A Simple Aptamer SERS Sensor Based on Mesoporous Silica for the Detection of Chlorpyrifos
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials and Instruments
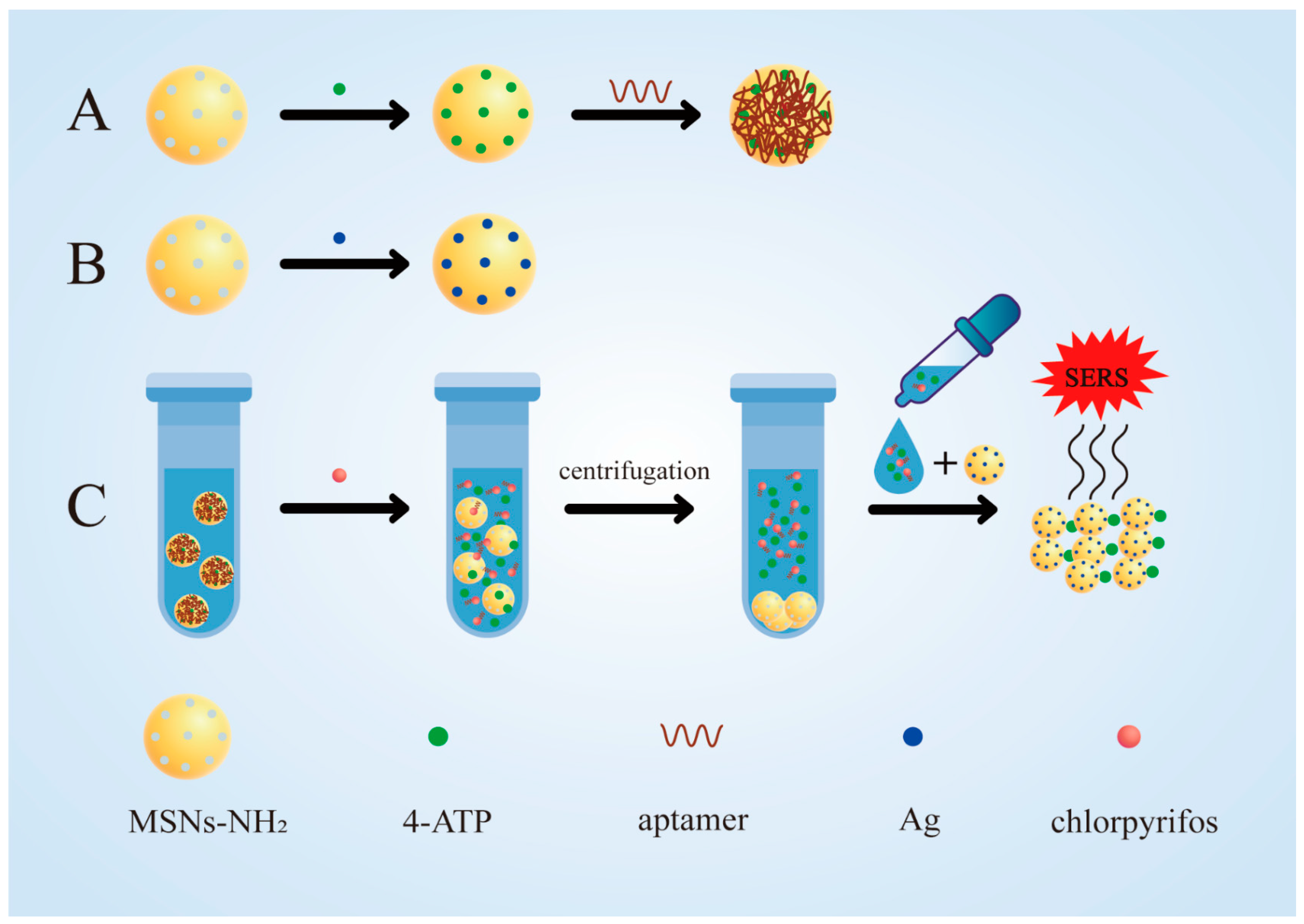
2.2. Synthesis of MSNs-NH2 and 4-ATP@MSNs-NH2
2.3. Synthesis of 4-ATP@MSNs-NH2@aptamers
2.4. Synthesis of Ag@MSNs
2.5. Feasibility Analysis
2.6. Sensing Analysis of Chlorpyrifos
2.7. Pretreatment of Actual Samples
3. Results and Discussion
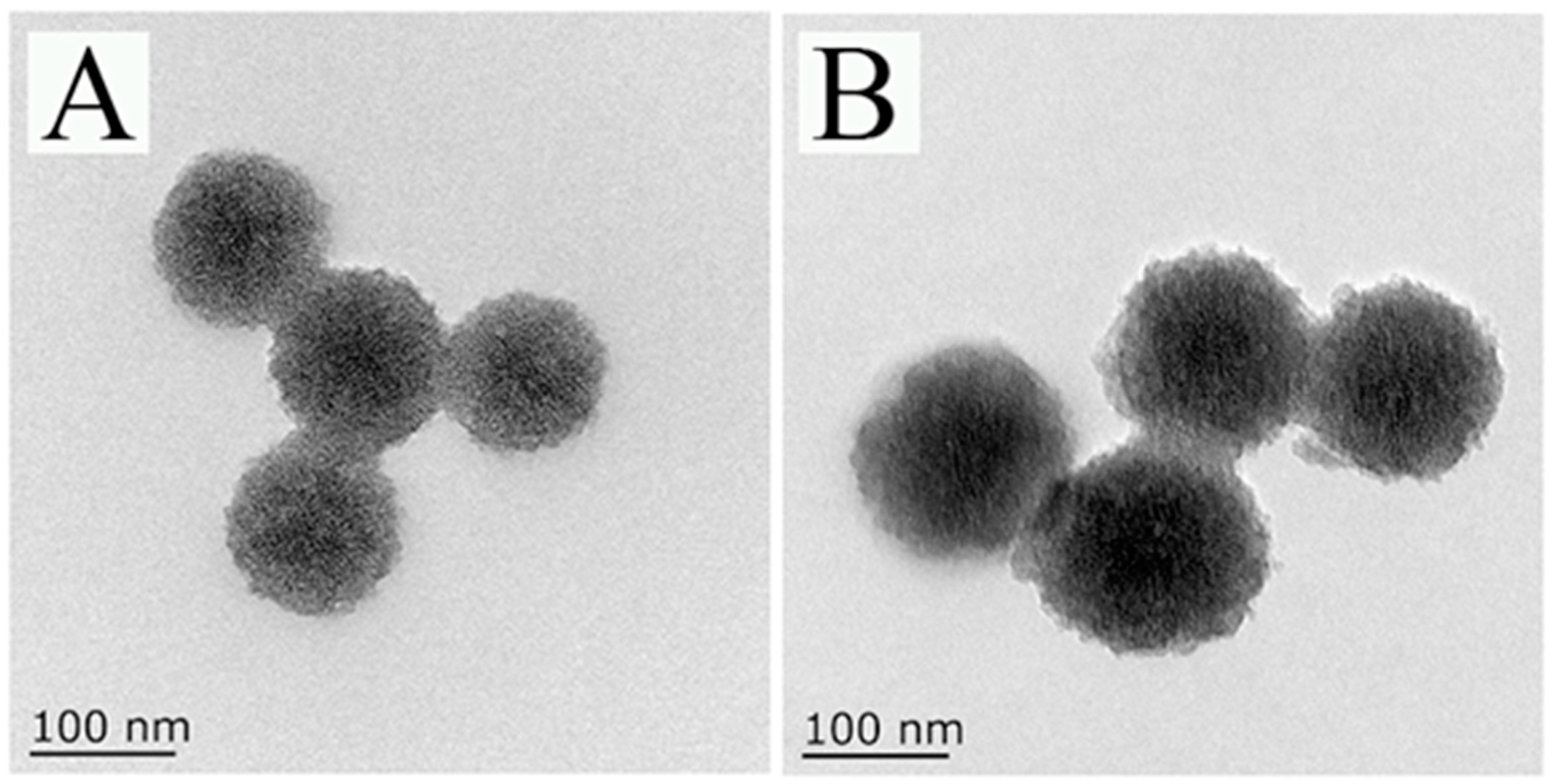
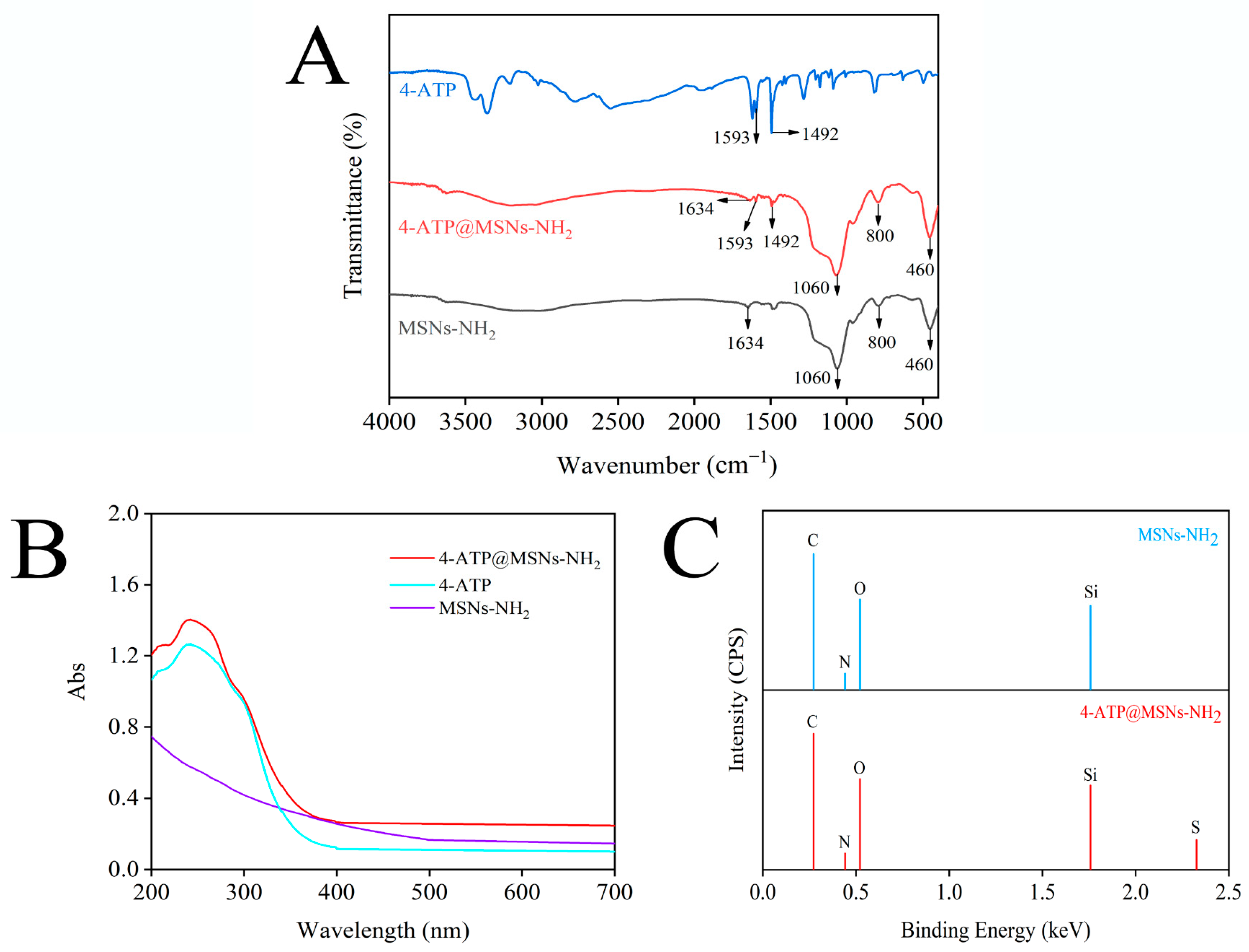
3.1. Characterization of MSNs-NH2 and 4-ATP@MSNs-NH2
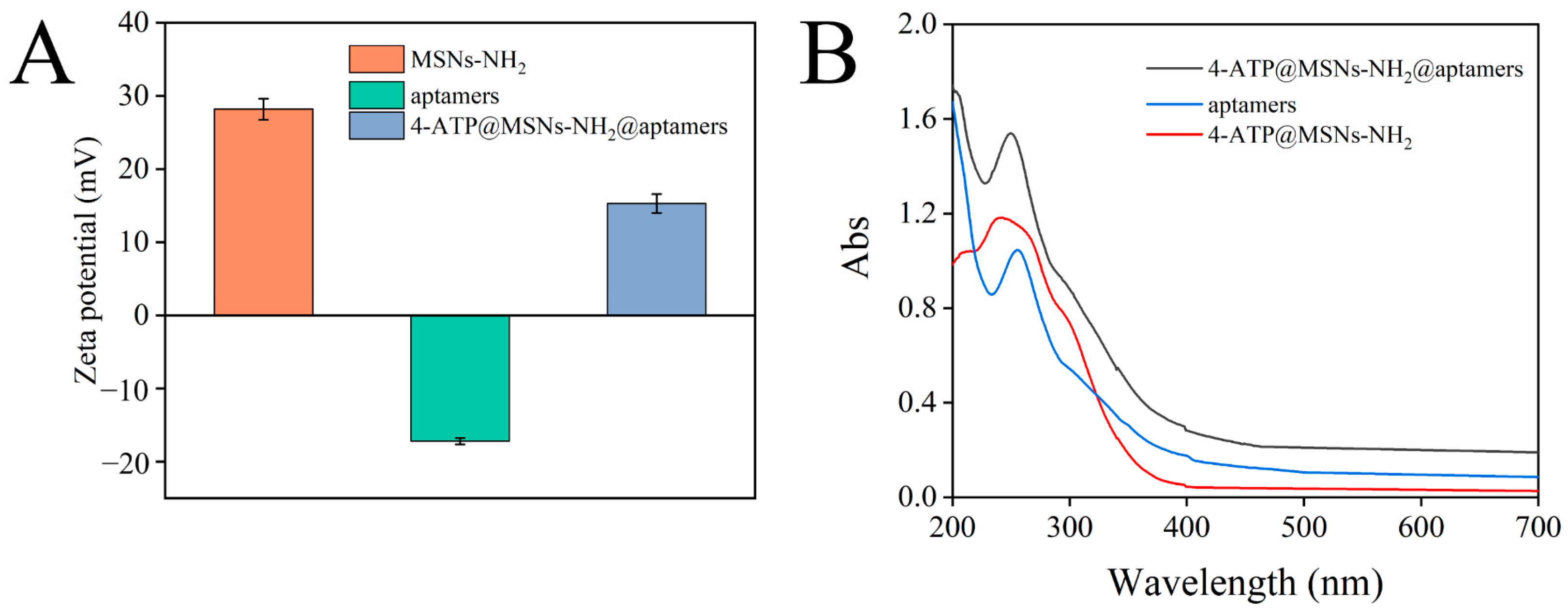
3.2. Characterization of 4-ATP@MSNs-NH2@aptamers
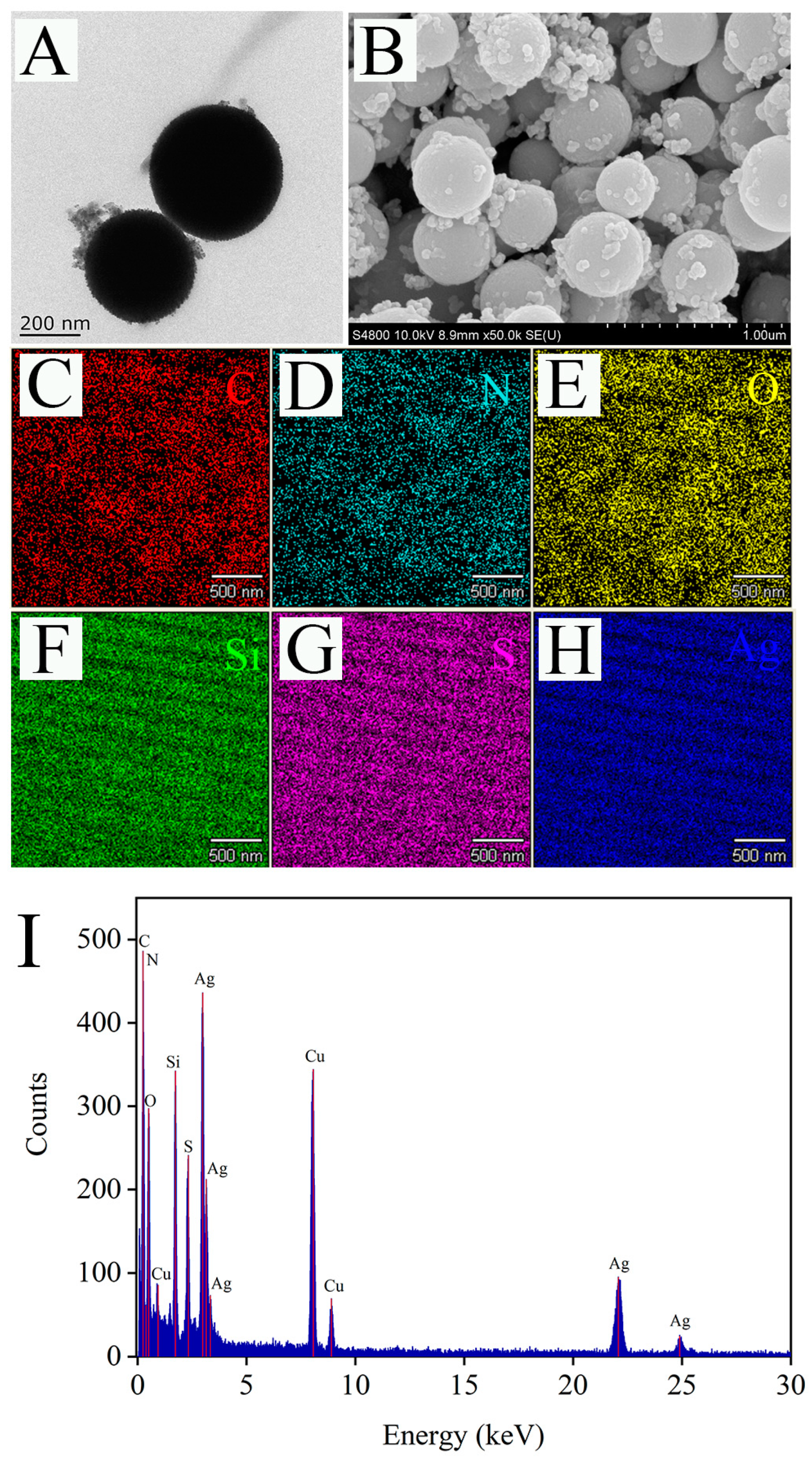
3.3. Characterization and Activity Analysis of Ag@MSNs
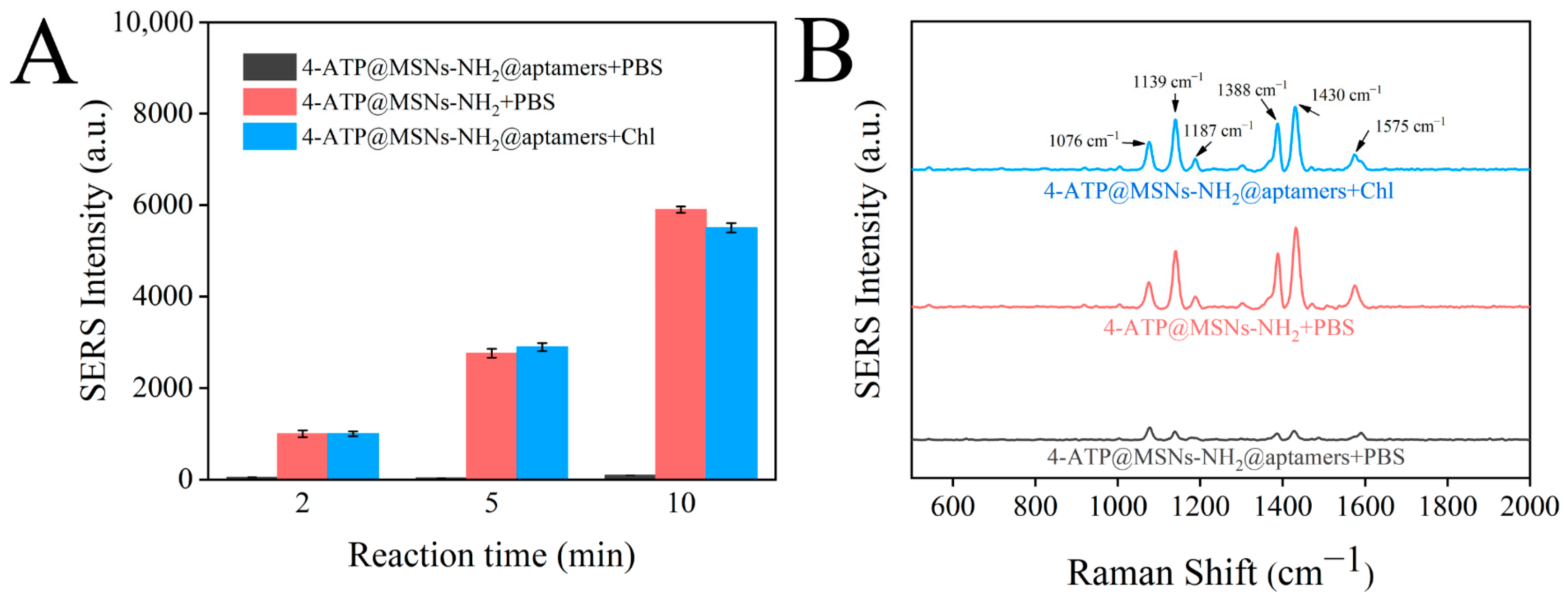
3.4. Feasibility Analysis
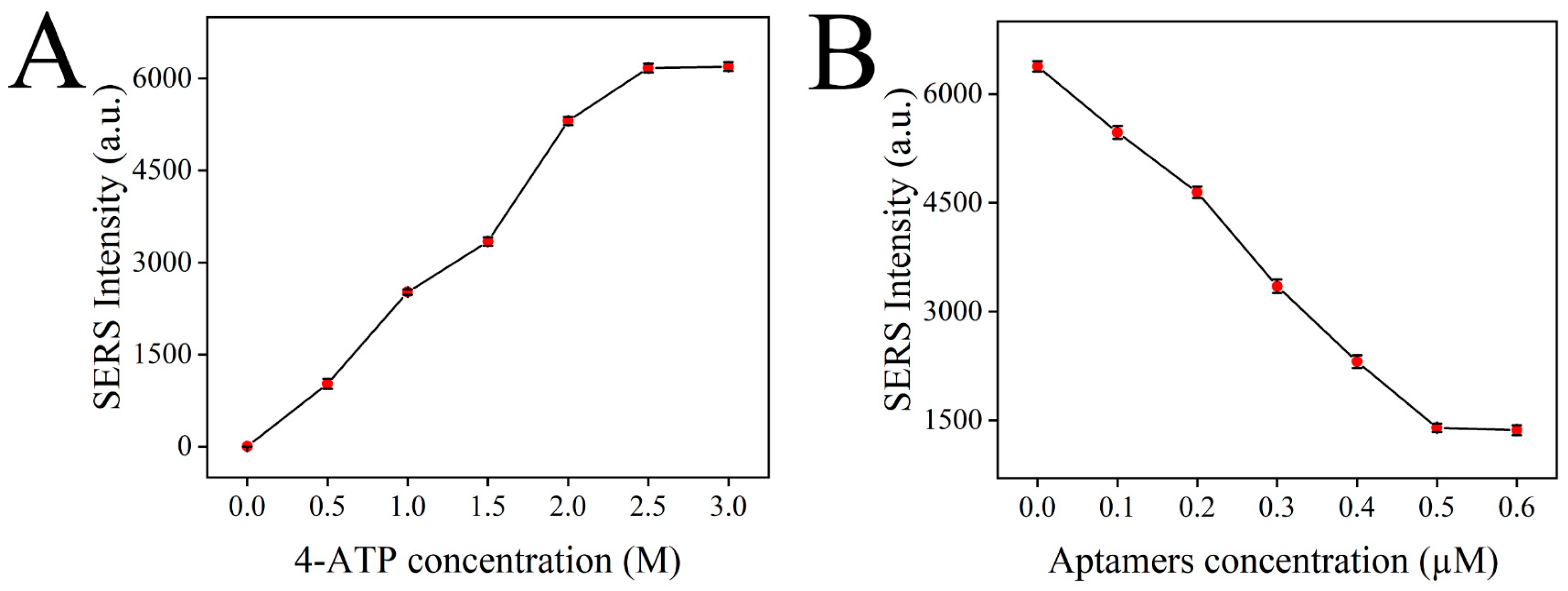
3.5. Optimization of Test Conditions
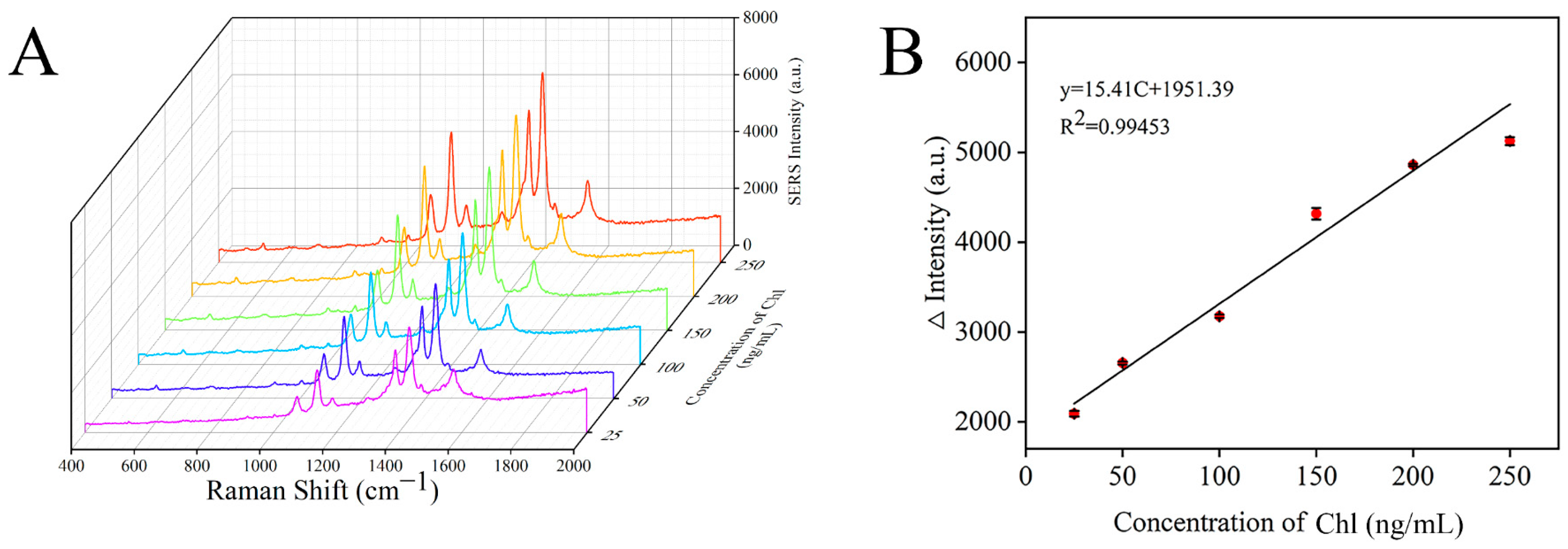
3.6. Preparation of SERS Sensor
| Method | Linear Range (ng/mL) | LOD (ng/mL) |
|---|---|---|
| Bioenzyme sensor [3] | 0–100 | 29.42 |
| Gas chromatography-mass spectrometry [10] | 100–1000 | 100 |
| High-performance liquid chromatography [11] | 800–80,000 | 890 |
| immunochromatographic assay [32] | − | 10 |
| dual-readout immunochromatographic assay [33] | 0.1–50 | 0.033 |
| cytometric bead array method [34] | 5.14–49.53 | 1.09 |
| Fluorescence immunoassay [35] | 9.77–1250 | 4.9 |
