Abstract
Edible coatings as carriers for protective lactic acid bacteria (LAB) can enhance hygienic quality to dairy products. Thus, the aim of this study was to improve the quality of artisanal acid-curd cheese by applying liquid acid whey protein concentrate based edible coating with entrapped indigenous antimicrobial Lactobacillus helveticus MI-LH13. The edible fresh acid-curd cheese coating was composed of 100% (w/w) liquid acid whey protein concentrate (LAWPC), apple pectin, sunflower oil, and glycerol containing 6 log10 CFU/mL of strain biomass applied on cheese by dipping. The cheese samples were examined over 21 days of storage for changes of microbiological criteria (LAB, yeast and mould, coliform, enterobacteria, and lipolytic microorganism), physicochemical (pH, lactic acid, protein, fat, moisture content, and colour), rheological, and sensory properties. The coating significantly improved appearance and slowed down discolouration of cheese by preserving moisture during prolonged storage. The immobilisation of L. helveticus cells into the coating had no negative effect on their viability throughout 14 days of storage at 4 °C and 23 °C. The application of coating with immobilised cells on cheeses significantly decreased the counts of yeast up to 1 log10 CFU/g during 14 days (p < 0.05) of storage and suppressed growth of mould for 21 days resulting in improved flavour of curd cheese at the end of storage. These findings indicate that LAWPC-pectin formulation provided an excellent matrix to support L. helveticus cell viability. Acting as protective antimicrobial barrier in fresh cheeses, this bioactive coating can reduce microbial contamination after processing enabling the producers to extend the shelf life of this perishable product.
1. Introduction
Edible coatings are natural biodegradable layers on foods retaining the appearance, physicochemical properties, and freshness during their storage and serving as an improvement to conventional packaging [1]. Many protein-based edible coatings intended for cheese protection contain sweet whey proteins that in a majority of studies [2,3,4,5,6,7] are purchased in powdered form along with other coating ingredients using water as a solvent [8]. Acid whey, on the other hand, a main waste product of acid curd production, causes devastating environmental problems and, therefore, calls for reintroducing it back into production. Cheese factories, responding to the sustainability and environmental demands, are searching for the ways to repurpose acid whey, preferably in its liquid form [9]; therefore, ultrafiltration is most widely used by cheese factories to acquire liquid acid whey concentrate (LAWPC) from acid whey. Employing LAWPC with specific nutritional value and functional properties [10,11] as a basis for acid whey protein coating, and as a solvent for other coating ingredients, provides an opportunity to avoid subsequent energy-consuming spray drying process, which brings along environmental, health, and economic benefits [12,13,14]. In a liquid form, though it contains only 2.4–3.2% of whey protein—not ideal for coating production—thus, there is a need to supplement it with biopolymers (such as agro-industrial waste pectin) and lipids to make it work as a protectant layer on cheese surface.
Prolonging the shelf life of the product along with reducing food waste is another emerging need of modern society [15]. Fresh acid-curd or farmer’s cheese has long been a well-known artisanal dairy product, which, due to prevailing manual operations, has a short shelf life of 8–10 days [14]. Mould contamination of such cheese during storage is a problem for the small dairy farmers wrapping cheese to be sold in paper, which does not add up to the shelf life of this cheese [16]. It was reported that the shelf life of a product can be prolonged not only by reducing respiration with the help of main edible coating components [17] but also by addition of antimicrobial protectants to the coating formulations. Indigenous, probiotic-type lactic acid bacteria (LAB) are one of them, demonstrating protective properties [18,19,20] and antimicrobial potential [21]. Various immobilisation or encapsulation technologies have been widely studied underlining the impact of materials, environmental conditions, and individual strain properties on the survival of such LAB [22,23,24]. Preparation of bioactive coatings with protective LAB incorporated is an innovative field in the dairy industry, and therefore, only a small number of studies on this topic are available [25]. Coating formulations supporting LAB survival [26] could be a good aid in not only ensuring microbiological safety but also enhancing the flavour of such type of cheese.
To date, there is no research carried out on the application of LAWPC and pectin as a base in edible coating production and as a vehicle for protective LAB strains. Therefore, the aim of this study was to improve the quality of artisanal acid-curd cheese by applying liquid acid whey protein concentrate pectin-based edible coating with entrapped indigenous antimicrobial Lactobacillus helveticus MI-LH13. Simultaneously, the survival of L. helveticus MI-LH13 immobilised in the edible coating and the strain’s impact on the characteristics of this cheese were evaluated.
The production of edible coating by incorporating protective lactobacilli as natural antimicrobial agents in a matrix based on LAWPC could be an excellent opportunity to reuse whey in its liquid form in the factory that produced it. Applying produced bioprotective coating on fresh curd cheese by spraying or dipping could be an alternative or improvement to conventional cheese packaging, enhancing cheese sensory perception, preventing spoilage, and prolonging its shelf-life.
2. Materials and Methods
2.1. Materials
Liquid acid whey protein concentrate (LAWPC) was supplied from dairy plant AB Kauno pienas, Lithuania. LAWPC was produced by ultra-filtrating fresh acid bovine whey. The LAWPC (12.34% dry matter, presented 2.24% protein, 0.5% fat, and 5.55% other solids, pH 4.69) was frozen at −20 °C until use. Before coating preparation, the LAWPC was thawed at 4 °C.
Plasticiser glycerol (99% purity), thickener and emulsifier apple pectin, surfactant tween 80, and sunflower oil were supplied by Sigma-Aldrich, Darmstadt, Germany.
Indigenous L. helveticus MI-LH13 previously isolated from raw bovine milk was stored at −80 °C in MRS broth (Merck, Germany) in the presence of 30% glycerol. Antimicrobial properties of this strain and some of the technological properties relevant to the dairy industry were tested in vitro before the study [27].
The strain was revitalised in MRS broth (Biolife, Milano, Italy) by growing for 18 h at 30 °C until reaching 6 log10 CFU/mL. The biomass of the strain was harvested by centrifugation at 4000 rpm for 15 min at 4 °C.
Fresh acid-curd cheese (4.00% fat, 3.00% protein, 4.50% lactose, 8.10% non-fat solids, pH 6.60) determined with a milk analyser (Milko-Skan, FOSS ELEKTRIK, Hillerød, Denmark) was purchased from local artisanal dairy factory. It was made by a conventional method: standardised bovine milk was pasteurised, cooled down to 28 °C, and then commercial starter was added. After casein coagulation (12 h), the soured milk was heated up to 50 ± 1 °C (90 min) to form the curd. The curd was placed into cotton bags and pressed (approx. 30 min) until the required firmness of cheese was achieved. The resultant cheese samples (100 ± 5 g) were manually taken out of bags and stored in a refrigerator at 4 ± 1 °C for 24 h until coating application.
