Oil Penetration of Batter-Breaded Fish Nuggets during Deep-Fat Frying: Effect of Frying Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Organic/Biological Materials
2.1.2. Chemicals
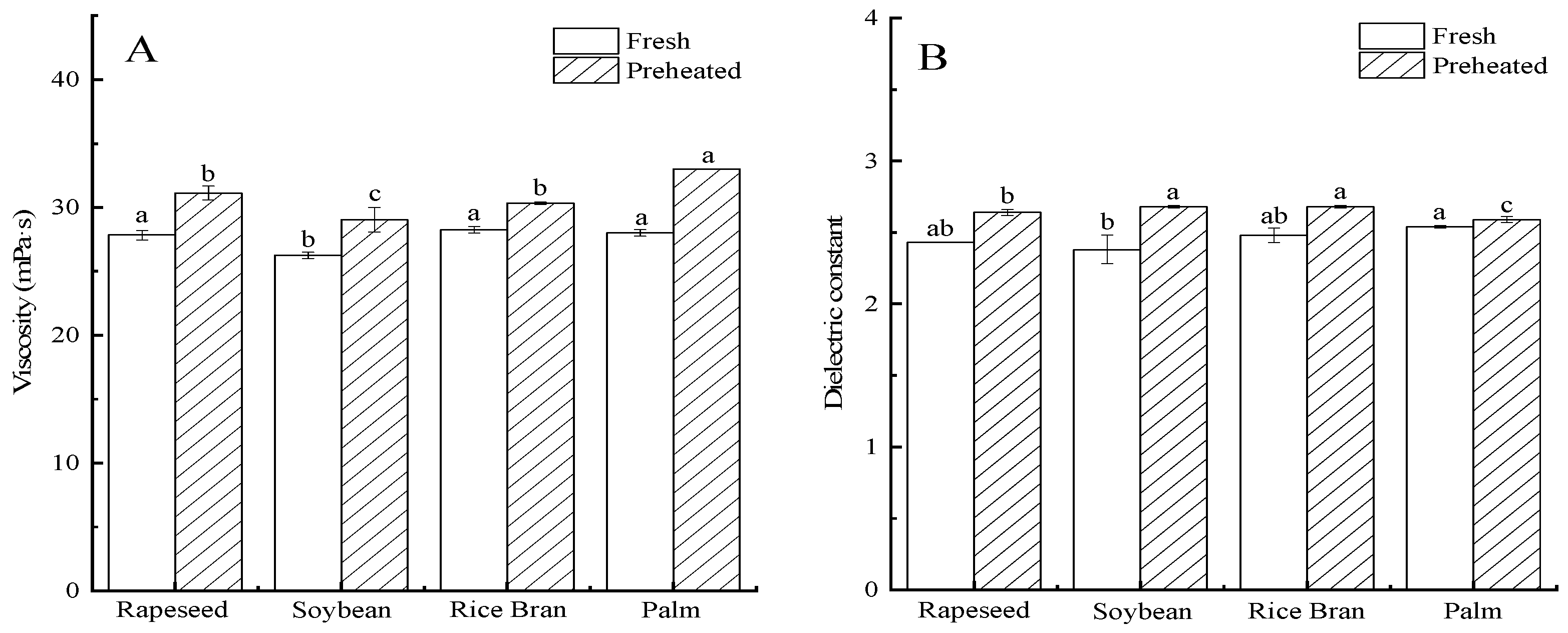
2.2. Fatty Acid Composition, Viscosity, and Dielectric Constant of Frying Oils
2.2.1. Fatty Acid Composition
2.2.2. Viscosity
2.2.3. Dielectric Constant
2.3. Preparation of BBFNs
2.4. Frying Process
2.5. Analysis of Moisture Content
2.6. Analysis of Fat Content
2.6.1. Standard Curve
2.6.2. Surface Oil
2.6.3. Penetrated Surface Oil
2.7. Instrumental Surface Color Analysis
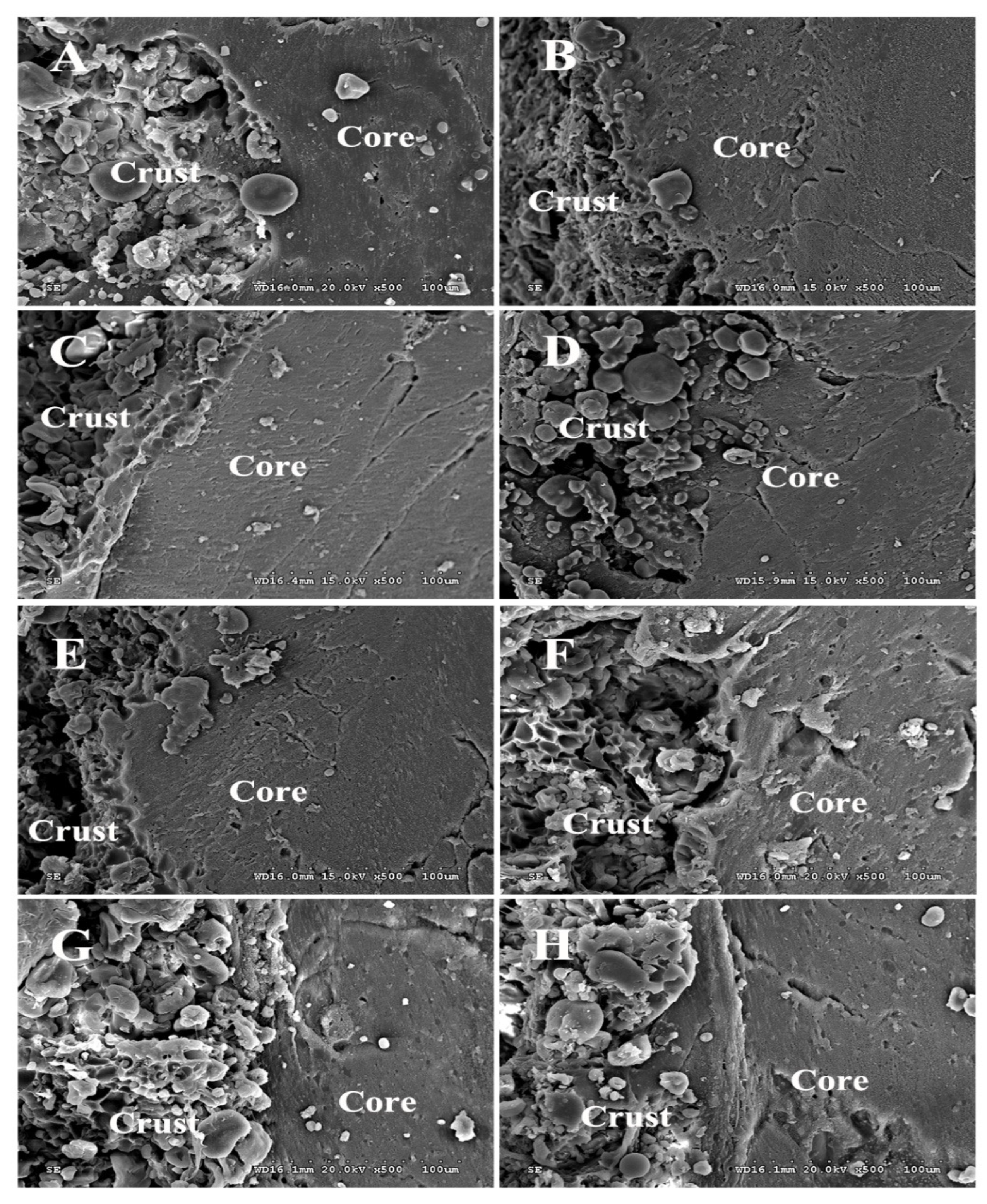
2.8. Scanning Electron Microscopic (SEM) Analysis
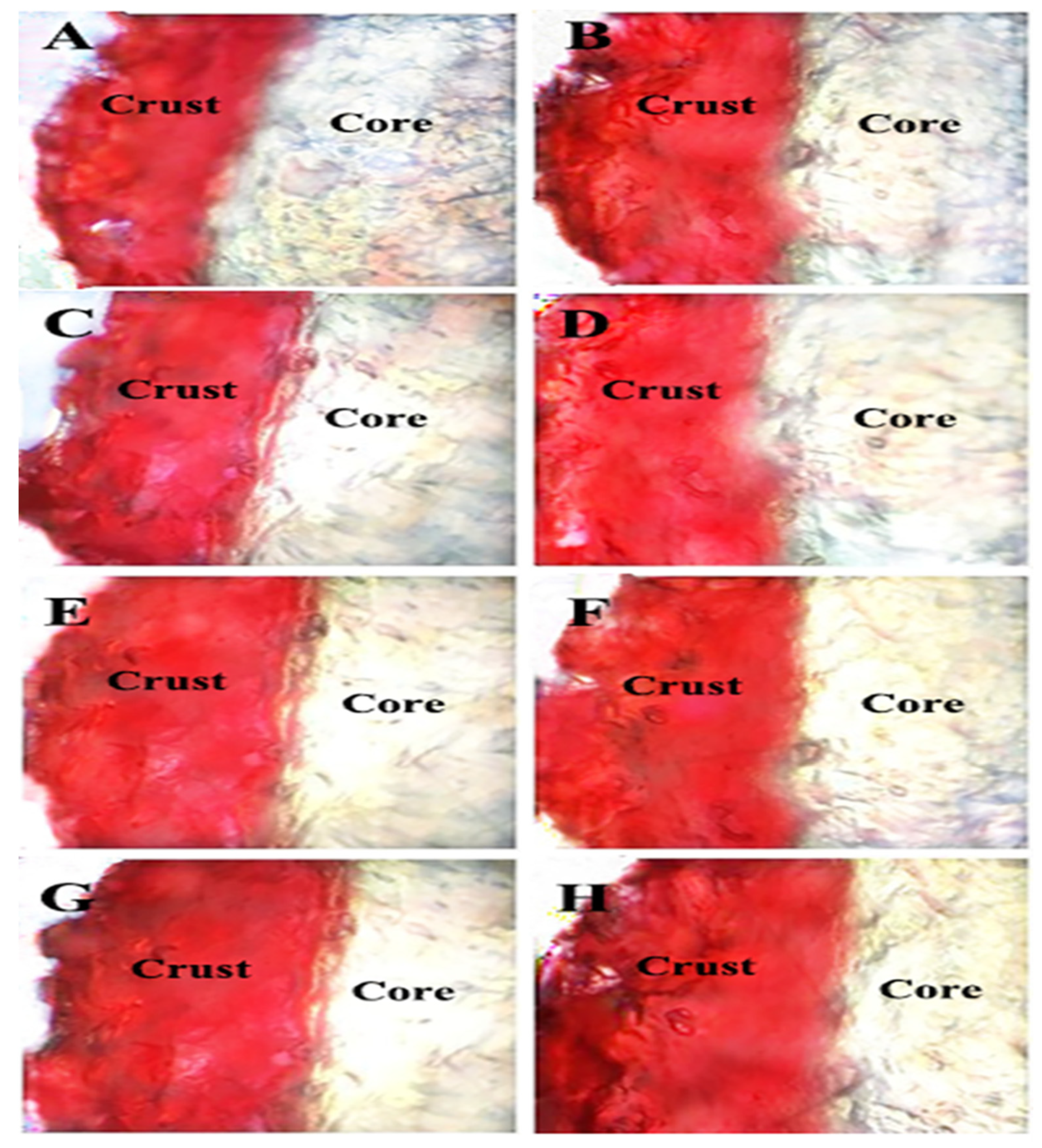
2.9. Oil Transport via Optical Microscopy
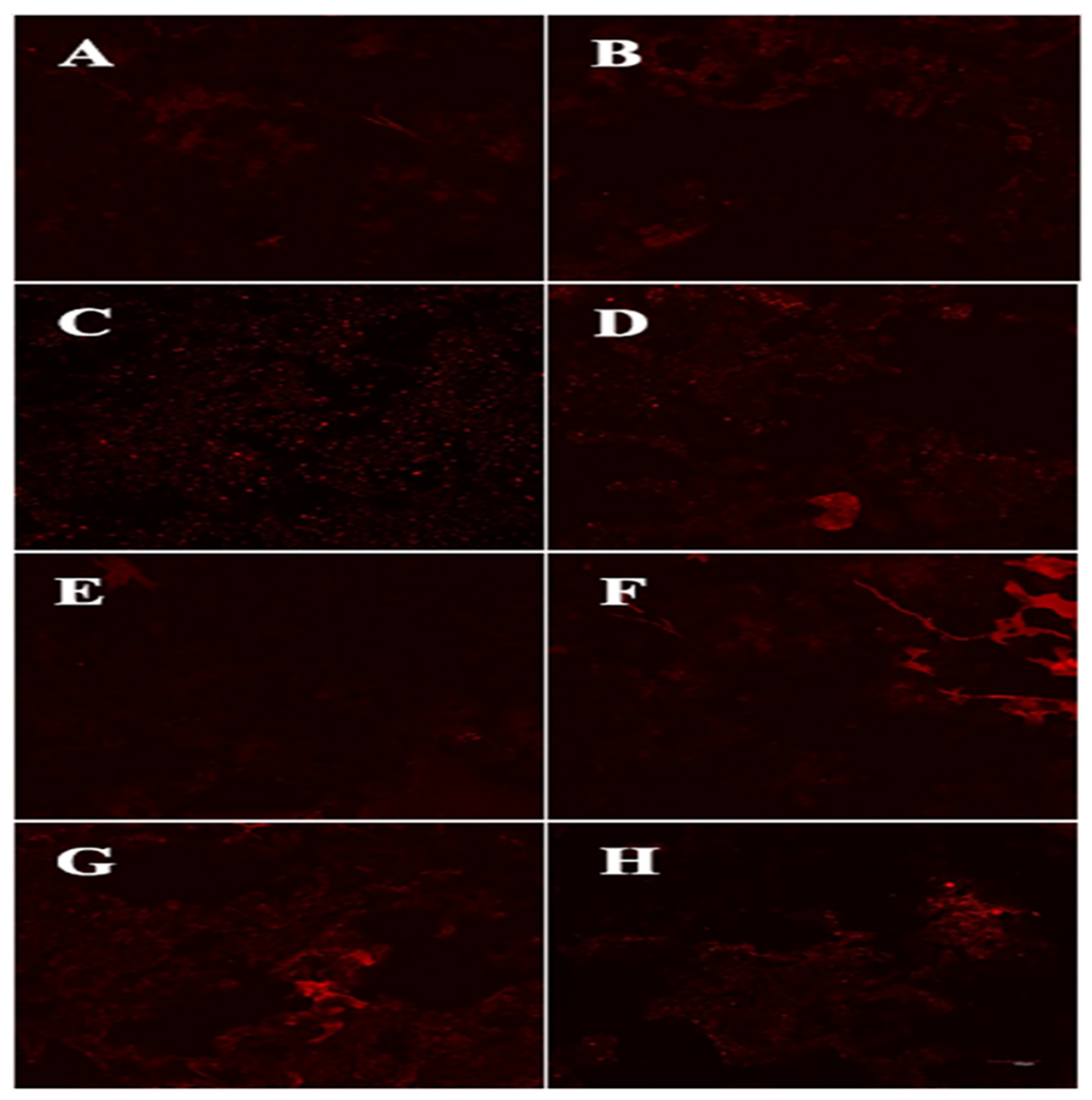
2.10. Fat Distribution via a Confocal Laser Scanning Microscope (CLSM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition, Viscosity, and Dielectric Constant of Frying Oils
3.2. Moisture and Fat Contents of Fried BBFNs
3.3. Color Characteristics of Fried BBFNs
3.4. SEM Analysis
3.5. Oil Transport Revealed by Optical Microscopy
3.6. Fat distribution Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brannan, R.G.; Pettit, K. Reducing the oil content in coated and deep-fried chicken using whey protein. Lipid Technol. 2015, 27, 131–133. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, W.W.; Jia, Z.B.; Tao, F. Physiochemical properties of short-term frying oil for chicken wing and its oxidative stability in an oil-in-water emulsion. Food Sci. Nutr. 2020, 8, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, F.X.; Song, D.D.; Gao, H.Q. China Fishery Statistical Yearbook 2021; China Agricultural Press: Beijing, China, 2021. [Google Scholar]