| Surface-enhanced Raman [36] | − | 175.29 |
| This work | 25–250 | 19.87 |
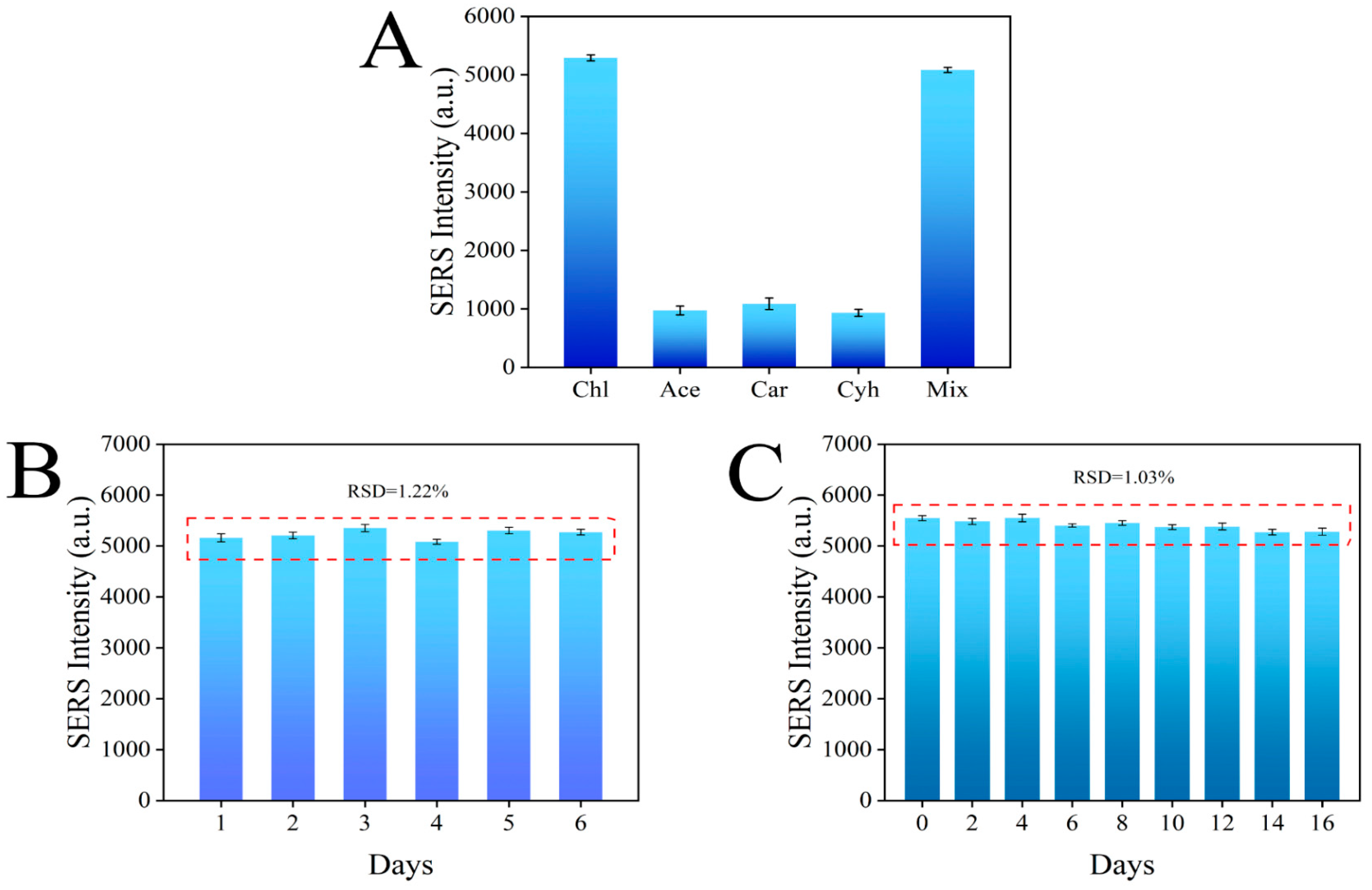
3.7. Specificity, Reproducibility, and Stability of the Sensor
3.8. Actual Sample Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic interactions between peroxidase-mimic silver NanoZymes and chlorpyrifos-specific aptamers enable highly-specific pesticide sensing in river water. Anal. Chim. Acta 2019, 1083, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hou, C.; Lei, J.; Huo, D.; Luo, X.; Dong, L. A novel device based on a fluorescent cross-responsive sensor array for detecting pesticide residue. Meas. Sci. Technol. 2016, 27, 115104. [Google Scholar] [CrossRef]
- Dorneles, A.L.; Rosa-Fontana, A.S.; Dos Santos, C.F.; Blochtein, B. Larvae of stingless bee Scaptotrigona bipunctata exposed to organophosphorus pesticide develop into lighter, smaller and deformed adult workers. Environ Pollut. 2021, 272, 116414. [Google Scholar] [CrossRef] [PubMed]
- Anli, E.; Vural, N.; Vural, H.; Gucer, Y. Application of solid-phase micro-extraction (SPME) for determining residues of chlorpyrifos and chlorpyrifos-methyl in wine with gas chromatography (GC). J. Inst. Brew. 2007, 113, 213–218. [Google Scholar] [CrossRef]
- Wan, P.; Santerre, C.R.; Brown, P.B.; Deardorff, D.C. Chlorpyrifos residues before and after cooking of catfish fillets. J. Food Sci. 2003, 68, 12–15. [Google Scholar] [CrossRef]