2.2. Coating and Film Preparation
Coating formulations were prepared according to the method described by Ramos et al. [5] with some modifications. In order to obtain the coating solution, 5% of glycerol (w/w), 2% of pectin (w/w), 0.2% of Tween (w/w), and 2% of sunflower oil (w/w) were added to the LAWPC, homogenised (15,000 rp/s, 3 min) for good dispersion, and solution was pasteurised in water bath (85 °C, 10 min). After cooling it down to 35 °C, L. helveticus biomass (0.2 g/100 g, respectively) was thoroughly mixed in.
Films were produced from the coating solution by pouring 5 mL of it in Petri dishes and letting them dry at 37 °C for 24 h.
To evaluate the survival of incorporated strain, the coating solution was kept at room temperature (23 ± 1 °C) and refrigerated (4 ± 1 °C) for 14 days. Films were kept in Petri dishes at 4 °C for 60 days.
2.3. Coating Application on Acid-Curd Cheese
Two coating formulations, plain coating (C) and coating with L. helveticus incorporated (C + Lh), were prepared and immediately applied by dipping fresh acid-cured cheese into the coating solution for a few seconds. Coated cheeses were placed on perforated metal trays to dry off for 60 min (12–14 °C) and then placed into paper bags and stored refrigerated at 4 ± 1 °C for 21 days in previously sanitised perforated plastic boxes.
2.4. Cheese Analyses
Cheese samples were analysed in triplicate on days 1, 7, 14, 18, and 21 for physicochemical, rheological, and microbiological changes. pH was directly measured with a pH meter (Sartorius Professional meter for pH Measurement, Goettingen, Germany). Titratable acidity, expressed as a percentage of lactic acid (g/100 g cheese), was determined according to the standard method ISO 11869:2012 [28].
Dry matter, moisture, acidity, fat, and protein determinations in samples were performed according to prescribed methods: dry matter and moisture ISO 5534:2004 [29], fat ISO 1735:2004 [30], lactose ISO 22662:2007 [31], and protein ISO 8968-3:2004 [32].
Colour characteristics (where L* = lightness, a* = red–yellow colour, and b* = blue–green colour) of cheese were assessed using a CIE L*a*b* system (1996) (Chromameter CR-400, Konica Minolta, Tokyo, Japan). A standard white plate was used to calibrate the equipment, with colour coordinates Lstandard = 97.6, astandard = 0.01, and bstandard = 1.60. The total colour difference (ΔE) was calculated as follows:
where L0, a0, and b0 were values of day 1; and L, a, and b were the values measured throughout the storage period. Three readings were taken for each triplicate.
ΔE = [(L − L0)2 + (a − a0)2 + (b − b0)2]1/2,
Textural properties of cheese samples were evaluated with the texture analyser CT3 (Brookfield, Middleboro, MA, USA) with a TA4/1000 cylinder (diameter of 38.1 mm D, 20 mm L, a stroke speed of 1 mm/s, and a strike depth of 10 mm).
For microbiological analysis, viable counts of microorganisms commonly found in this type of cheese were determined in triplicate on the selective media for each species at days 1, 7, 14, 18, and 21 of cheese storage. Microorganisms were enumerated according to the prescribed methods: total mesophilic LAB count ISO 15214:1998 [33], enterobacteria count ISO 21528-2:2017 [34], coliform count ISO 4832:2006 [35], and yeast and mould count ISO 6611:2004 [36]. Lipolytic bacteria were enumerated on MRS agar overlaid with lard as described by Tuynenburg Muys and Willemse (1965) [37].
Sensory analysis was conducted on days 1, 7, 14, and 18 of cheese storage upon previous confirmation of microbiological safety. Sensory evaluation was carried out in the sensory room by a trained panel of 7 members (both sexes, ages ranging between 20 and 50 years old). The panel had previously been selected and trained by guidelines of ISO 8586:2012 [38]. Prior to assessment, samples were coded with 3-digit randomised numbers and served at room temperature. Cheese samples (5 × 2 × 2 cm blocks) were randomly presented to the panel members in identical plastic plates. The scorecard for the sensory evaluation of curd cheese was designed according to Bodyfelt et al. (1988) [39] as follows: 50 points for flavour, 40 points for body and texture, and 10 points for the appearance with the overall perception of 100 points. Cheeses were considered to be acceptable if at least an overall score of 65 points was obtained.
2.5. Statistical Analysis
All data processing and analysis were performed by SPSS statistical package (Chicago, IL, USA, SPSS Inc., SPSS 24). The data were analysed using descriptive statistics (Explore) and two-way analysis of variance (ANOVA). Differences between pairs of means were assessed on the basis of confidence intervals using the Tukey test. All the statistical analysis was performed at 95% level of significance.