- Ananey-Obiri, D.; Matthews, L.; Azahrani, M.H.; Ibrahim, S.A.; Galanakis, C.M.; Tahergorabi, R. Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 2018, 80, 167–174. [Google Scholar] [CrossRef]
- Mellon, M. Mechanism and reduction of fat uptake in deep-fat fried foods. Trends Food Sci. Technol. 2003, 14, 364–373. [Google Scholar] [CrossRef]
- Cui, L.L.; Chen, J.W.; Wang, Y.H.; Xiong, Y.L. The effect of batter characteristics on protein-aided control of fat absorption in deep-fried breaded fish nuggets. Foods 2022, 11, 147. [Google Scholar] [CrossRef]
- Esfarjani, F.; Khoshtinat, K.; Zargaraan, A.; Mohammadi-Nasrabadi, F.; Salmani, Y.; Saghafi, Z.; Hosseini, H.; Bahmaei, M. Evaluating the rancidity and quality of discarded oils in fast food restaurants. Food Sci. Nutr. 2019, 7, 2302–2311. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Kaur, A.; Singh, N. Changes in chemical properties and oxidative stability of refined vegetable oils during short-term deep-frying cycles. J. Food Process. Preserv. 2020, 44, e14445. [Google Scholar] [CrossRef]
- Crosby, G. Do cooking oils present a health risk? Food Technol. (Chic.) 2018, 72, 50–57. [Google Scholar]
- Mitrea, L.; Teleky, B.E.; Leopold, L.F.; Nemes, S.A.; Plamada, D.; Dulf, F.V.; Pop, I.D.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Brannan, R.G.; Mah, E.; Schott, M.; Yuan, S.; Casher, K.L.; Myers, A.; Herrick, C. Influence of ingredients that reduce oil absorption during immersion frying of battered and breaded foods. Eur. J. Lipid Sci. Technol. 2014, 116, 240–254. [Google Scholar] [CrossRef]
- Touffet, M.; Trystram, G.; Vitrac, O. Revisiting the mechanisms of oil uptake during deep-frying. Food. Bioprod. Process. 2020, 123, 14–30. [Google Scholar] [CrossRef]
