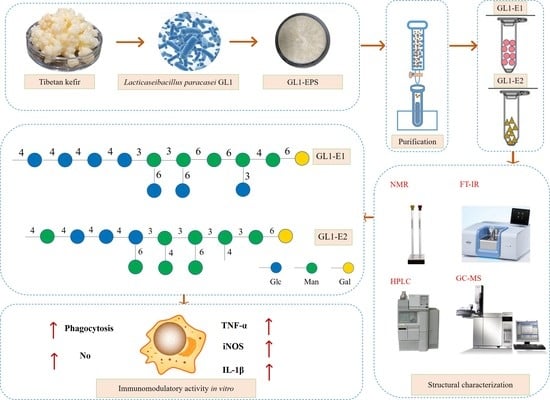
Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Chemicals
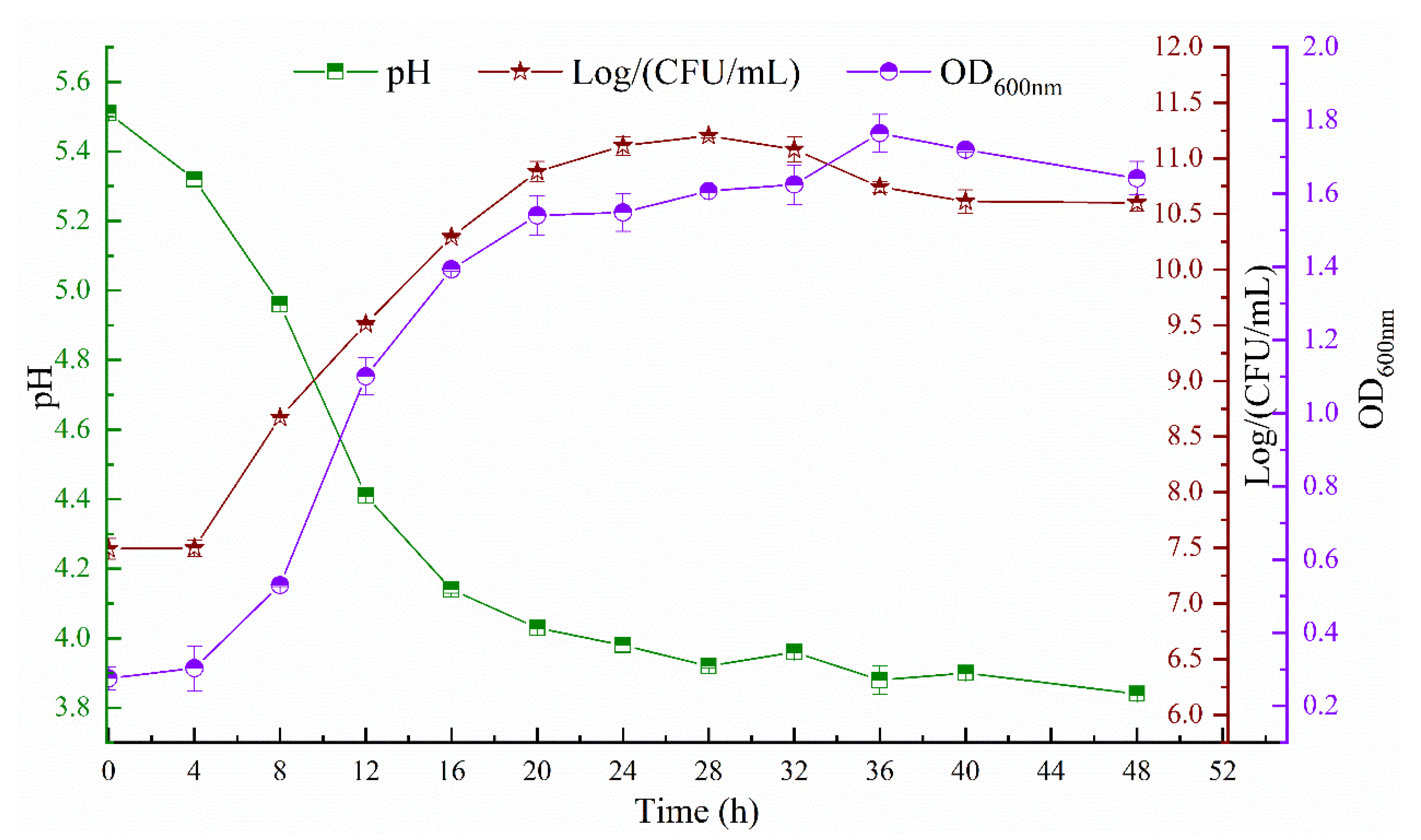
2.2. Determination of pH and Bacterial Growth
2.3. Extraction of Crude GL1-EPS
2.4. Chemical Characterization of GL1-EPS
2.4.1. UV–Vis Analysis and FT-IR Analysis
2.4.2. Measurement of Molecular Weight (Mw)
2.4.3. Monosaccharide Composition Analysis
2.4.4. Methylation Analysis
2.4.5. 1D and 2D NMR Spectra Analysis
2.4.6. SEM Analysis
2.4.7. Differential Scanning Calorimetry (DSC) Analysis
2.5. Immunomodulatory Effects of GL1-EPS
2.5.1. The Viability of RAW 264.7 Cells
2.5.2. Effect of GL1-EPS on Macrophage Phagocytosis
2.5.3. Effect of GL1-EPS on NO Production
2.5.4. Determination of TNF-α, IL-1β, and iNOS Production
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bacterial Growth
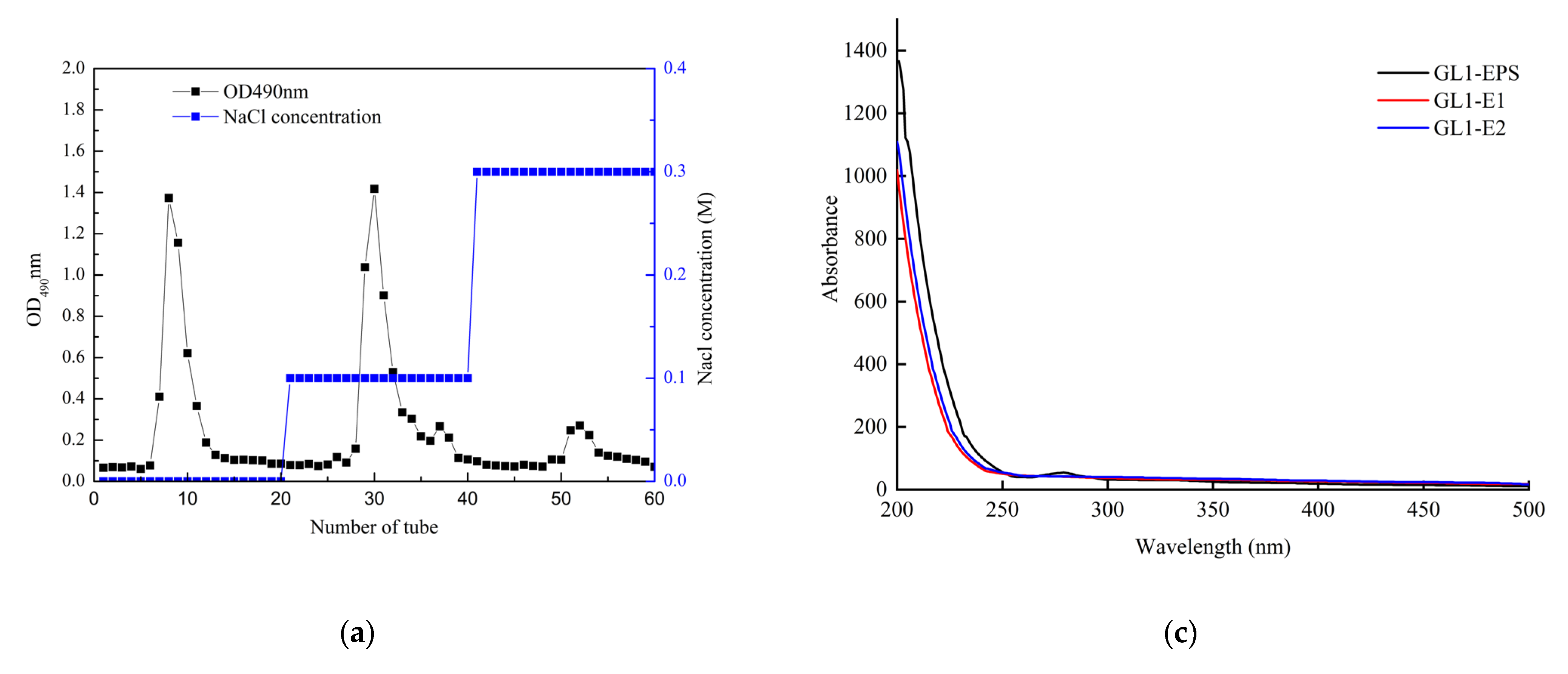
3.2. Extraction of Crude GL1-EPS
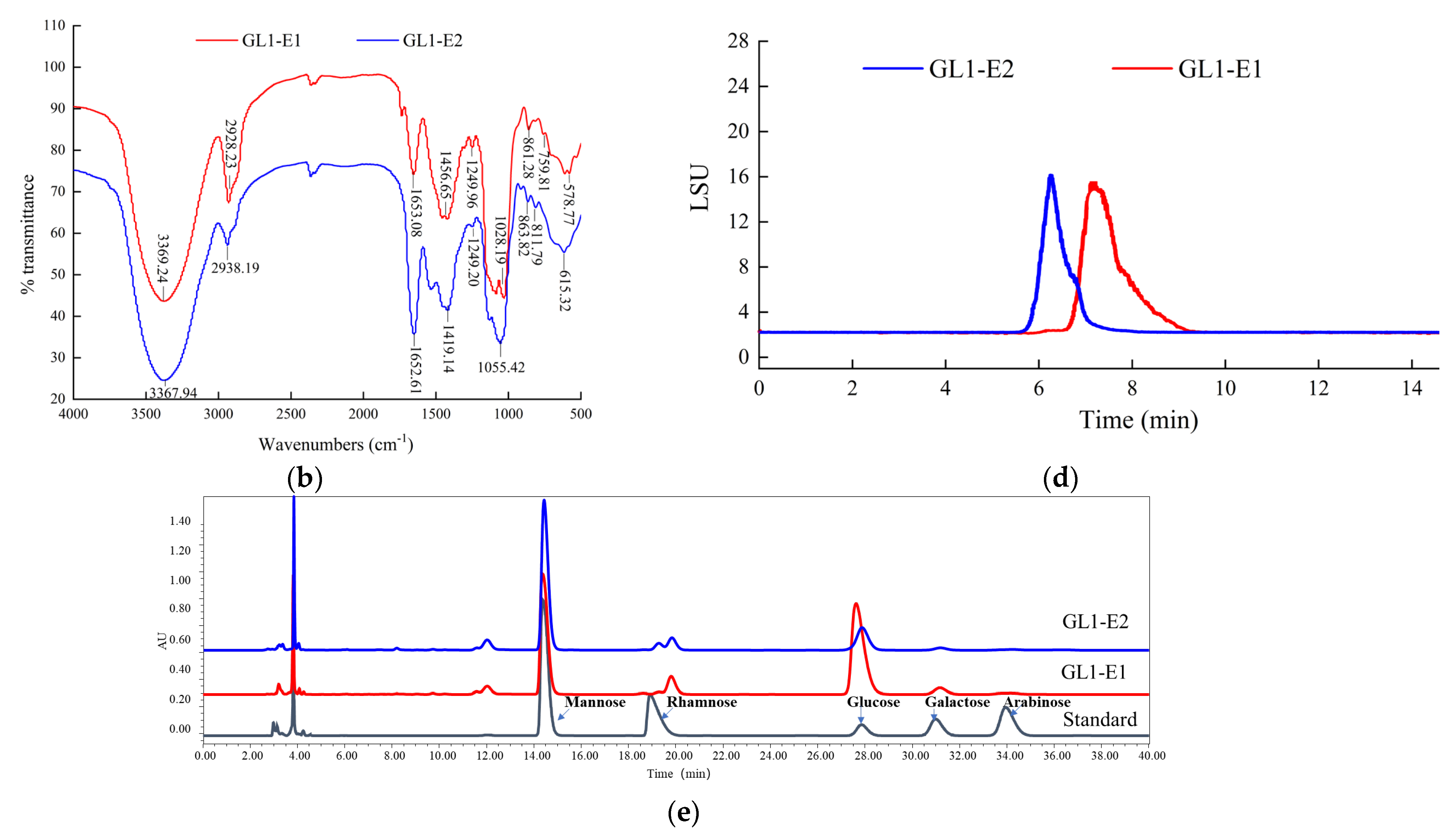
3.3. UV and FTIR Spectrum Analysis of GL1-EPS
3.4. Mw and Monosaccharide Composition of GL1-EPS
3.5. Methylation Analysis
3.6. 1D and 2D NMR Spectra Analysis
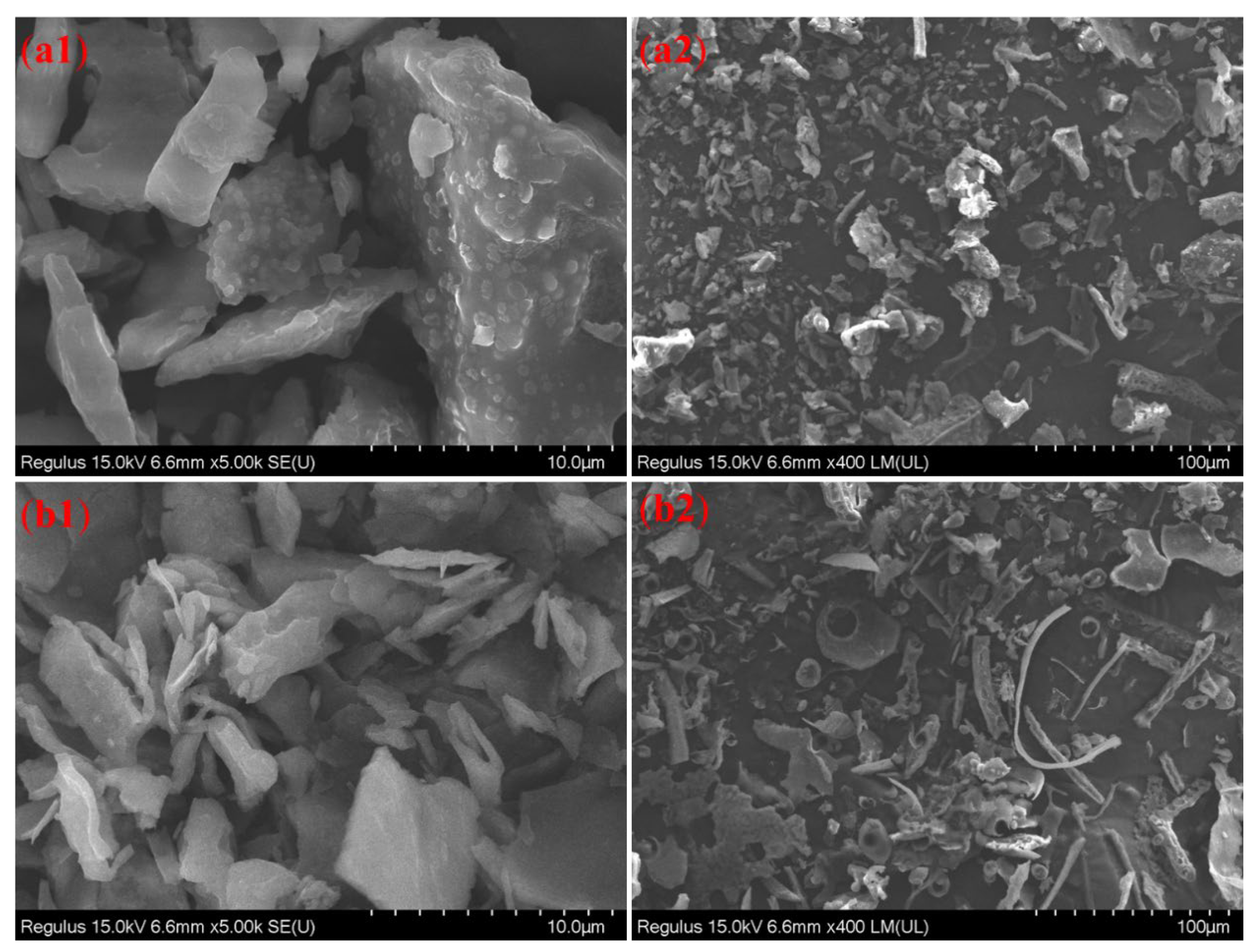
3.7. SEM Analysis
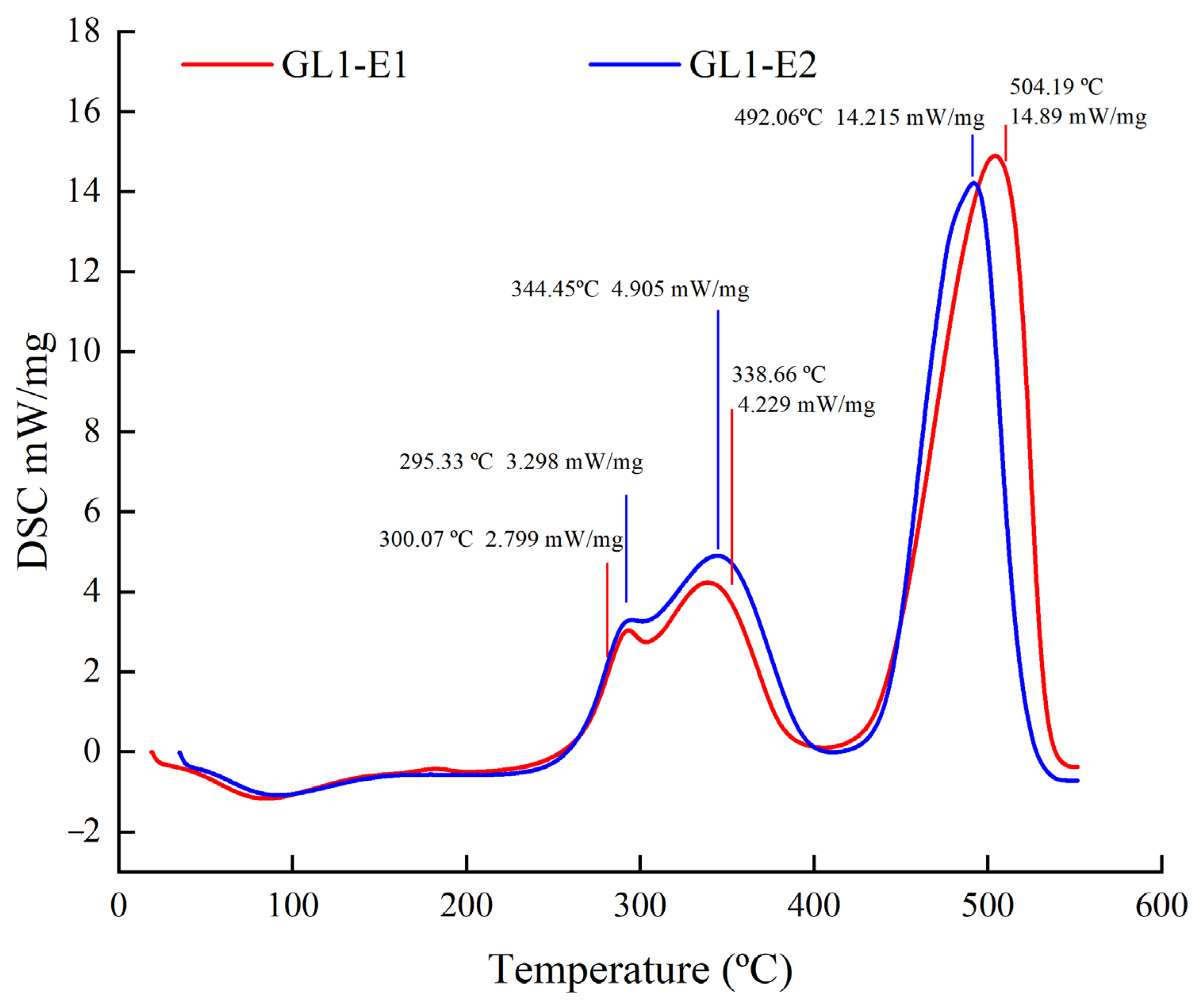
3.8. DSC Analysis
3.9. Immunomodulatory Effects of GL1-E1 and GL1-E2
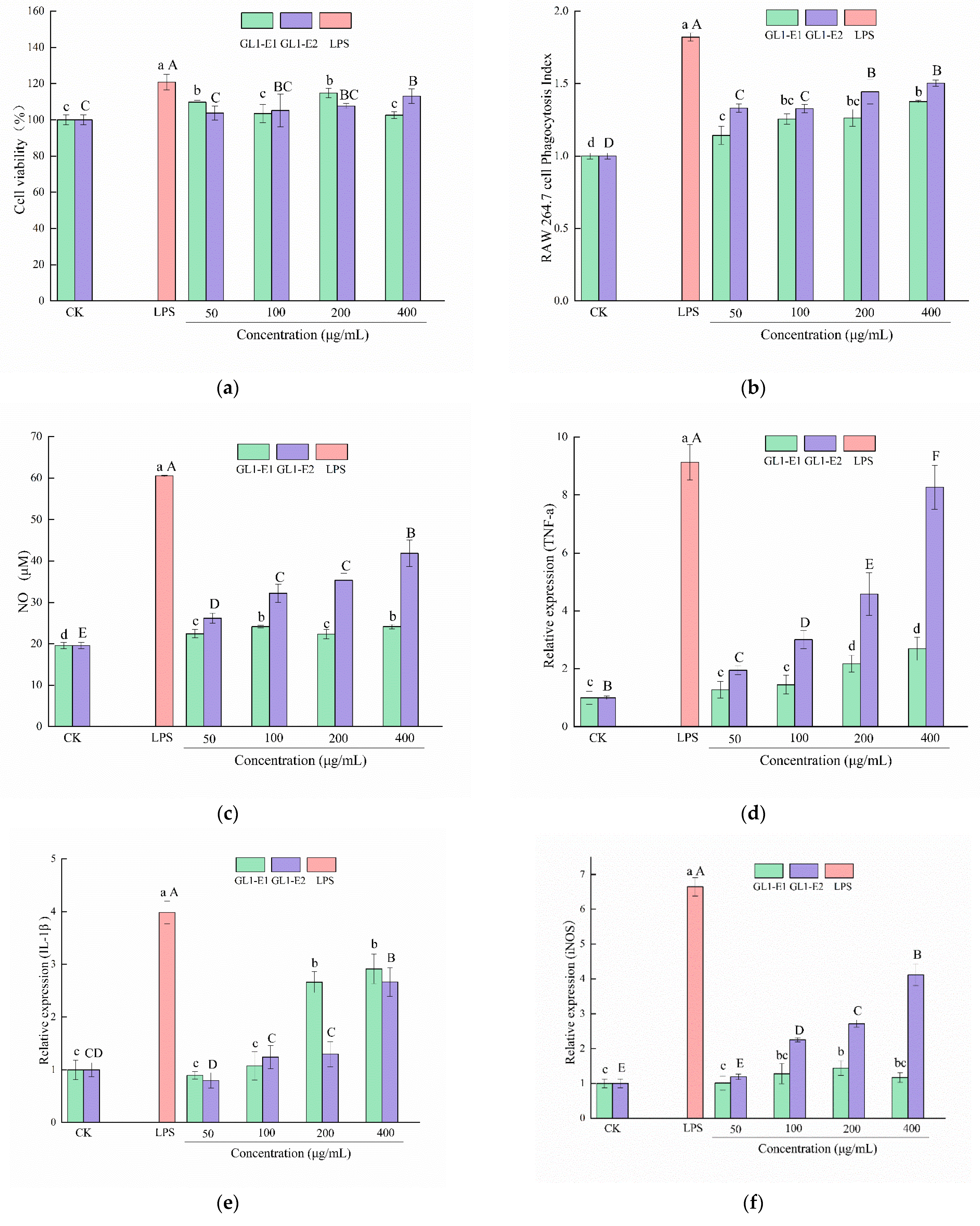
3.9.1. Cytotoxicity Assay of GL1-E1 and GL1-E2 on RAW264.7 Cells
3.9.2. Effect of GL1-E1 and GL1-E2 on the Phagocytic Capacity of RAW264.7 Cells
3.9.3. Effect of GL1-E1 and GL1-E2 on the NO Production of RAW264.7 Cells