3. Results and Discussion
3.1. Survival of L. helveticus in the Coating and Film
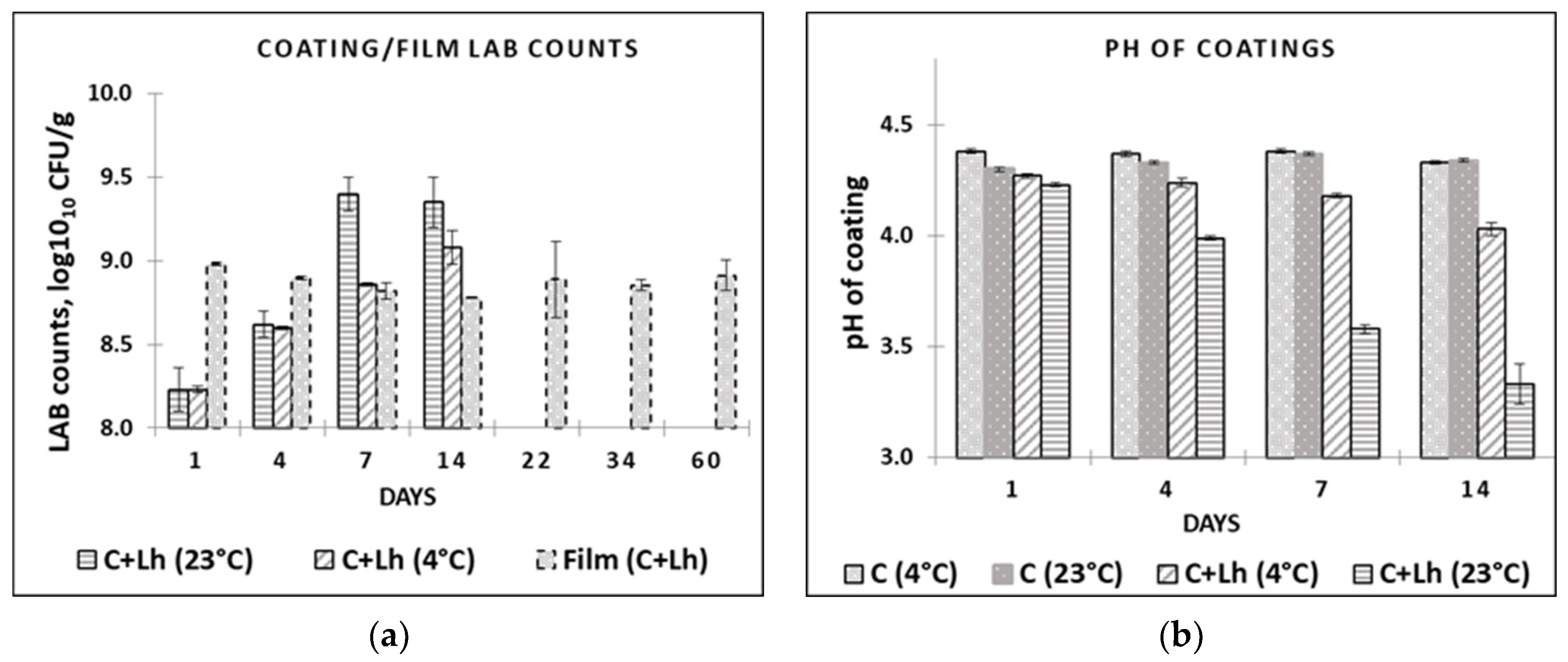
The impact of temperature on the survival of L. helveticus during storage in the coating solution (C + Lh) and the pH changes are shown in Figure 1a,b, respectively. L. helveticus grew to higher CFU at 23 °C (log10 9.4) compared with 4 °C (log10 9.0) during the 14 days, breaking down glucose that corresponded to faster decline in pH during storage at 23 °C, resulting in pH of 3.6 compared with 4.0 at 4 °C (p ≤ 0.05). This is in agreement with Perreira et al. (2016) [40] reporting temperature and strain dependencies when evaluating the survival rates of Bifidobacterium animalis Bb-12® and Lactobacillus casei-01 in whey protein isolate coating at different temperatures (4 °C, 23 °C). In our study, LAB immobilised in the film demonstrated viability loss of ca. 0.5 log cycle within 14 days of storage at 4 °C that was kept stable thereafter (8.88 ± 0.03 on day 1 and 8.32 ± 0.03 on day 60; (p ≤ 0.05; Figure 1a). Perreira et al. (2016) [40] reported viability loss (3 log cycles, reaching 106 CFU/g film until 60 d) at both 23 °C and 4 °C, noting the most marked decrease at 23 °C for both strains, with B. animalis demonstrating less decrease in its cell numbers (108 CFU/g film). The combination of L. helveticus, low temperature [40], glycerol, whey proteins, and pectin as protectants [41] in LAWPC-pectin-based film ensured the survival of protective strain that could be used for the quality maintenance of perishable products. Since the initial low pH of AWPC (4.69) did not interfere with the survival of the strain in the coating and film, it can be recommended as a basis for coating preparations reducing negative environmental impact of acid whey as well.
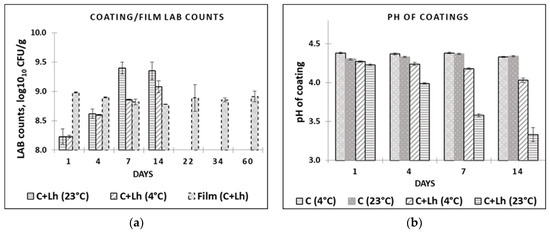
Figure 1.
LAB counts (a) in coating solutions and films and pH; (b) in coating solutions at different temperatures (at 4 °C and 23 °C) during their storage. C—plain coating; C + Lh—coating solution with L. helveticus.
3.2. Cheese Storage Trial
3.2.1. Physicochemical Profile
Data in Table 1 illustrate the chemical changes in the coated curd cheeses during prolonged storage at 4 °C (23 days). We observed no impact of storage and treatment factors’ interaction on dry matter (DM) and moisture parameters, and only individual factors significantly impacted these parameters. Storage day, treatment, and their interaction had an impact on all other cheese components (p ≤ 0.005).

Table 1.
Chemical compositions of curd cheese treatments during cold storage.
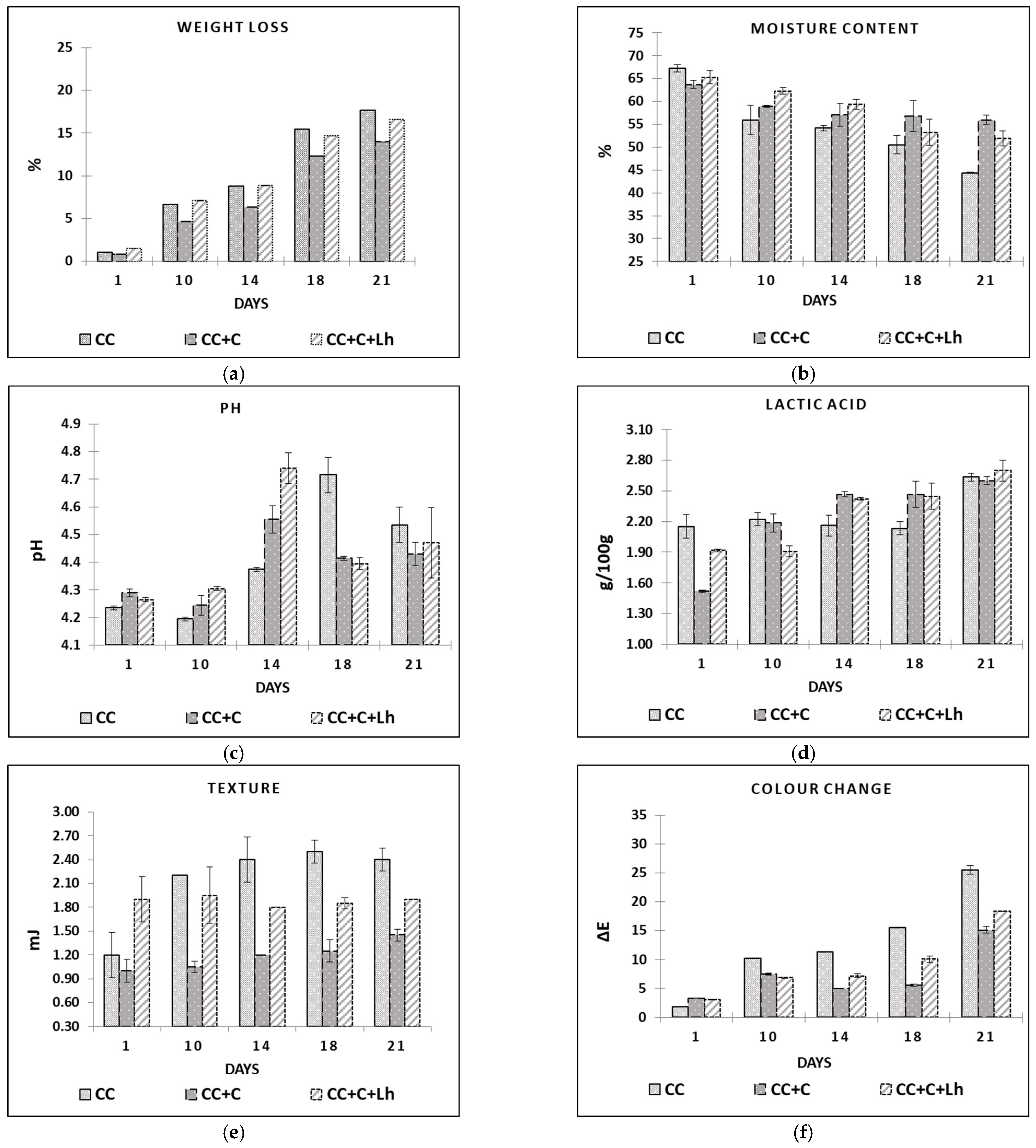