- Brannan, R.G.; Myers, A.S.; Herrick, C.S. Reduction of fat content during frying using dried egg white and fiber solutions. Eur. J. Lipid Sci. Technol. 2013, 115, 946–955. [Google Scholar] [CrossRef]
- Ziaiifar, A.M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 2008, 43, 1410–1423. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Fan, D. Effect of ultrasonic on deterioration of oil in microwave vacuum frying and prediction of frying oil quality based on low field nuclear magnetic resonance (LF-NMR). Ultrason. Sonochem. 2019, 51, 77–89. [Google Scholar] [CrossRef]
- Xu, L.R.; Yang, F.; Li, X.; Zhao, C.W.; Jin, Q.Z.; Huang, J.H.; Wang, X.G. Kinetics of forming polar compounds in frying oils under frying practice of fast food restaurants. LWT Food Sci. Technol. 2019, 115, 108307. [Google Scholar] [CrossRef]
- Li, X.D.; Li, J.W.; Wang, Y.; Cao, P.R.; Liu, Y.F. Effects of frying oils’ fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem. 2017, 237, 98–105. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Review: Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. J. Colloid. Interface Sci. 2006, 128–130, 267–272. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of intermittent frying on chemical properties, fatty acid composition, and oxidative stability of 10 different vegetable oil blends. J. Food Process. Pres. 2021, 45, e16015. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen, J.W.; Yuan, Z.J.; Liao, E.; Xia, W.S.; Wang, H.B.; Xiong, Y.L. The effect of the wheat starch/wheat protein ratio in a batter on fat absorption and quality attributes of fried battered and breaded fish nuggets. J. Food Sci. 