3.9.4. Effects of GL1-E1 and GL1-E2 on Cytokines Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir frontiers in microbiology. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L.W. Comparative analysis of the microbial community composition between Tibetan kefir grains and milks. Food Res. Int. 2018, 116, 137–144. [Google Scholar] [CrossRef]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biot. 2002, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Gimzewski, J. Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int. J. Oncol. 2014, 44, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Golowczyc, M.A.; De Antoni, G.L.; Perez, P.F.; Humen, M.; Serradell, M.D. Administration of kefir-fermented milk protects mice against Giardia intestinalis infection. J. Med. Microbiol. 2013, 62, 1815–1822. [Google Scholar] [CrossRef][Green Version]
- Golnar, R.; Shahnaz, S.D.; Fatemeh, F.R. Comparison of two types of gels in improving burn wound. J. Med. Biol. Sci. 2014, 1, 28–32. [Google Scholar]
- Tsai, C.C.; Ke, P.C.; Hsu, T.K.; Hsieh, Y.M. Oral administration of multiple lactic acid bacteria strains suppressed allergic responses IgE in an ovalbumin-induced allergy BALB/c mouse model. Afr. J. Microbiol. Res. 2012, 6, 1206–1212. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New perspectives for Lactobacilli exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohyd. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Wu, M.H.; Pan, T.M.; Wu, Y.J.; Chang, S.J.; Chang, M.S.; Hu, C.Y. Exopolysaccharide activities from probiotic bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 1104–1110. