Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Snack Production
2.3. In Vitro Digestibility of Starch
2.4. Protein In Vitro Digestibility
2.5. Phenolic Compounds
2.5.1. Extractable Phenolic Compounds (EPP)
2.5.2. Hydrolyzable Phenolic Compounds (HPP)
2.6. Antioxidants
2.6.1. ABTS
2.6.2. DPPH
2.7. Determination of Carotenoid Content by Spectrophotometric Method
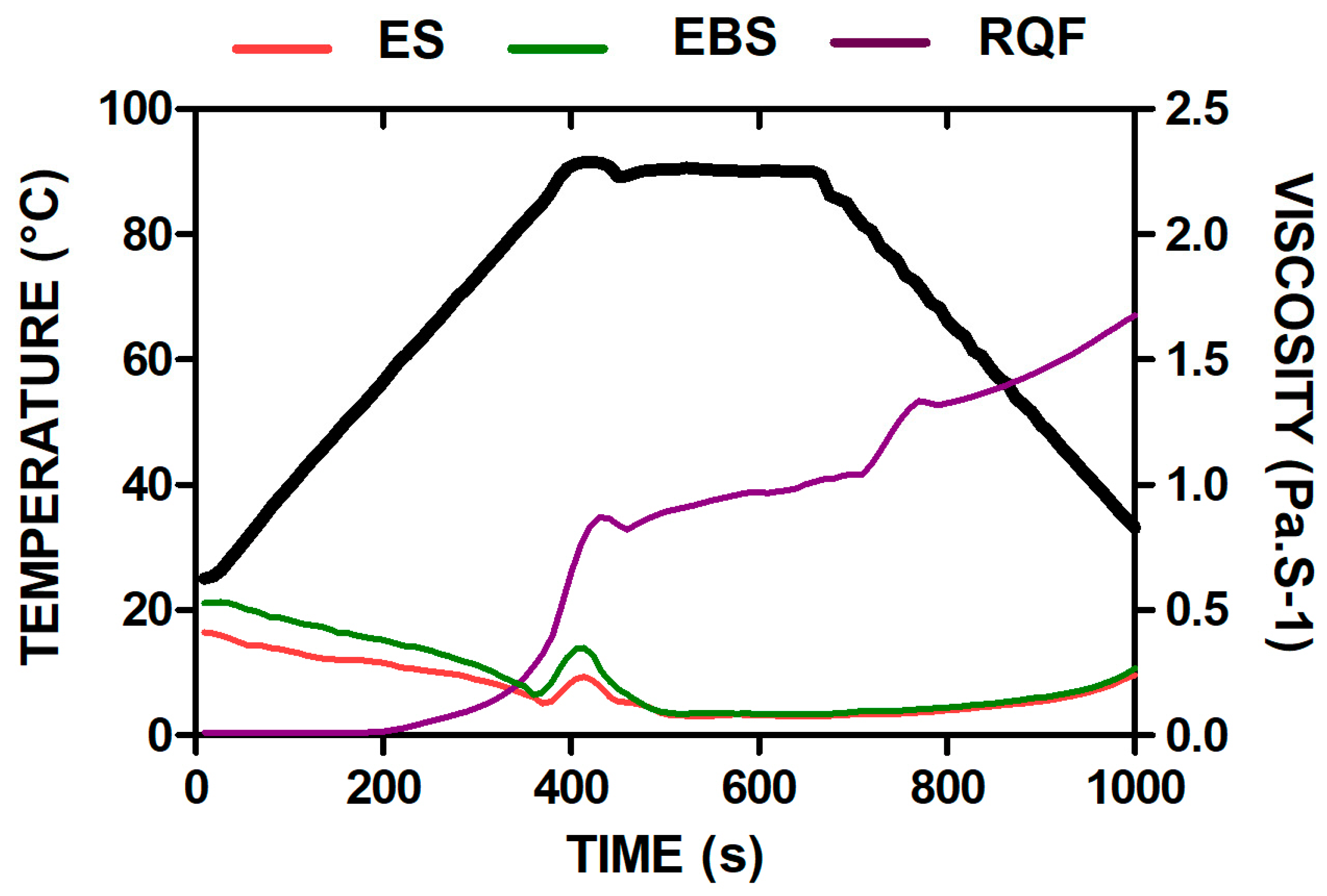
2.8. Pasting Properties
2.9. Color
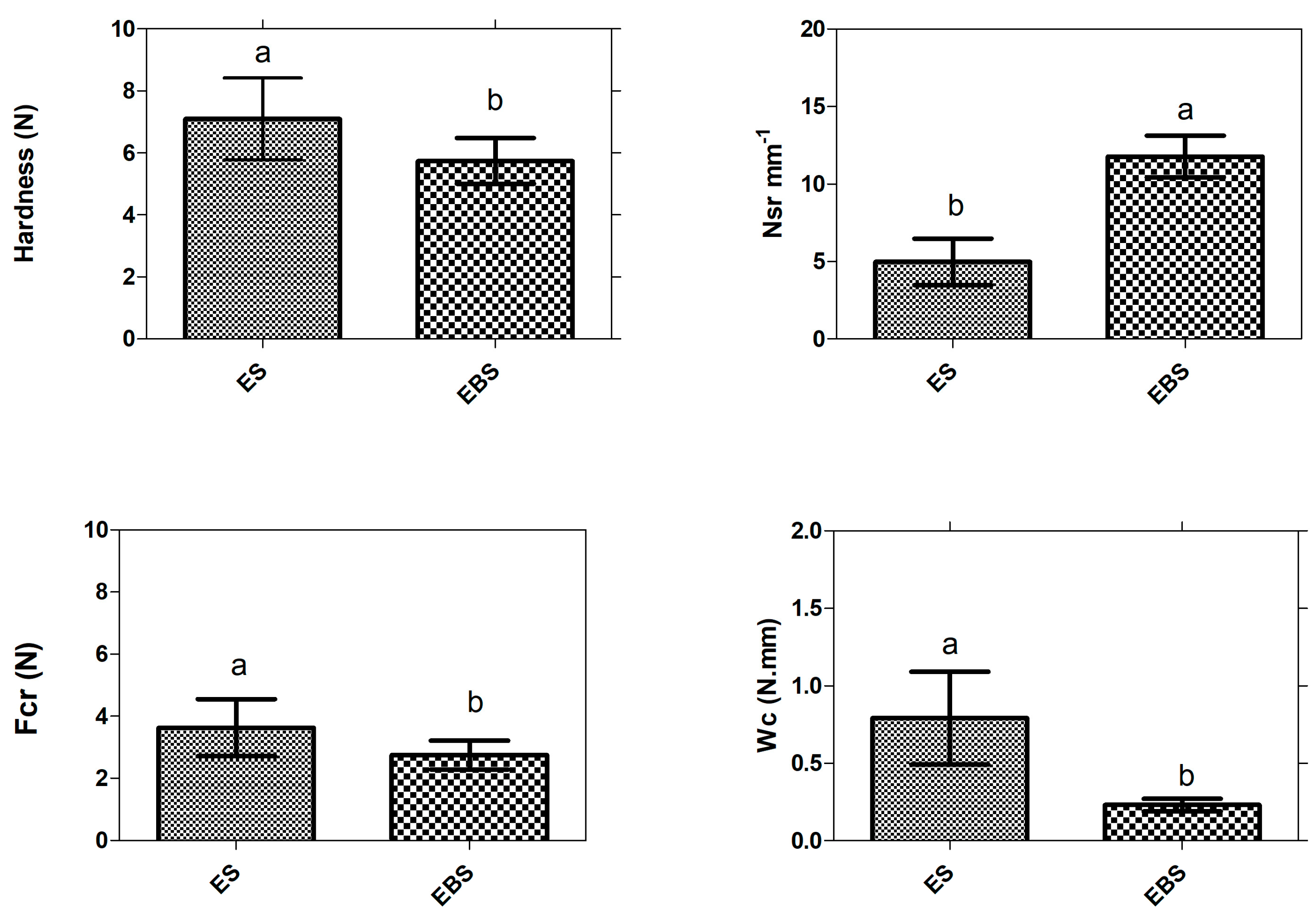
2.10. Textural Properties
2.11. Statistical Analyses
3. Results
4. Discussion
4.1. Carbohydrate and Protein Digestibility
4.2. Effect of Processing on EPP and HPP
4.3. Effect of Processing on the Antioxidant Activities and Carotenoids
4.4. Pasting
4.5. Color
4.6. Texture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.R.G.; Singh, P. Paediatrics and International Child Health Coeliac disease Coeliac disease. Paediatr. Int. Child Health 2018, 39, 23–31. [Google Scholar] [CrossRef]
- Tursi, A. Gastrointestinal Motility Disturbances in Celiac Disease. J. Clin. Gastroenterol. 2004, 38, 642–645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volta, U.; Tovoli, F.; Caio, G. Disease in patients with Type 1 diabetes mellitus Clinical and immunological features of celiac disease in patients with Type 1 diabetes mellitus. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef]
- Gallagher, E. Formulation and nutritional aspects of gluten-free cereal products and infant foods. In Gluten-Free Cereal Products and Beverages; Elsevier Inc.: Cork, Irlanda, 2008; pp. 322–341. [Google Scholar]
- Abdel-Aal, E.-S.M. Functionality of Starches and Hydrocolloids in Gluten-Free Foods. In Gluten-Free Food Science and Technology; Wiley Blackwell: London, UK, 2009; pp. 200–224. [Google Scholar] [CrossRef]
- MADR. En los Últimos 4 años, la Quinua ha Tenido un Crecimiento de más del 150% en áreas de Producción. Ministerio de Agricultura y Desarrollo Rural (MADR) de Colombia, Bogotá, Colombia. Available online: https://www.minagricultura.gov.co/noticias/Paginas/En-los-últimos-4-años,-la-quinua-ha-tenido-un-crecimiento-de-más-del-150-en-áreas-de-producción-.aspx (accessed on 1 September 2022).