2020, 85, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.H.; Chen, J.W.; Xie, D.; Xia, W.S.; Xu, W.; Xiong, Y.L. Effect of Xanthan gum/Soybean fiber ratio of the batter on oil absorption and quality attributes of fried breaded fish nuggets. J Food Sci. 2018, 83, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, J.W.; Zhai, J.L.; Wang, H.B.; Xia, W.S.; Xiong, Y.L. Reduction of the fat content of battered and breaded fish balls during deep-fat frying using fermented bamboo shoot dietary fiber. LWT-Food Sci. Technol. 2016, 73, 425–431. [Google Scholar] [CrossRef]
- Gao, H.X.; Yu, J.; Chen, N.; Zeng, W.C. Effects and mechanism of tea polyphenols on the quality of oil during frying process. J. Food Sci. 2020, 85, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Rubalya Valantina, S. Measurement of dielectric constant: A recent trend in quality analysis of vegetable oil—A review. Trends Food Sci. Technol. 2021, 113, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Measurement of dielectric constant and loss factor of the dielectric material at microwave frequencies. Prog. Electromagn. Res. 2007, 69, 47–54. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; No. 922. 06. 2005; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Bouchon, P.; Aguilera, J.M.; Pyle, D.L. Structure oil-absorption relationships during deep-fat frying. J. Food Sci. 2003, 68, 2711–2716. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC international; No. 925. 09. 2005; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Salehi, F. Color changes kinetics during deep fat frying of carrot slice. Heat. Mass Transfer. 2018, 54, 3421–3426. [Google Scholar] [CrossRef]