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.H.; Kang, I.B.; Kim, H.; Song, K.Y.; Kim, H.S.; Seo, K.H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control. 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; He, C.C.; Yang, Y.W.; Yang, Z.N. Isolation and characterization of exopolysaccharide-producing Lactobacillus plantarum SKT109 from Tibet kefir. Pol. J. Food Nutr. Sci. 2015, 65, 269–279. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Zhao, L.Q. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Peluso, I.; Fina, D.; Caruso, R.; Stolfi, C.; Caprioli, F.; Fantini, M.C.; Caspani, G.; Grossi, E.; Di Iorio, L.; Paone, F.M.; et al. Lactobacillus paracasei subsp paracasei B21060 suppresses human T-cell proliferation. Infect. Immun. 2007, 75, 1730–1735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ocana, V.S.; Holgado, A.A.P.D.; Nader-Macias, M.E. Growth inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus paracasei subsp. paracasei isolated from the human vagina. Fems. Immunol. Med. Mic. 1999, 23, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, X.F.; Jin, H.N.; Man, C.X.; Jiang, Y.J. The effect of optimized carbon source on the synthesis and composition of exopolysaccharides produced by Lactobacillus paracasei. J. Dairy Sci. 2021, 104, 4023–4032. [Google Scholar] [CrossRef]
- Xu, R.H.; Ma, S.M.; Wang, Y.; Liu, L.S.; Li, P.L. Screening, identification and statistic optimization of a novel exopolysaccharide producing Lactobacillus paracasei HCT. Afr. J. Microbiol. Res. 2010, 4, 783–795. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Dardis, C.; Gagliarini, N.; Garrote, G.L.; Abraham, A.G. Exopolysaccharides from Lactobacillus paracasei isolated from kefir as potential bioactive compounds for microbiota modulation. Front. Microbiol. 