In the beginning of the experiment, both coated cheese treatments, with (CC + C + Lh) and without L. helveticus (CC + C), were significantly higher in fat and lactose content compared with control cheese (CC). Furthermore, the dry matter, protein, and fat contents of all cheese treatments significantly increased (p ≤ 0.0001) during the storage period mainly due to loss in moisture content (Figure 2b), while the use of coatings minimised moisture losses and hence improved the barrier properties (Figure 2b). The ability to preserve moisture during storage of the product is the most wanted parameter of edible coating [42]. Applied coating had no effect on initial moisture content—at day 1, no significant differences were noticed among samples. However, significant moisture loss was seen in uncoated control sample (CC, −19.7%, p < 0.05), whereas coated CC + C sample significantly lost less moisture (−13.9%, p < 0.05) during the 21 days of storage. This is in agreement with Mileriene et al. (2021) [43] stating that uncoated samples of acid-curd cheese significantly dried off faster than their coated counterparts. The addition of living strain to the coating, though, had a negative effect on its barrier properties. According to Ye et al. (2018) [44], the dispersion of bacterial cells in the film-forming solution destroys intermolecular interactions in the solution during film formation and increases the volume of voids in the living bacteria containing films, thereby slightly decreasing the tensile strength of the films but significantly increasing water vapour permeability.
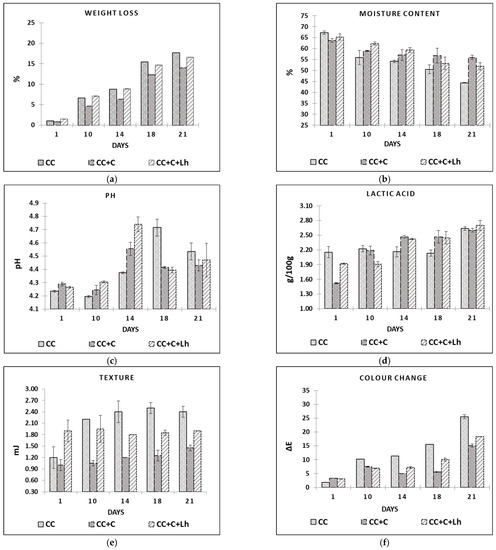
Figure 2.
Weight loss (a), moisture content (b), pH (c), lactic acid content (d), texture (e), and colour change (f) in control acid-curd cheese (CC), coated acid-curd cheese (CC + C), coated acid-curd cheese with L. helveticus (CC + C + Lh) during 23 days of storage at 4–6 °C (mean values ± SD).
The storage day, sample factors, and their interaction had a significant impact (p ≤ 0.0001) on instrumentally measured acidity, texture, and colour parameters (Figure 2a–f).
Low pH (4.3–4.8), distinctive wedge-like shape with semi-soft texture, and whitish colour are the main qualities of acid-curd cheese. The variation in pH as storage time elapsed is depicted in Figure 2c. At day 1, pH in all samples ranged from 4.24 (CC) to 4.27 (CC + C + Lh) and 4.29 (CC + C), indicating a small but significant impact of acid whey base coating on the curd cheese pH (p < 0.05). In a course of naturally occurring proteolysis [1,45], pH values increased and then decreased in all cheese samples. This process was faster in case of coated samples reaching the pH peak on day 14 compared with control ones reaching it on day 18. Between coated samples, the ripening process was more intense in the sample with lactobacilli incorporated into the coating (4.56 ± 0.05 for CC + C and 4.71 ± 0.06 for CC + C + Lh, p < 0.05). We can speculate that the coating presence contributed to cheese proteolysis by preserving the moisture in cheese (Figure 2b) and thus favouring the growth or survival of LAB (Figure 3a) that resulted in significantly lower pH (Figure 2c) and higher acidity values (Figure 2d; (p < 0.05)). Furthermore, the addition of L. helveticus strain to the coating significantly sped up the above-mentioned process (p < 0.01), showing that the immobilisation in coating may be an effective way for microorganism survival [26].