- Alam, S.; Srivastava, N.; Iqbal, N.; Saini, M.K.; Kumar, J. Magnetic solid-phase extraction (MSPE) using magnetite-based core-shell nanoparticles with silica network (SiO2) coupled with GC-MS/MS analysis for determination of multiclass pesticides in water. J. AOAC Int. 2021, 104, 633–644. [Google Scholar] [CrossRef]
- Lee, J.E.; Jang, S.H.; Hur, S.H.; Bang, H.Y.; Bae, I.-k.; Kim, H.J. LC-MS/MS and GC-MS/MS cross-checking analysis method for 247 pesticide residues in sweet pepper (Capsicum annuum). Int. J. Food Prop. 2021, 24, 1758–1776. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Tran, L.-H.; Van, T.-K.; Le, D.-V. Determination of chlorpyrifos pesticide residue in bell peppers grown in dalat (Vietnam) by GC-MS/MS using QuEChERS extraction. J. Anal. Chem. 2022, 77, 604–610. [Google Scholar] [CrossRef]
- Tay, B.Y.P.; Wai, W.H. A gas chromatography-mass spectrometry method for the detection of chlorpyrifos contamination in palm-based fatty acids. J. Am. Oil Chem. Soc. 2021, 98, 881–887. [Google Scholar] [CrossRef]
- Mauldin, R.E.; Primus, T.M.; Buettgenbach, T.A.; Johnston, J.J.; Linz, G.M. A simple HPLC method for the determination of chlorpyrifos in black oil sunflower seeds. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 339–348. [Google Scholar] [CrossRef]