2020, 11, 583254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.Y.; Xu, D.L.; Tang, N.Y.; Rui, X.; Zhang, Q.Q.; Chen, X.H.; Dong, M.S.; Li, W. Biosynthesis of exopolysaccharide and structural characterization by Lacticaseibacillus paracasei ZY-1 isolated from Tibetan kefir. Food Chem. Mol. Sci. 2002, 3, 100054. [Google Scholar] [CrossRef]
- Wang, X.M.; Li, W.P.; Xu, M.J.; Tian, J.J.; Li, W. The microbial diversity and biofilm-forming characteristic of two traditional Tibetan kefir grains. Foods 2022, 11, 12. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Ohashi, Y.; Amamiya, M.; Moriya, N.; Ohmori, H.; Sekiyama, Y. Fermentation of onion (Allium cepa L.) peel by lactic acid bacteria for production of functional food. J. Food Meas. Charact. 2020, 14, 142–149. [Google Scholar] [CrossRef]
- Niamah, A.K. Ultrasound treatment (low frequency) effects on probiotic bacteria growth in fermented milk. Future Food 2019, 7, 103. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.H.; Jiang, M.; Rui, X.; Dong, M.S. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohyd. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.J.; Wang, X.M.; Zhang, X.L.; Zhang, C.P.; Chen, X.H.; Dong, M.S.; Rui, X.; Zhang, Q.Q.; Fang, Y.; Li, W. Isolation, structural characterization and neuroprotective activity of exopolysaccharide from Paecilomyces cicada TJJ1213. Int. J. Biol. Macromol. 