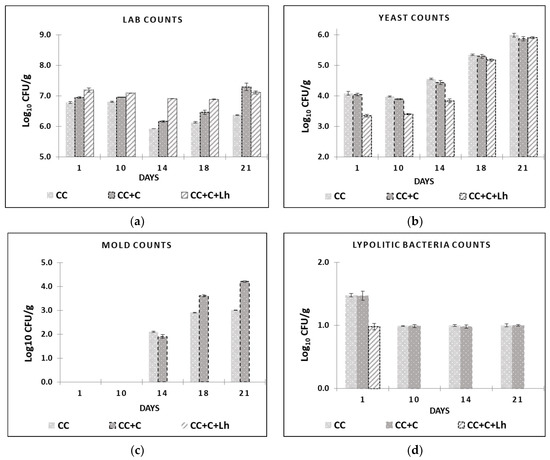
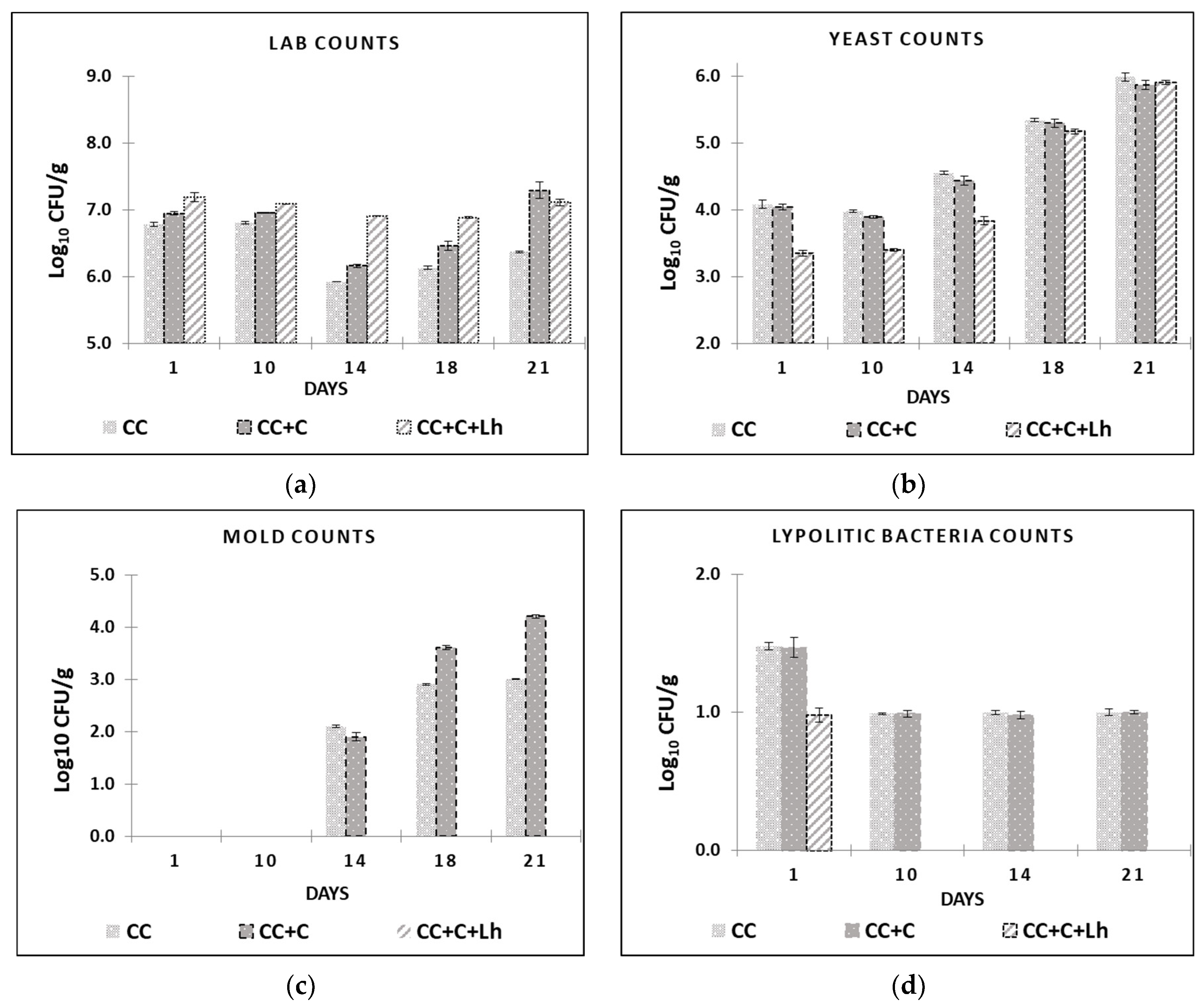
Figure 3.
Lactic acid bacteria (LAB) (a), yeast (b), and mould (c) counts and lipolytic bacteria (d), are presented for control acid-curd cheese (CC), coated acid-curd cheese (CC + C), and coated acid-curd cheese with L. helveticus (CC + C + Lh) during 23 days of storage at 4–6 °C (mean values ± SD).
The concentration of lactic acid (g/100 g) is presented in Figure 2d. At day 1, the concentration of lactic acid in fresh control curd cheeses (CC) was higher (2.15± 0.11; p < 0.05) compared with coated CC + C (1.52 ± 0.01) and CC + C + Lh (1.52 ± 0.02) samples; the samples switched places from day 14 with coated samples demonstrating higher (p < 0.05) lactic acid content compared with the control one, indicating growth of lactic acid bacteria in this cheese. Lactic acid produced by LAB consequently helps to draw out moisture from the cheese mass [46], and therefore, we observed more intense moisture (Figure 2b) and weight loss (Figure 2a of uncoated cheese (CC)) from day 10 compared with coated ones CC + C and CC + C + Lh (p < 0.05). Moreover, the moisture loss in the uncoated sample led to the harder texture (Figure 2e; p < 0.01) and discoloration (Figure 2f; p < 0.001). Cheese coated with plain coating (CC + C) lost the least weight, had the softest texture, and demonstrated the lowest change in colour compared with the other samples (p < 0.05). Addition of L. helveticus to the coating not only significantly reduced moisture-retaining properties of the coating but also contributed to cheese weight loss and colour change. It was reported by Ma, Jiang, Ahmed, Quin, and Liu (2018) [44] that the addition of LAB can change the spatial structure of the molecules, destroy intermolecular interactions, and enlarge the intermolecular space, which could decrease coating’s barrier properties. Moisture loss in curd cheese visually results in its colour change from white to yellow due to surface drying. Colour is an important sensory attribute of food products, and thus, consumers avoid cheese that is discoloured [47]. Instrumental colour determination revealed that, at day 1, there were no significant differences among samples. During the storage, however, uncoated control samples had significantly (p < 0.05) higher values of b* coordinates (data not shown) than coated ones, which means that uncoated samples were more yellow than coated counterparts. Overall colour change (∆E, calculated by comparing L*a*b* values of day 1 with values of days 10, 14, 18, and 21) of cheeses during storage is shown in Figure 2f. Uncoated samples showed the highest changes in colour during all storage. Between coated samples, CC + C had significantly lower (p < 0.05) ∆E values than the bacteria-supplemented one (CC + C + Lh). With regard to our previous study on liquid whey protein (LWPC) coated acid-curd cheese, our recent findings allow us to conclude that liquid sweet and acid whey protein-based coatings have similar potential to improve cheese quality by preserving moisture, and naturally cheese colour and appearance [43].