- Masís-Mora, M.; Beita-Sandí, W.; Rodríguez-Yáñez, J.; Rodríguez-Rodríguez, C.E. Validation of a methodology by LC-MS/MS for the determination of triazine, triazole and organophosphate pesticide residues in biopurification systems. J. Chromatogr. B 2020, 1156, 122296. [Google Scholar] [CrossRef]
- Khan, N.S.; Pradhan, D.; Choudhary, S.; Saxena, P.; Poddar, N.K.; Jain, A.K. Immunoassay-based approaches for development of screening of chlorpyrifos. J. Anal. Sci. Technol. 2021, 12, 32. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Abd El-Aty, A.M.; Wang, Y.; Cao, Z.; Jia, H.; Salvador, J.P.; Hacimuftuoglu, A.; Cui, X.; Zhang, Y.; et al. A highly sensitive bio-barcode immunoassay for multi-residue detection of organophosphate pesticides based on fluorescence anti-quenching. J. Pharm. Anal. 2022, 12, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, O.; Canoura, J.; Yu, H.; Liu, Y.; Xiao, Y. Innovative engineering and sensing strategies for aptamer-based small-molecule detection. TrAC Trends Anal. Chem. 2019, 121, 115699. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Sha, J.; Li, Z.; Wang, W.; Zhang, H. High affinity truncated aptamers for ultra-sensitive colorimetric detection of bisphenol A with label-free aptasensor. Food Chem. 2020, 317, 126459. [Google Scholar] [CrossRef]
- Cui, H.; Yang, J.; Lu, H.; Li, L.; Zhu, X.; Ding, Y. Near-infrared carbon dots for cell imaging and detecting ciprofloxacin by label-free fluorescence sensor based on aptamer. Microchim. Acta 2022, 189, 170. [Google Scholar] [CrossRef] [PubMed]