2021, 183, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, C.; Hayakawa, A.; Matsumoto, K.; Katoh, T.; Yamamoto, K.; Hisa, K. Exopolysaccharides produced by Leuconostoc mesenteroides strain NTM048 as an immunostimulant to enhance the mucosal barrier and influence the systemic immune response. J. Agric. Food Chem. 2015, 63, 7009–7015. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xiao, L.Y.; Tian, J.J.; Wang, X.M.; Zhang, X.L.; Fang, Y.; Li, W. Structural characterization, rheological properties and protection of oxidative damage of an exopolysaccharide from Leuconostoc citreum 1.2461 fermented in soybean whey. Foods 2022, 11, 2283. [Google Scholar] [CrossRef]
- Tian, J.J.; Zhang, C.P.; Wang, X.M.; Rui, X.; Zhang, Q.Q.; Chen, X.H.; Dong, M.S.; Li, W. Structural characterization and immunomodulatory activity of intracellular polysaccharide from the mycelium of Paecilomyces cicadae TJJ1213. Food Res. Int. 2021, 147, 110515. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.D.; Tang, W.Z.; Ji, J.; Rui, X.; Chen, X.H.; Jiang, M.; Zhou, J.Z.; Zhang, Q.Q.; Dong, M.S. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Wang, X.M.; Xu, M.J.; Xu, D.L.; Ma, K.; Zhang, C.L.; Wang, G.X.; Dong, M.S.; Li, W. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohyd. Polym. 2022, 296, 119971. [Google Scholar] [CrossRef]
- Suvakanta, D.; Narsimha, M.P.; Pulak, D.; Joshabir, C.; Biswajit, D. Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chem. 2014, 149, 76–83. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.K.; Song, P.Y.; Wang, X.; Shi, W.Y. Effects of salvia miltiorrhiza polysaccharides on lipopolysaccharide-induced inflammatory factor release in RAW264.7 cells. J. Interf. Cytok. Res. 2018, 1, 29–37. [Google Scholar] [CrossRef]
