; Yang, C.; Du, G.H. Avasimibe exerts anticancer effects on human glioblastoma cells via inducing cell apoptosis and cell cycle arrest. Acta. Pharmacol. Sin. 2021, 42, 97–107. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Nasr, F.A.; Qamar, W.; Noman, O.M.; Khan, J.M.; Al-Mishari, A.A.; Ali, S.A. Janerin induces cell cycle arrest at the G2/M phase and promotes apoptosis involving the MAPK pathway in THP-1, leukemic cell line. Molecules 2021, 26, 7555. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Mir, R.H.; Wani, T.U.; Alsharif, K.F.; Alam, W.; Albrakati, A.; Saso, L.; Khan, H. The regulation of endoplasmic reticulum stress in cancer: Special focuses on luteolin patents. Molecules 2022, 27, 2471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, L.; Zhang, G.; Ji, G.; Xu, H. The role and therapeutic implication of endoplasmic reticulum stress in inflammatory cancer transformation. Am. J. Cancer Res. 2022, 12, 2277–2292. [Google Scholar] [PubMed]
- Al Mughairbi., F.; Nawaz, R.; Khan, F.; Hassan, A.; Mahmood, N.; Ahmed, H.T.; Alshamali, A.; Ahmed, S.; Bashir, A. Neuroprotective effects of Bhilawanol and Anacardic acid during glutamate-induced neurotoxicity. Saudi Pharm. J. 2021, 29, 1043–1049. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, Y.; Guo, J.; Liu, Y.X.; Zhong, J.; Liu, Y.Q.; Pan, L.R.; Guo, Y.; Tan, Y.; Yu, X.J. Effect of pristimerin on apoptosis through activation of ROS/endoplasmic reticulum (ER) stress-mediated noxa in colorectal cancer. Phytomedicine 2021, 80, 153399. [Google Scholar] [CrossRef]
- Chang, C.Y.; Pan, P.H.; Wu, C.C.; Liao, S.L.; Chen, W.Y.; Kuan, Y.H.; Wang, W.Y.; Chen, C.J. Endoplasmic reticulum stress contributes to gefitinib-induced apoptosis in glioma. Int. J. Mol. Sci. 2021, 22, 3934. [Google Scholar] [CrossRef]
- Park, M.N.; Park, H.; Rahman, M.A.; Kim, J.W.; Park, S.S.; Cho, Y.; Choi, J.; Son, S.R.; Jang, D.S.; Shim, B.S.; et al. BK002 induces miR-192-5p-mediated apoptosis in castration-resistant prostate cancer cells via modulation of PI3K/CHOP. Front. Oncol. 2022, 12, 791365. [Google Scholar] [CrossRef]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.C.; Yu, X.J.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef]
- Batbold, U.; Liu, J.J. Chemosensitization Effect of Seabuckthorn (Hippophae rhamnoides L.) Pulp Oil via Autophagy and Senescence in NSCLC Cells. Foods 2022, 11, 1517. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Mc Glorthan, L.; Paucarmayta, A.; Casablanca, Y.; Maxwell, G.L.; Syed, V. Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis 2021, 26, 184–194. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting bcl-2 in cancer: Advances, challenges, and perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Pervaiz, S. TNF receptor superfamily-induced cell death: Redox-dependent execution. FASEB J. 2006, 20, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.S.; Friedmann, K.S.; Mang, S.; Knörck, A.; Hoth, M.; Kummerow, C. Natural killer cells induce distinct modes of cancer cell death: Discrimination, quantification, and modulation of apoptosis, necrosis, and mixed forms. J. Biol. Chem. 2018, 293, 16348–16363. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, M.Y.; Wu, J.; Chen, F.H.; Wang, Y.; Ma, Y.J.; Dai, Z.J. Glioma progression is suppressed by Naringenin and APO2L combination therapy via the activation of apoptosis in vitro and in vivo. Investig. New Drugs 2020, 38, 1743–1754. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Hong, J.Q.; Song, Y.M.; Xie, J.Y.; Xie, J.H.; Chen, Y.; Li, P.; Liu, D.Y.; Hu, X.B.; Yu, Q. Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans. Foods 2022, 11, 1590. [Google Scholar] [CrossRef]
- Fan, H.; He, Y.J.; Xiang, J.Q.; Zhou, J.; Wan, X.Y.; You, J.W.; Du, K.L.; Li, Y.; Cui, L.; Wang, Y.T.; et al. ROS generation attenuates the anti-cancer effect of CPX on cervical cancer cells by inducing autophagy and inhibiting glycophagy. Redox Biol. 2022, 53, 102339. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Sun, X.; Chen, P.; Chen, X.; Yang, W.; Chen, X.; Zhou, W.; Huang, D.; Cheng, Y.F. KIF4A enhanced cell proliferation and migration via Hippo signaling and predicted a poor prognosis in esophageal squamous cell carcinoma. Thorac. Cancer 2021, 12, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, D.; Liu, F.; Chen, Y. Rhein inhibits the progression of chemoresistant lung cancer cell lines via the Stat3/Snail/MMP2/MMP9 pathway. Biomed. Res. Int. 2022, 2022, 7184871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Ma, R.-H.; Zhang, X.-X.; Thakur, K.; Zhang, J.-G.; Khan, M.R.; Busquets, R.; Wei, Z.-J. Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo. Foods 2022, 11, 3415. https://doi.org/10.3390/foods11213415
Ye L, Ma R-H, Zhang X-X, Thakur K, Zhang J-G, Khan MR, Busquets R, Wei Z-J. Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo. Foods. 2022; 11(21):3415. https://doi.org/10.3390/foods11213415
Chicago/Turabian StyleYe, Lei, Run-Hui Ma, Xiu-Xiu Zhang, Kiran Thakur, Jian-Guo Zhang, Mohammad Rizwan Khan, Rosa Busquets, and Zhao-Jun Wei. 2022. "Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo" Foods 11, no. 21: 3415. https://doi.org/10.3390/foods11213415
APA StyleYe, L., Ma, R.-H., Zhang, X.-X., Thakur, K., Zhang, J.-G., Khan, M. R., Busquets, R., & Wei, Z.-J. (2022). Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo. Foods, 11(21), 3415. https://doi.org/10.3390/foods11213415