3.2.2. Microbiological Profile
Mesophilic LAB along with lesser counts of potential spoilage microorganisms such as enterococci, enterobacteria, and yeast and mould can be present in fresh artisanal acid-curd cheese [48]. Many manual manufacturing operations and absence of modern plastic packaging by artisanal cheese processors negatively affect cheese hygienic quality and end up with short shelf life (8–10 days). In our study, 1–1.5 log10 CFU/g of coliforms and enterobacteria were detected in all cheeses on day 1, with no statistical difference among samples (p > 0.05, data not shown). During the rest of the storage period, these indicator microorganisms were no longer detectable (<1 log10 CFU/g) in any of the cheese samples.
The results of mesophilic LAB, lipolytic bacteria, yeast, and mould are shown in Figure 3. Initial mesophilic LAB counts were 7.5 log10 CFU/g for control cheese. These counts were similar to those reported for similar cheese types [43,48]. Total LAB counts dropped down for approx. 1 log10 unit in control (CC) and plain coated (CC + C) cheese samples on day 14 (Figure 3a). From there, these samples underwent a slight but significant increase (p ≤ 0.05) in LAB counts, registering close to 6.4 log10 CFU/g for control (CC) and 7,3 log10 CFU/g for plain coated CC + C at the end of the storage. No effect of storage on LAB counts was observed in L. helveticus supplemented sample (CC + C + Lh), but in coated cheeses, LAB counts increased after day 14 and were significantly higher than the control cheese (p ≤ 0.05) at the end of storage.
Lipolytic microorganisms (aerobic and anaerobic bacteria of the genera Pseudomonas and Clostridium) and mould fungi are the most common contaminants of dairy products responsible for visible or non-visible defects, such as off-odour and off-flavour [49]. Lipolytic microorganisms cause damage to food products; thus, according to the instruction of microbiological control [50], it is recommended for dairy factories to not exceed 2 log10 units CFU/g limit of such psychrotrophic spoilage microflora. In our study, significant inhibition of spoilage microorganisms was achieved by protective strain incorporated in the coating (Figure 3b–d). Our coating with L. helveticus (CC + C + Lh) was able to suppress the growth of yeast for up to 1.0 log10 units for 14 days of storage (p ≤ 0.05) and prevent mould growth for 21 days compared with other samples where the mould appeared on cheese surface as early as day 14 (Figure 2c). It also suppressed lipolytic bacteria growth on day 1; these microorganisms were no longer detectable (<1 log10 CFU/g) in CC + C + Lh samples during the rest of the storage period compared with control and plain coated cheeses samples (1.5–1 log10 CFU/g; Figure 3d). This is in agreement with Guimarães et al. (2020) [51], where whey protein coatings containing L. buchneri cells prevented microbial spoilage in cheese for at least 30 days.
3.2.3. Sensory Profile
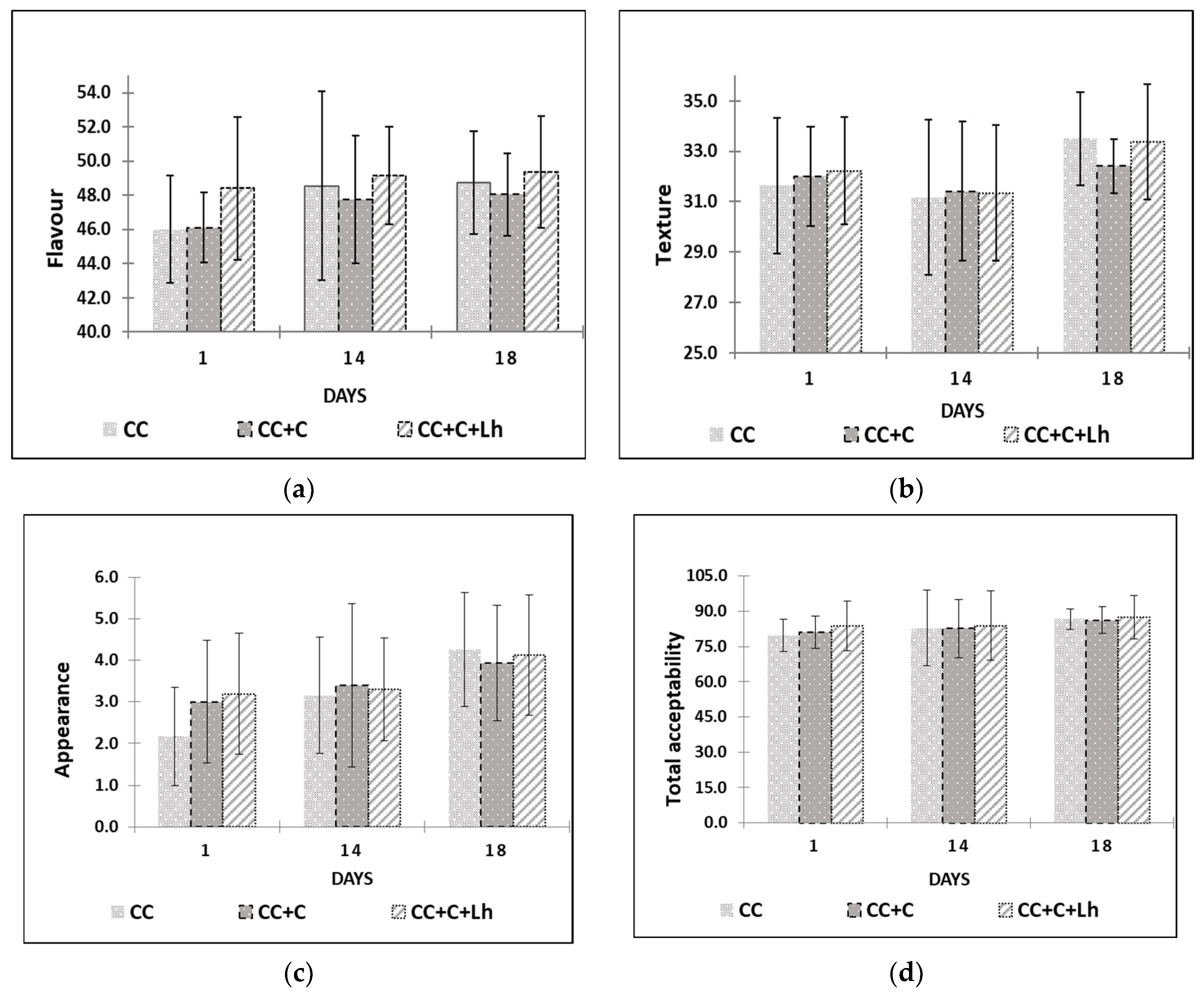
Edible films and coatings can contain flavouring substances, improving the sensory properties of foods [16]; thus, through sensory trials (Figure 4), we intended to assess the influence of our edible coatings on flavour, body and texture, appearance, and overall acceptability of acid-curd cheese. The results of the factorial analysis revealed no single treatment factor impact on any of sensory parameters of curd cheese. The storage day factor and storage and treatment interaction, though, had a significant impact on the perception of cheese flavour (the interaction factor; p ≤ 0.001), on overall acceptability, and on cheese texture (storage factor; p ≤ 0.01).