- Hu, L.Z.; Toyoda, K.; Ihara, I. Dielectric properties of edible oils and fatty acids as a function of frequency, temperature, moisture and composition. J. Food Eng. 2008, 88, 151–158. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, Y.; Zhang, N.; Gao, Y.; Yu, X. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 2021, 364, 130451. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.Y.; Huang, X.Y.; Sun, Y.H.; Chen, Q.S.; Wei, Z.J.; Lv, R.Q. Intelligent Evaluation of Total Polar Compounds (TPC) Content of Frying Oil Based on Fluorescence Spectroscopy and Low-Field NMR. Food Chem. 2020, 342, 128242. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; He, Q.X.; Qiu, Y.X.; Liu, H.F.; Wu, J.H.; Liu, G.Q.; Brennan, C.; Brennan, A.M.; Zhu, L.X. Food matrixes play a key role in the distribution of contaminants of lipid origin: A case study of malondialdehyde formation in vegetable oils during deep-frying. Food Chem. 2021, 347, 129080. [Google Scholar] [CrossRef] [PubMed]
- Fasina, O.O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil Chem. Soc. 2006, 83, 899–903. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Lee, W.J.; Akoh, C.C.; Li, A.; Wang, Y. Modification of palm-based oil blend via interesterification: Physicochemical properties, crystallization behaviors and oxidative stabilities. Food Chem. 2021, 347, 129070. [Google Scholar] [CrossRef]
- Hwang, H.S.; Ballb, J.C.; Dolla, K.M.; Andersonb, J.E.; Vermilliona, K. Investigation of polymers and alcohols produced in oxidized soybean oil at frying temperatures. Food Chem. 2020, 317, 126379. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Xu, Z.Y.; Tan, C.P.; Liu, Y.F.; Xu, Y.J. Biohazard and dynamic features of different polar compounds in vegetable oil during thermal oxidation. LWT Food Sci. Technol. 2021, 146, 111450. [Google Scholar] [CrossRef]