- Shen, T.; Wang, G.C.; You, L.; Zhang, L.; Ren, H.W.; Hu, W.C.; Qiang, Q.; Wang, X.F.; Jia, L.L.; Gu, Z.Z.; et al. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X.J. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohyd. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhang, Z.H.; Qiu, L.; Zhang, F.; Xu, X.P.; Wei, H.; Tao, X.Y. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy. Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Rui, X.; Chen, X.H.; Jiang, M.; Dong, M.S. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Wang, C.T.; Jia, S.S.; Wang, B.Y.; Zhou, K.; Chen, S.J.; Yang, Y.; Liu, S.L. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from Sichuan paocai. Int. J. Biol. Macromol. 2018, 115, 820–828. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.H.; Han, T.; Zhang, H.X. Purification and structure study on exopolysaccharides produced by Lactobacillus paracasei KL1-Liu from Tibetan kefir. Adv. Mater. Res. 2013, 781–784, 1513–1518. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.L.; Zhu, Y.T.; Yang, Y.; Wang, C.T. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Lamosa, P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef]
- Wu, J.N.; Chen, X.T.; Qiao, K.; Su, Y.C.; Liu, Z.Y. Purification, structural elucidation, and in vitro antitumor effects of novel polysaccharides from Bangia fuscopurpurea. Food Sci. Hum. Well. 2021, 10, 63–71. [Google Scholar] [CrossRef]