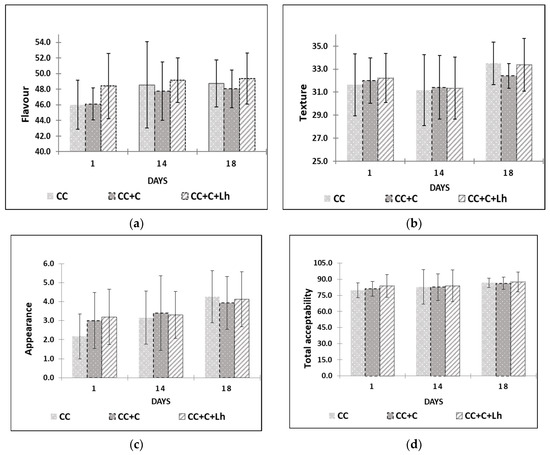
Figure 4.
Flavour (a), body and texture (b), appearance (c), and overall acceptability (d) are presented for control curd cheese (CC), coated curd cheese (CC + C), and coated cheese with L. helveticus (CC + C + Lh) during 18 days of sensory evaluation (mean values ± SD).
The scores of overall acceptability, flavour, and texture increased throughout the storage of cheese (p ≤ 0.05). Cheese samples coated with L. helveticus (CC + C + Lh) received the highest scores for flavour throughout the storage (p ≤ 0.001) compared with other samples. Plain coating based on sweet whey protein concentrate when used on the same type of cheese in our previous study [43] did not indicate any significant effect of the coating on the flavour of cheese. Fresh acid-curd cheese is known for slight dull and indistinctive flavour. Addition of sweet and sour tasting coating with lactobacilli incorporated positively affected cheese flavour and was described by the panellists as “more tasteful”. During the storage, the growth of spoilage microflora in control (CC) and in cheese with plain coating (CC + C) resulted in various off-odours and off-flavours lowering scores of the flavour parameter. Sensory defects in fresh curd cheese are very often caused by microbial contamination [49]; thus, bioprotective LAB cultures are employed to prevent the spoilage and preserve the flavour of the product.
With regard to appearance, coated cheese samples were perceived as better (p ≤ 0.05) than those of uncoated samples in the beginning of storage due to “fresher look” noted by the panellists. There were no significant differences among samples at the end of testing on day 18 in neither appearance nor total acceptability.
4. Conclusions
The presence of antimicrobial edible coating on fresh acid-curd cheeses was evaluated in order to represent small-scale East European acid-curd cheese producers that sell cheese unpacked or only wrapped in paper. Applied coating significantly improved appearance and slowed down colour changes by preserving moisture in cheese. The coating with incorporated indigenous L. helveticus decreased the growth of spoilage microorganisms during prolonged cheese storage, thus significantly improving the flavour of cheese.
In general, the incorporation of LAWPC as an acid-curd cheese by-product back into dairy production as a base of edible coating is quite beneficial for both small and big scale manufacturers. LAWPC-based edible coating could be prepared immediately after acid-curd cheese production reusing leftover whey and other agro-industrial waste ingredients obtained with minimal investments. This type of active edible coating with antimicrobial effect could be an excellent addition to both package-free and vacuum-packaged cheeses produced by manufacturers aiming for sustainability, enhanced quality, and extended shelf life of the final product.
Author Contributions
L.S.: data curation, formal analysis, investigation, visualisation, writing—original draft, project administration, and supervision. J.M.: conceptualisation, formal analysis, investigation, visualisation, and writing—original draft. A.V.: data curation, formal analysis, investigation, visualisation, and writing—original draft. E.A.: formal analysis, investigation, and writing—review and editing. E.S.: methodology and writing—review and editing. I.R., E.T., S.M.-B., L.A., I.C. and L.L.: writing—review and editing. J.S.: methodology and writing—review and editing. M.M.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The project “The edible coating formulated with liquid acid whey protein and bioactive compounds, and biopackaging for safety and quality of probiotic cheese” (Biocoat) benefits from a 974 thousand € grant from Iceland, Liechtenstein, and Norway through the EEA Grants. The aim of the project is to develop an edible coating formulated with liquid acid whey protein concentrate and bioactive compounds, in combination with biodegradable packaging to ensure safety, extend the shelf life, and enhance functionality of probiotic cheese. Project contract with the Research Council of Lithuania (LMTLT) No is S-BMT-21-10 (LT08-2-LMT-K-01-046).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Isfari, D.; Lara, U.G. Cheese whey as potential resource for antimicrobial edible film and active packaging production. Foods Raw Mater. 2019, 7, 229–239. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and Characterization of Coating Based on Protein Nanofibers and Polyphenol and Application for Salted Duck Egg Yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Jalilzadeh, A. The Effect of Whey Protein-Based Edible Coating Containing Natamycin and Lysozyme-Xanthan Gum Conjugate on the Shelf Life of Ultrafiltrated White Cheese|Request PDF. Available online: https://www.researchgate.net/publication/351226502_The_effect_of_whey_protein-based_edible_coating_containing_natamycin_and_lysozyme-xanthan_gum_conjugate_on_the_shelf_life_of_ultrafiltrated_white_cheese (accessed on 13 October 2022).