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Peñas, E.; Uberti, F.; Di Lorenzo, C.; Ballabio, C.; Brandolini, A.; Restani, P. Biochemical and Immunochemical Evidences Supporting the Inclusion of Quinoa (Chenopodium quinoa Willd.) as a Gluten-free Ingredient. Plant Foods Hum. Nutr. 2014, 69, 297–303. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Ellis, H.J.; Šuligoj, T.; Herencia, L.I.; Ciclitira, P.J. Variable activation of immune response by quinoa (Chenopodium quinoa Willd.) prolamins in celiac disease. Am. J. Clin. Nutr. 2012, 96, 337–344. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Ghumman, A.; Mudgal, S.; Singh, N.; Ranjan, B.; Kaur, A.; Rana, J.C. Physicochemical, functional and structural characteristics of grains, flour and protein isolates of Indian quinoa lines. Food Res. Int. 2020, 140, 109982. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Effect of processing on in vitro digestibility of protein and starch in quinoa seeds. Int. J. Food Sci. Technol. 2007, 29, 449–456. [Google Scholar] [CrossRef]
- Değirmencioğlu, N.; Gürbüz, O.; Herken, E.N.; Yıldız, A.Y. The impact of drying techniques on phenolic compound, total phenolic content and antioxidant capacity of oat flour tarhana. Food Chem. 2016, 194, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kataria, A.; Singh, B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). LWT 2022, 160, 113256. [Google Scholar] [CrossRef]
- García-Parra, M.; Roa-Acosta, D.; García-Londoño, V.; Moreno-Medina, B.; Bravo-Gomez, J. Structural Characterization and Antioxidant Capacity of Quinoa Cultivars Using Techniques of FT-MIR and UHPLC/ESI-Orbitrap MS Spectroscopy. Plants 2021, 10, 2159. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A Systematic Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef]
- Song, J.; Shao, Y.; Chen, X.; Li, X. Release of characteristic phenolics of quinoa based on extrusion technique. Food Chem. 2020, 374, 128780. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2011, 133, 1–9. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.-S.M. The Impact of Milling and Thermal Processing on Phenolic Compounds in Cereal Grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef]
- Félix-Medina, J.V.; Gutiérrez-Dorado, R.; López-Valenzuela, J.A.; López-Ángulo, G.; Quintero-Soto, M.F.; Perales-Sánchez, J.X.K.; Montes-Ávila, J. Nutritional, antioxidant and phytochemical characterization of healthy ready-to-eat expanded snack produced from maize/common bean mixture by extrusion. LWT 2021, 142, 111053. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, U.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves—The influence of protein–phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef]