- Inam, A.; Angeli, M.A.C.; Douaki, A.; Shkodra, B.; Lugli, P.; Petti, L. An aptasensor based on a flexible screen-printed silver electrode for the rapid detection of chlorpyrifos. Sensors 2022, 22, 2754. [Google Scholar] [CrossRef]
- Shuanggen, H.; Yan, W.; Jianping, H.; Muhua, L.; Ruimei, W.; Yuan, F.; Xiaobin, W. Rapid detection of malathion residues in Chinese cabbage by surface-enhanced Raman spectroscopy. Trans. Chin. Soc. Agric. Eng. 2016, 32, 296–301. [Google Scholar] [CrossRef]
- Mikac, L.; Kovačević, E.; Ukić, S.; Raić, M.; Jurkin, T.; Marić, I.; Gotić, M.; Ivanda, M. Detection of multi-class pesticide residues with surface-enhanced Raman spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 252, 119478. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Fang, X.; Shang, Y.; Gu, H.; Jia, W.; Yang, G.; Gu, Y.; Qu, L. Rapid and non-invasive surface-enhanced Raman spectroscopy (SERS) detection of chlorpyrifos in fruits using disposable paper-based substrates charged with gold nanoparticle/halloysite nanotube composites. Microchim. Acta 2022, 189, 197. [Google Scholar] [CrossRef]
- Peng, Y.; Niu, Z.; Huang, W.; Chen, S.; Li, Z. Surface-enhanced Raman scattering studies of 1,10-Phenanthroline adsorption and its surface complexes on a gold electrode. J. Phys. Chem. B 2005, 109, 10880–10885. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ge, H.; Zhao, K.; Yu, L.; Zhang, C. Determination of sildenafil citrate added in traditional Chinese medicines and health care products by thin layer chromatographic surface-enhanced Raman spectroscopic method. Chin. J. Pharm. Anal. 2014, 34, 1241–1246. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented mesoporous silica nanoparticles. Chem. Mater. 2016, 29, 371–388. [Google Scholar] [CrossRef]
- Truong, P.L.; Duyen, V.T.C.; Toi, V.V.; Hien, M.D. Rapid detection of tebuconazole based on aptasensor and aggregation of silver nanoparticles. J. Nanomater. 2021, 2021, 5532477. [Google Scholar] [CrossRef]
- Kong, X.P.; Zhang, B.H.; Wang, J. Multiple roles of mesoporous silica in safe pesticide application by nanotechnology: A Review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef]
- Asgari, S.; Wu, G.; Aghvami, S.A.; Zhang, Y.; Lin, M. Optimisation using the finite element method of a filter-based microfluidic SERS sensor for detection of multiple pesticides in strawberry. Food Addit. Contam. Part A 2021, 38, 646–658. [Google Scholar] [CrossRef]
- Jiao, A.; Dong, X.; Zhang, H.; Xu, L.; Tian, Y.; Liu, X.; Chen, M. Construction of pure worm-like AuAg nanochains for ultrasensitive SERS detection of pesticide residues on apple surfaces. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 209, 241–247. [Google Scholar] [CrossRef]