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Henriques, M.; Gomes, D.; Pereira, C. Whey Protein Edible Coatings: Recent Developments and Applications. In Food Engineering Series; Springer: New York, NY, USA, 2016; pp. 177–196. [Google Scholar]
- Saklani, P.S.P.; Nath, S.; Das, S.K.; Singh, S.M. A Review of Edible Packaging for Foods. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2885–2895. [Google Scholar] [CrossRef]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation 2020, 6, 19. [Google Scholar] [CrossRef]
- Madadlou, A.; Abbaspourrad, A. Bioactive whey peptide particles: An emerging class of nutraceutical carriers. Crit. Rev. Food Sci. Nutr. 2018, 58, 1468–1477. [Google Scholar] [CrossRef]
- Arbizu-Berrocal, S.; Talcott, S.; Noratto, G.; Chew, B.; Talcott, S. Assessing Cheese Whey Components for Their Potential to Improve Intestinal Health (P06-097-19). Curr. Dev. Nutr. 2019, 3, 615. [Google Scholar] [CrossRef]
- Fang, T.; Guo, M. Physicochemical, texture properties, and microstructure of yogurt using polymerized whey protein directly prepared from cheese whey as a thickening agent. J. Dairy Sci. 2019, 102, 7884–7894. [Google Scholar] [CrossRef]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Borges, A.R.; Pereira, C.J.D. Liquid whey protein concentrates as primary raw material for acid dairy gels. Food Sci. Technol. 2019, 40, 361–369. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Lauciene, L.; Kasetiene, N.; Sekmokiene, D.; Andruleviciute, V.; Malakauskas, M. Quality and nutritional characteristics of traditional curd cheese enriched with thermo-coagulated acid whey protein and indigenous Lactococcus lactis strain. Int. J. Food Sci. Technol. 2021, 56, 2853–2863. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 1–61. [Google Scholar] [CrossRef]
- Bagheripoor, N.; Khoshgozaran-Abras, S.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M.; Mollakhalili, N.; Jazaeri, S. Application of Active Edible Coatings to Improve the Shelf-life of Cheese. Food Sci. Technol. Res. 2018, 24, 949–962. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Gas barrier and wetting properties of whey protein isolate-based emulsion films. Polym. Eng. Sci. 2019, 59, E375–E383. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Costa, E.; Silva, S.; Gomes, A.; Pintado, M. Characterization of Edible Films Based on Alginate or Whey Protein Incorporated with Bifidobacterium animalis subsp. lactis BB-12 and Prebiotics. Coatings 2019, 9, 493. [Google Scholar] [CrossRef]
- Siracusa, V.; Karpova, S.; Olkhov, A.; Zhulkina, A.; Kosenko, R.; Iordanskii, A. Gas Transport Phenomena and Polymer Dynamics in PHB/PLA Blend Films as Potential Packaging Materials. Polymers 2020, 12, 647. [Google Scholar] [CrossRef]
- Šipailienė, A.; Petraitytė, S. Encapsulation of Probiotics: Proper Selection of the Probiotic Strain and the Influence of Encapsulation Technology and Materials on the Viability of Encapsulated Microorganisms. Probiotics Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Haji, F.; Cheon, J.; Baek, J.; Wang, Q.; Tam, K.C. Application of Pickering emulsions in probiotic encapsulation-A review. Curr. Res. Food Sci. 2022, 5, 1603–1615. [Google Scholar] [CrossRef]
- Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Panattoni, A.; Labella, R.; Ghelardi, E. Microbiological Quality and Resistance to an Artificial Gut Environment of Two Probiotic Formulations. Foods 2021, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Olivo, P.M.; Scapim, M.R.D.S.; Maia, L.F.; Miazaki, J.; Rodrigues, B.M.; Madrona, G.S.; Bankuti, F.I.; Pozza, M.S.D.S. Probiotic Coating for Ripened Cheeses with Lactobacillus Acidophilus and Lactobacillus Helveticus Inclusion. J. Agric. Stud. 2020, 8, 152. [Google Scholar] [CrossRef]
- Šalomskienė, J.; Abraitiene, A.; Jonkuvienė, D.; Macioniene, I.; Repečkienė, J. Selection of enhanced antimicrobial activity posing lactic acid bacteria characterised by (GTG)5-PCR fingerprinting. J. Food Sci. Technol. 2015, 52, 4124–4134. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 11869:2012; Fermented Milks—Determination of Titratable Acidity—Potentiometric Method. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 5534:2004; Cheese and Processed Cheese. Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 1735:2004; Cheese and Processed Cheese Products. Determination of Fat Content. Gravimetric Method. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 22662:2007; Milk and Milk Products—Determination of Lactose Content by High-Performance Liquid Chromatography (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8968-3:2004; Milk—Determination of Nitrogen Content—Part 3: Block-Digestion Method (Semi-Micro Rapid Routine Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 6611:2004; Milk and Milk Products—Enumeration of Colony-Forming Units of Yeasts and/or Moulds—Colony-Count Technique at 25 Degrees C. International Organization for Standardization: Geneva, Switzerland, 2004.
- Muys, G.T.; Willemse, R. The detection and enumeration of lipolytic microorganisms by means of a modified Eykman-plate method. Antonie Van Leeuwenhoek 1965, 31, 103–112. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Bodyfelt, F.W.; Tobias, J.; Trout, G.M. The Sensory Evaluation of Dairy Products; Van Nostrand Reinhold: New York, NY, USA, 1988; pp. 1–7, 59–88. [Google Scholar]
- Pereira, J.O.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, P.; Mariniello, L.; Giosafatto, V.L.; Esposito, M.; Sabbah, M.; Porta, R. Dairy Whey Protein-Based Edible Films and Coatings for Food Preservation. In Food Packaging and Preservation; Elsevier: Cambridge, MA, USA, 2018; pp. 439–456. [Google Scholar]
- Mileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of liquid whey protein concentrate–based edible coating enriched with cinnamon carbon dioxide extract on the quality and shelf life of Eastern European curd cheese. J. Dairy Sci. 2021, 104, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ma, D.; Qin, W.; Liu, Y. Physical and Antibacterial Properties of Sodium Alginate—Sodium Carboxymethylcellulose Films Containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef] [PubMed]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Lauciene, L.; Andruleviciute, V.; Kasetiene, N.; Sekmokiene, D.; Malakauskas, M. Effect of Indigenous Lactococcus lactis on physicochemical and sensory properties of thermo-coagulated acid whey protein. J. Food Process. Preserv. 2021, 45, e15420. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.A.; Delahunty, C.M. Sensory Character of Cheese And Its Evaluation. In Cheese: Chemistry, Physics and Microbiology: Fourth Edition; Elsevier Inc.: Cambridge, MA, USA, 2017; Volume 1, pp. 517–545. ISBN 9780122636530. [Google Scholar]
- Pappa, E.C.; Bontinis, T.G.; Tasioula-Margari, M.; Samelis, J. Microbial Quality and Biochemical Changes of Fresh Soft, Acid-Curd Xinotyri Cheese Made from Raw or Pasteurized Goat Milk. Food Technol. Biotechnol. 2017, 55, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Šalomskienė, J.; Mačionienė, I. Instruction of Microbiological Control for Milk Processing Plants. 2nd Updated and Supplemented Edition, 2nd ed.; JSC Smaltija Publishing House: Kaunas, Lithuania, 2004. [Google Scholar]
- Guimarães, A.; Ramos, Ó.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active Whey Protein Edible Films and Coatings Incorporating Lactobacillus Buchneri for Penicillium Nordicum Control in Cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).