- Rahimi, J.; Ngadi, M.O. Surface ruptures of fried batter as influencing by batter formulation. J. Food Eng. 2015, 152, 50–56. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Ye, Z.; Li, Y.D.; Li, J.W.; Liu, Y.F. Comparative study of the oxidation stability of high oleic oils and palm oil during thermal treatment. J. Oleo Sci. 2020, 69, 573–584. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 18–77. [Google Scholar]
- Fayle, S.E.; Gerrard, J.A. The Maillard Reaction; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Lalam, S.; Sandhu, J.S.; Takhar, P.S.; Thompson, L.D.; Alvarado, C. Experimental study on transport mechanisms during deep fat frying of chicken nuggets. LWT Food Sci. Technol. 2013, 50, 110–119. [Google Scholar] [CrossRef]
- Bouchon, P.; Hollins, P.; Pearson, M.; Pyle, D.L.; Tobin, M.J. Oil distribution in fried potatoes monitored by infrared microspectroscopy. J. Food Sci. 2001, 66, 918–923. [Google Scholar] [CrossRef]
- Dhital, S.; Baier, S.K.; Gidley, M.J.; Stokes, J.R. Microstructural properties of potato chips. Food Struct. 2018, 16, 17–26. [Google Scholar] [CrossRef]
- Asokapandian, S.; Swamy, G.J.; Hajjul, H. Deep fat frying of foods: A critical review on process and product parameters. Cri. Rev. Food Sci. Nutr. 2019, 60, 3400–3413. [Google Scholar] [CrossRef] [PubMed]
| Type of Fatty Acid | Rapeseed Oil | Soybean Oil | Rice Bran Oil | Palm Oil |
|---|---|---|---|---|
| Dodecanoic acid (C12:0) | - | - | - | 0.2 ± 0.00 |
| Myristic acid (C14:0) | - | 0.1 ± 0.00 | 0.3 ± 0.00 | 0.9 ± 0.01 |
| Palmitic acid (C16:0) | 4.6 ± 0.03 | 11.6 ± 0.05 | 17.2 ± 0.00 | 39.3 ± 0.20 |