- Patel, U.B.; Mehta, V.N.; Kumar, M.A.; Kailasa, S.K. 4-Aminothiophenol functionalized gold nanoparticles as colorimetric sensors for the detection of cobalt using UV–Visible spectrometry. Res. Chem. Intermed. 2012, 39, 771–779. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Wu, H.F. Semiconductor cadmium sulphide nanoparticles as matrices for peptides and as co-matrices for the analysis of large proteins in matrix-assisted laser desorption/ionization reflectron and linear time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Lee, E.-H.; Kim, K.-O.; Lee, Y.T.; Hammock, B.D.; Lee, H.-S. Competitive immunochromatographic assay for the detection of the organophosphorus pesticide chlorpyrifos. Anal. Chim. Acta 2011, 693, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, H.; Lu, Q.; Wang, W.; Song, Y.; Tu, X.; Zhu, C.; Smith, J.N.; Du, D.; Fu, Z.; Lin, Y. Dual-Readout Immunochromatographic Assay by Utilizing MnO2 Nanoflowers as the Unique Colorimetric/Chemiluminescent Probe. Anal. Chem. 2018, 90, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Luo, J.; Dou, X.; Yang, M.; Fan, Z. A sensitive cytometric bead array for chlorpyrifos using magnetic microspheres. Microchem. J. 2020, 156, 104847. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, L.; Liu, C.; Li, Q.; Luo, J.; Yang, M. Fluorometric competitive immunoassay for chlorpyrifos using rhodamine-modified gold nanoparticles as a label. Microchim. Acta 2018, 185, 41. [Google Scholar] [CrossRef]