- Li, X.W.; Lv, S.; Shi, T.T.; Liu, K.; Li, Q.M.; Pan, L.H.; Zha, X.Q.; Luo, J.P. Exopolysaccharides from yoghurt fermented by Lactobacillus paracasei: Production, purification and its binding to sodium caseinate. Food Hydrocolloid. 2020, 102, 105635. [Google Scholar] [CrossRef]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confuse. Carbohyd. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Z.; Ma, K.; Zhang, C.L.; Chen, X.H.; Wang, G.X.; Yang, L.; Dong, M.S.; Rui, X.; Zhang, Q.Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohyd. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.B.; Wu, T.; Min, W.H.; Yang, Z.N. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Xiu, L.; Sheng, S.X.; Hu, Z.P.; Liu, Y.; Li, J.W.; Zhang, H.C.; Liang, Y.C.; Du, R.P.; Wang, X. Exopolysaccharides from Lactobacillus kefiri as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection. Int. J. Biol. Macromol. 2020, 161, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tang, X.F.; Shuai, X.X.; Jiang, C.J.; Liu, X.; Wang, L.F.; Yao, Y.F.; Nie, S.P.; Xie, M.Y. Mannose receptor mediates the immune response to ganoderma atrum polysaccharides in macrophages. J. Agric. Food Chem. 2017, 65, 348–357. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.J.; Wang, G.Q.; Zhang, H.; Zhu, S.; Ai, L.Z. Bioactive exopolysaccharides from a S-thermophilus strain: Screening, purification and characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.N.J.; Nowak, D.; Schumacher, A.; Plenagl, S.; Langwisch, S.; Tiegs, G.; Reinhold, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Kitazawa, H.; Barba, F.J.; Berthelot, L.; Umair, M.; Zhu, Q.C.; He, Z.D.; Zhao, L.Q. Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk. Food Chem. 2022, 377, 131954. [Google Scholar] [CrossRef]