- Latinreco, M.J.K. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef] [PubMed]
- Waramboi, J.G.; Gidley, M.J.; Sopade, P.A. Carotenoid contents of extruded and non-extruded sweetpotato flours from Papua New Guinea and Australia. Food Chem. 2013, 141, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Kimura, M.; Godoy, H.T.; Amaya-Farfan, J. Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. J. Food Compos. Anal. 2008, 21, 445–463. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus Technical Monograph: Washington, DC, USA; International Food Policy Research Institute: Cal, Colombia, 2004; Volume 2, pp. 8–19. [Google Scholar]
- Muñoz-Pabon, K.S.; Parra-Polanco, A.S.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Bravo-Gomez, J.E. Physical and Paste Properties Comparison of Four Snacks Produced by High Protein Quinoa Flour Extrusion Cooking. Front. Sustain. Food Syst. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Karkle, E.L.; Alavi, S.; Dogan, H. Cellular architecture and its relationship with mechanical properties in expanded extrudates containing apple pomace. Food Res. Int. 2012, 46, 10–21. [Google Scholar] [CrossRef]
- Öztürk, S.; Mutlu, S. Physicochemical Properties, Modifications, and Applications of Resistant Starches. In Starches for Food Application; Elsevier Inc.: Sakarya, Turkey, 2019; pp. 299–314. [Google Scholar]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Properties of starch and dietary fibre in raw and processed quinoa (Chenopodium quinoa, Willd) seeds. Plant. Foods Hum. Nutr. 1994, 45, 223–246. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Investigations on Functional and Thermo-Mechanical Properties of Gluten Free Cereal and Pseudocereal Flours. Foods 2022, 11, 1857. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Cui, L.-H.; You, X.-Y.; Jiang, Z.-H.; Qu, W.-H.; Liu, P.-D.; Ma, D.-Y.; Cui, Y.-Y. Effects of repeated and continuous dry heat treatments on the physicochemical and structural properties of quinoa starch. Food Hydrocoll. 2020, 113, 106532. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.; Maskan, M. Effect of Extrusion Cooking on Functional Properties and in vitro Starch Digestibility of Barley-Based Extrudates from Fruit and Vegetable By-Products. J. Food Sci. 2009, 74, 2. [Google Scholar] [CrossRef] [PubMed]