| Palmitoleic acid (16:1) | 0.2 ± 0.01 | 0.1 ± 0.00 | - | 0.2 ± 0.00 |
| Heptadecanoic acid (C17:0) | - | 0.1 ± 0.00 | - | - |
| Octadecanoic acid (C18:0) | 2.1 ± 0.01 | 5.0 ± 0.03 | 1.4 ± 0.01 | 4.7 ± 0.05 |
| Oleic acid (C18:1) | 60.4 ± 0.06 | 24.5 ± 0.01 | 40.7 ± 0.05 | 43.1 ± 0.07 |
| Linoleic acid (C18:2) | 20.8 ± 0.01 | 49.3 ± 0.02 | 37.3 ± 0.02 | 10.9 ± 0.12 |
| Arachidic acid (C20:0) | 0.7 ± 0.01 | 0.5 ± 0.02 | 0.6 ± 0.01 | 0.4 ± 0.01 |
| Linolenic acid (C18:3) | 9.5 ± 0.04 | 7.6 ± 0.53 | 1.5 ± 0.00 | 0.2 ± 0.05 |
| Eicosenoic acid (C20:1) | 1.2 ± 0.03 | 0.2 ± 0.00 | 0.6 ± 0.01 | 0.2 ± 0.01 |
| Octadecadienoic acid (C18:2) | - | 0.1 ± 0.00 | 0.1 ± 0.00 | - |
| Heneicosanoic acid (C21:0) | - | - | 0.4 ± 0.04 | - |
| Docosanoic acid (C22:0) | 0.5 ± 0.04 | 0.5 ± 0.02 | - | - |
| Lignoceric acid (C24:0) | - | 0.2 ± 0.00 | - | - |
| Saturated fatty acid | 7.8 ± 0.08 d | 17.9 ± 0.11 c | 19.9 ± 0.06 b | 45.4 ± 0.27 a |
| Unsaturated fatty acid | 92.2 ± 0.15 a | 81.8 ± 0.56 b | 80.1 ± 0.07 c | 54.6 ± 0.21 d |
| Monounsaturated fatty acid | 61.9 ± 0.10 a | 24.8 ± 0.01 d | 41.3 ± 0.05 c | 43.5 ± 0.08 b |
| Polyunsaturated fatty acid | 30.3 ± 0.05 c | 57.0 ± 0.55 a | 38.8 ± 0.02 b | 11.1 ± 0.13 d |
| Oil Type | Preheating Duration (h) | Moisture Content (%) | Fat Content (%, dw) | Color Characteristics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Crust | Core | Surface Oil | Penetrated Surface Oil | Total | L* | a* | b* | ||
| Rapeseed oil | 0 | 15.3 ± 0.20 c | 68.4 ± 3.4 a | 5.0 ± 0.02 c | 6.3 ± 0.02 g | 12.1 ± 0.03 e | 48.2 ± 0.38 bc | 6.0 ± 0.67 e | 26.2 ± 0.30 d |
| 10 | 13.4 ± 0.23 e | 62.2 ± 2.8 c | 5.5 ± 0.03 a | 6.5 ± 0.01 e | 12.8 ± 0.02 d | 46.5 ± 1.7 d | 6.7 ± 0.25 d | 27.8 ± 0.26 b | |