- Park, H.; Park, J.; Lee, G.; Kim, W.; Park, J. Detection of chlorpyrifos using bio-inspired silver nanograss. Materials 2022, 15, 3454. [Google Scholar] [CrossRef]

| Sample | Spiked Amount (µg/kg) | Measured Amount (ng/mL) | Recovery Rate (%) | RSD (%) |
|---|---|---|---|---|
| Apple | 0 | ND | ND | ND |
| 15 | 51.1 | 102.2 | 1.24 | |
| 30 | 90.08 | 90.08 | 1.71 | |
| 60 | 198.26 | 99.13 | 1.11 | |
| Tomato | 0 | ND | ND | ND |
| 15 | 50.31 | 100.63 | 0.64 | |
| 30 | 99.42 | 99.42 | 3.32 | |
| 60 | 200.15 | 100.07 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Shi, Q.; He, K.; Wu, J.; Zhu, Z.; Feng, J. A Simple Aptamer SERS Sensor Based on Mesoporous Silica for the Detection of Chlorpyrifos. Foods 2022, 11, 3331. https://doi.org/10.3390/foods11213331
Dong S, Shi Q, He K, Wu J, Zhu Z, Feng J. A Simple Aptamer SERS Sensor Based on Mesoporous Silica for the Detection of Chlorpyrifos. Foods. 2022; 11(21):3331. https://doi.org/10.3390/foods11213331
Chicago/Turabian StyleDong, Sa, Qiuyun Shi, Kangli He, Jianwei Wu, Zixin Zhu, and Jianguo Feng. 2022. "A Simple Aptamer SERS Sensor Based on Mesoporous Silica for the Detection of Chlorpyrifos" Foods 11, no. 21: 3331. https://doi.org/10.3390/foods11213331
APA StyleDong, S., Shi, Q., He, K., Wu, J., Zhu, Z., & Feng, J. (2022). A Simple Aptamer SERS Sensor Based on Mesoporous Silica for the Detection of Chlorpyrifos. Foods, 11(21), 3331. https://doi.org/10.3390/foods11213331