- Martinez, O.D.M.; Toledo, R.C.L.; Queiroz, V.A.V.; Pirozi, M.R.; Martino, H.S.D.; de Barros, F.A.R. Mixed sorghum and quinoa flour improves protein quality and increases antioxidant capacity in vivo. LWT 2020, 129, 109597. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Freire, D.T.; Boom, R.M.; Janssen, A.E.M. The Influence of Starch and Fibre on In Vitro Protein Digestibility of Dry Fractionated Quinoa Seed (Riobamba Variety). Food Biophys. 2018, 14, 49–59. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Sun, Y.; Ji, H.; Liu, F.; Peng, X.; Zhong, Y.; Geng, F.; Nie, S. In vitro digestion of eight types of wholegrains and their dietary recommendations for different populations. Food Chem. 2022, 370, 131069. [Google Scholar] [CrossRef]
- Gross, R.; Koch, F.; Malaga, I.; de Miranda, A.F.; Schoeneberger, H.; Trugo, L.C. Chemical Composition and Protein Quality of Some Local Andean Food Sources South American crops, such as potatoes (Solanum tuberosum), manioc. Food Chem. 1989, 34, 25–34. [Google Scholar] [CrossRef]
- Ye, G.; Wu, Y.; Wang, L.; Tan, B.; Shen, W.; Li, X.; Liu, Y.; Tian, X.; Zhang, D. Comparison of six modification methods on the chemical composition, functional properties and antioxidant capacity of wheat bran. LWT 2021, 149, 111996. [Google Scholar] [CrossRef]
- Gu, F.-L.; Kim, J.M.; Abbas, S.; Zhang, X.-M.; Xia, S.-Q.; Chen, Z.-X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Arribas, C.; Pereira, E.; Barros, L.; Alves, M.J.; Calhelha, R.C.; Guillamón, E.; Pedrosa, M.M.; Ferreira, I.C. Healthy novel gluten-free formulations based on beans, carob fruit and rice: Extrusion effect on organic acids, tocopherols, phenolic compounds and bioactivity. Food Chem. 2019, 292, 304–313. [Google Scholar] [CrossRef]
- Dilrukshi, H.N.; Torrico, D.D.; Brennan, M.A.; Brennan, C.S. Effects of extrusion processing on the bioactive constituents, in vitro digestibility, amino acid composition, and antioxidant potential of novel gluten-free extruded snacks fortified with cowpea and whey protein concentrate. Food Chem. 2022, 389, 133107. [Google Scholar] [CrossRef]
- Cueto, M.; Farroni, A.; Schoenlechner, R.; Schleining, G.; Buera, P. Carotenoid and color changes in traditionally flaked and extruded products. Food Chem. 2017, 229, 640–645. [Google Scholar] [CrossRef] [PubMed]