| Soybean oil | 0 | 14.5 ± 0.33 d | 64.4 ± 1.1 b | 3.5 ± 0.04 g | 6.8 ± 0.02 f | 11.3 ± 0.03 f | 45.9 ± 1.3 de | 7.2 ± 0.23 c | 26.0 ± 0.29 d |
| 10 | 13.2 ± 0.16 e | 58.2 ± 2.9 e | 5.0 ± 0.04 cd | 7.1 ± 0.04 de | 13.1 ± 0.03 c | 43.8 ± 1.5 f | 8.4 ± 0.32 a | 28.3 ± 0.53 ab | |
| Rice bran oil | 0 | 17.1 ± 0.25 a | 64.2 ± 1.7 b | 4.3 ± 0.01 e | 7.1 ± 0.04 d | 12.2 ± 0.03 e | 47.0 ± 1.1 c | 7.7 ± 0.06 b | 27.5 ± 0.17 bc |
| 10 | 14.2 ± 0.38 d | 61.1 ± 1.3 d | 5.4 ± 0.02 a | 7.9 ± 0.03 c | 14.1 ± 0.04 bd | 44.9 ± 1.2 e | 8.4 ± 0.40 a | 29.2 ± 0.08 a | |
| Palm oil | 0 | 16.1 ± 0.27 b | 60.6 ± 2.7 d | 4.9 ± 0.04 d | 9.0 ± 0.04 b | 14.2 ± 0.02 b | 50.6 ± 1.2 a | 5.6 ± 0.17 f | 25.0 ± 0.85 e |
| 10 | 12.2 ± 0.48 f | 57.5 ± 2.7 f | 5.3 ± 0.03 b | 9.4 ± 0.04 a | 15.3 ± 0.02 a | 48.1 ± 1.3 b | 7.7 ± 0.01 bc | 27.0 ± 0.19 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Chen, J.; Zhai, J.; Peng, L.; Hayes, D.G. Oil Penetration of Batter-Breaded Fish Nuggets during Deep-Fat Frying: Effect of Frying Oils. Foods 2022, 11, 3369. https://doi.org/10.3390/foods11213369
Cui L, Chen J, Zhai J, Peng L, Hayes DG. Oil Penetration of Batter-Breaded Fish Nuggets during Deep-Fat Frying: Effect of Frying Oils. Foods. 2022; 11(21):3369. https://doi.org/10.3390/foods11213369
Chicago/Turabian StyleCui, Lulu, Jiwang Chen, Jinling Zhai, Lijuan Peng, and Douglas G. Hayes. 2022. "Oil Penetration of Batter-Breaded Fish Nuggets during Deep-Fat Frying: Effect of Frying Oils" Foods 11, no. 21: 3369. https://doi.org/10.3390/foods11213369
APA StyleCui, L., Chen, J., Zhai, J., Peng, L., & Hayes, D. G. (2022). Oil Penetration of Batter-Breaded Fish Nuggets during Deep-Fat Frying: Effect of Frying Oils. Foods, 11(21), 3369. https://doi.org/10.3390/foods11213369