- James, L.E.A. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Santiago, Chile, 2009; Volume 58, pp. 2–24. [Google Scholar]
- Paznocht, L.; Burešová, B.; Kotíková, Z.; Martinek, P. Carotenoid content of extruded and puffed products made of colored-grain wheats. Food Chem. 2020, 340, 127951. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Review. Recent advances in lipid oxidation. J. Sci. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Jozinović, A.; Šubarić, D.; Ačkar, J.; Babić, J.; Miličević, B. Influence of spelt flour addition on properties of extruded products based on corn grits. J. Food Eng. 2016, 172, 31–37. [Google Scholar] [CrossRef]
- Guha, M.; Ali, S.Z.; Bhattacharya, S. Effect of barrel temperature and screw speed on rapid viscoanalyser pasting behaviour of rice extrudate. Int. J. Food Sci. Technol. 1998, 33, 259–266. [Google Scholar] [CrossRef]
- Yadav, G.P.; Dalbhagat, C.G.; Mishra, H.N. Effects of extrusion process parameters on cooking characteristics and physicochemical, textural, thermal, pasting, microstructure, and nutritional properties of millet-based extruded products: A review. J. Food Process Eng. 2022, 45, e14106. [Google Scholar] [CrossRef]
- Tiga, B.H.; Kumcuoglu, S.; Vatansever, M.; Tavman, S. Thermal and pasting properties of Quinoa—Wheat flour blends and their effects on production of extruded instant noodles. J. Cereal Sci. 2021, 97, 103120. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Amer, S.A.; Rizk, A.E. Production and evaluation of novel functional extruded corn snacks fortified with ginger, bay leaves and turmeric powder. Food Prod. Process. Nutr. 2022, 4, 4. [Google Scholar] [CrossRef]
- Arslan, N.; Toğrul, H. Moisture Sorption Isotherms for Crushed Chillies. Biosyst. Eng. 2005, 90, 47–61. [Google Scholar] [CrossRef]
- Katz, E.E.; Labuza, T.P. Effect of Water Activity on the Sensory Crispness and Mechanical Deformation of Snack Food Products. J. Food Sci. 1981, 46, 403–409. [Google Scholar] [CrossRef]
| Sample | Extractable Phenolic Compounds (EPP) (mg AG/g) | Hydrolyzable Phenolic Compounds (HPP) (mg AG/g) | ABTS (µmol de T/g) | DPPH (µmol de T/g) | Carotenoides µg β-Caroteno/g | RDS g/100 g | SDS g/100 g | TDS g/100 g | RS g/100 g | In Vitro Protein Digestibility g/100 g |
|---|---|---|---|---|---|---|---|---|---|---|
| RQF | 1.79 ± 0.06 a | 4.58 ± 0.10 b | 13.16 ± 0.20 c | 3.87 ± 0.21 b | 11.33 ± 0.82 a | 7.33 ± 0.00 c | 58.51 ± 0.00 a | 73.54 ± 0.07 b | 2.18 ± 0.03 a | 88.51 ± 0.12 c |
| ES | 0.75 ± 0.03 b | 7.23 ± 0.78 a | 19.72 ± 0.81 a | 5.32 ± 0.27 a | 8.39 ± 0.69 b | 77.33 ± 0.03 a | 2.08 ± 0.01 b | 79.21 ± 0.00 a | 1.07 ± 0.00 b | 93.82 ± 0.81 b |
| EBS | 0.81 ± 0.04 b | 6.98 ± 1.22 a | 16.10 ± 0.68 b | 5.30 ± 0.28 a | 7.40 ± 0.56 c | 73.61 ± 0.01 b | 2.72 ± 0.02 b | 79.44 ± 0.00 a | 0.09 ± 0.01 c | 94.58 ± 1.05 a |
| Sample | Peak Viscosity (Pa.s) | Peak Time (s) | Trouhg (Pa.s) | Final Viscosity (Pa.s) | Pasting Temperature (°C) | Breakdown | Setback (Pa.s) |
|---|---|---|---|---|---|---|---|
| Extruded samples | |||||||
| ES | 0.130 ± 0.006 a | 1033 ± 7.07 a | 0.076 ± 0.003 b | 0.19 ± 0.04 b | 90.72 ± 0.95 b | 0.118 ± 0.04 a | 0.183 ± 0.005 b |
| EBS | 0.158 ± 0.004 a | 1035 ± 0.72 a | 0.085 ± 0.002 b | 0.21 ± 0.00 b | 91.00 ± 0.00 b | 0.127 ± 0.02 a | 0.203 ± 0.013 b |
| Non-extruded samples | |||||||
| RQF | 0.127 ± 0.02 a | 565 ± 35.34 b | 1.220 ± 0.32 a | 1.13 ± 0.02 a | 94.15 ± 1.90 a | 0.082 ± 0.08 a | 1.125 ± 0.003 a |
 | L* | b* | b* | C* | h | ΔE |
| RQF | ||||||
| 86.65 a ± 0.13 | −0.16 c ± 0.01 | 15.06 c ± 0.08 | 15.06 ± 0.08 c | 90.61 ± 0.05 a | - | |
| ES | ||||||
| 64.15 c ± 0.17 | 4.13 b ± 0.07 | 18.81 b ± 0.25 | 19.25 ± 0.25 b | 77.62 ± 0.23 b | 23.23 | |
| EBS | ||||||
| 65.70 b ± 0.43 | 4.59 a ± 0.14 | 20.24 a ± 0.49 | 20.75 ± 0.40 a | 77.22 ± 0.31 c | 22.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Pabon, K.S.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Bravo-Gómez, J.E.; Ortiz-Gómez, V. Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. Foods 2022, 11, 3383. https://doi.org/10.3390/foods11213383
Muñoz-Pabon KS, Roa-Acosta DF, Hoyos-Concha JL, Bravo-Gómez JE, Ortiz-Gómez V. Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. Foods. 2022; 11(21):3383. https://doi.org/10.3390/foods11213383
Chicago/Turabian StyleMuñoz-Pabon, Karen Sofia, Diego Fernando Roa-Acosta, José Luis Hoyos-Concha, Jesús Eduardo Bravo-Gómez, and Vicente Ortiz-Gómez. 2022. "Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties" Foods 11, no. 21: 3383. https://doi.org/10.3390/foods11213383
APA StyleMuñoz-Pabon, K. S., Roa-Acosta, D. F., Hoyos-Concha, J. L., Bravo-Gómez, J. E., & Ortiz-Gómez, V. (2022). Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. Foods, 11(21), 3383. https://doi.org/10.3390/foods11213383