- Kang, H.; Choi, H.S.; Kim, J.E.; Han, N.S. Exopolysaccharide-Overproducing Lactobacillus paracasei KB28 Induces cytokines in mouse peritoneal macrophages via modulation of NF-kappa B and MAPKs. Appl. Microbiol. Biotechnol. 2011, 21, 1174–1178. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Lopez, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G.; Suarez, A.; Margolles, A.; Ruas-Madiedo, P. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and Bifidobacteria. Probiotics Antimicro. 2012, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Bleau, C.; Monges, A.; Rashidan, K.; Laverdure, J.-P.; Lacroix, M.; Van, M.-R.; Calsteren, M.; Millette, R.; Lamontagne, L. Intermediate chains of exopolysaccharides from Lactobacillus rhamnosus RW-9595M increase IL-10 production by macrophages. J. Appl. Microbiol. 2010, 108, 666–675. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wang, X.J.; Pan, W.S.; Shen, X.F.; He, Y.J.; Yin, H.L.; Zhou, K.; Zou, L.K.; Chen, S.J.; Liu, S.L. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2018, 121, 342–349. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Bittencourt, V.C.B.; Lopes, L.C.L.; Sassaki, G.; Barreto-Bergter, E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohyd. Res. 2012, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohyd. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
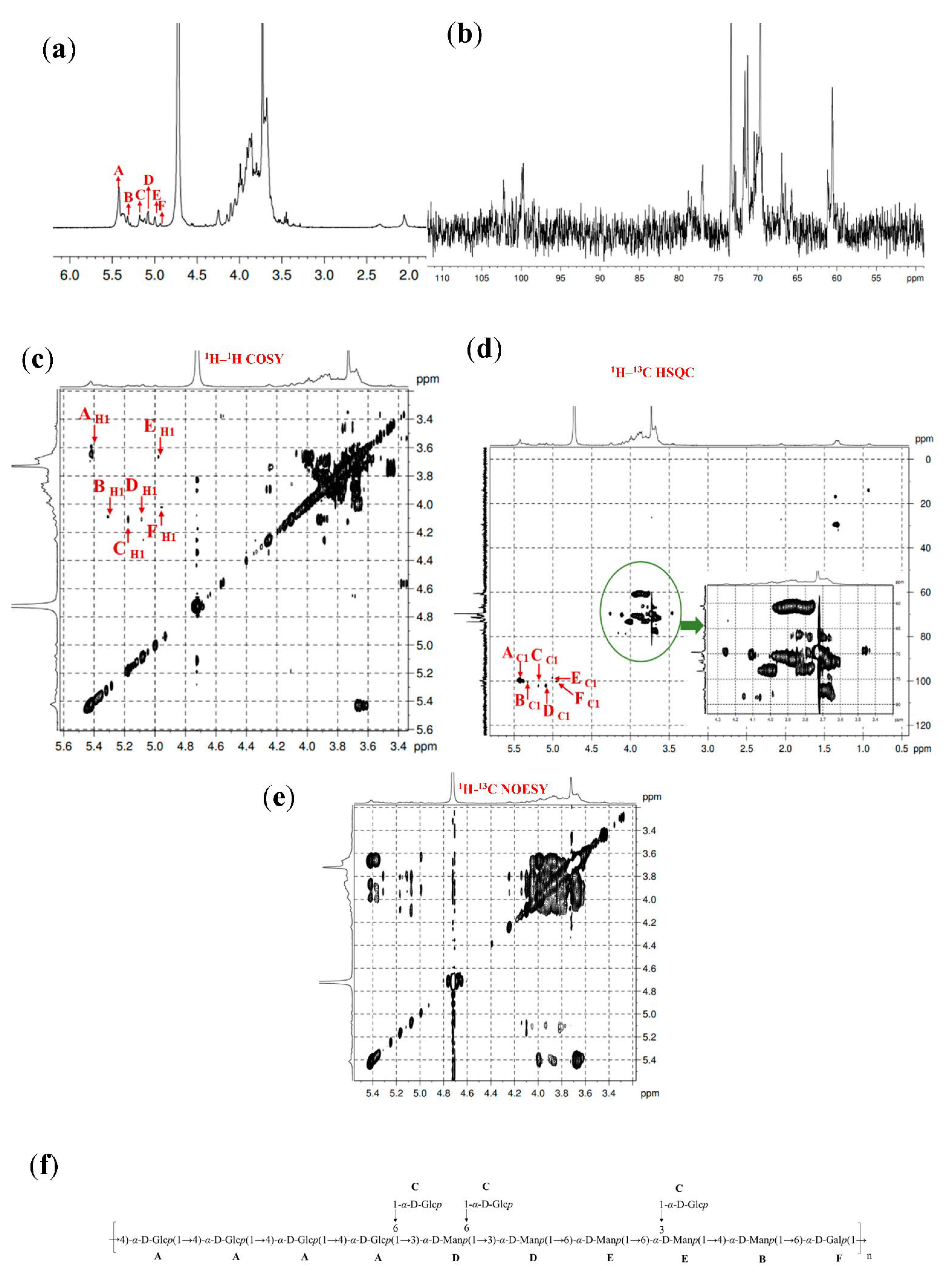
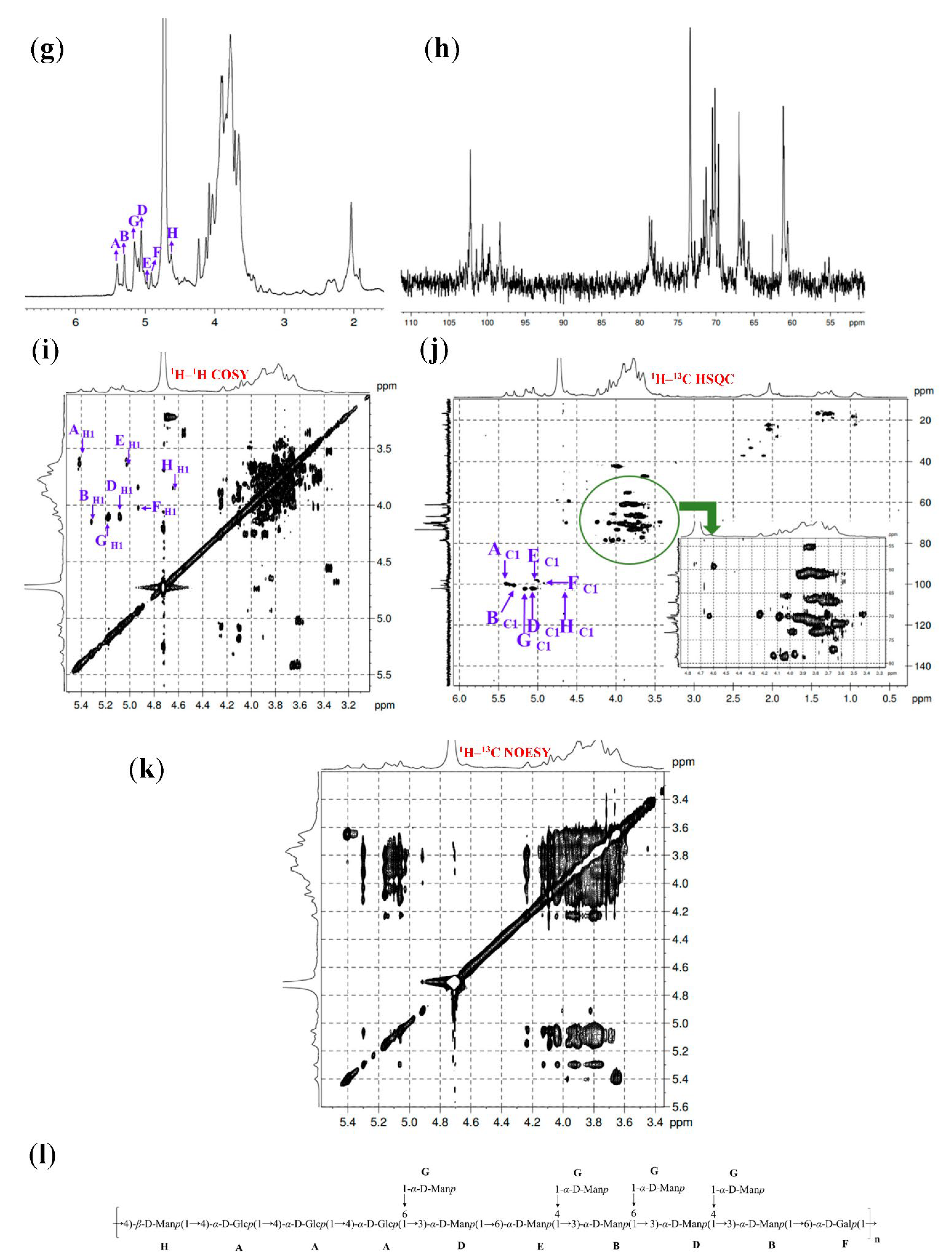
| Fractions | Methylation Sugar | Glycosidic Linkages | Mass Fractions (m/z) | Relative Molar Ratios |
|---|---|---|---|---|
| GL1-E1 | 2,3,4,6-Me4-Glc | D-Glcp-(1→ | 71, 87, 102, 117, 129, 145, 162, 205 | 2.6 |
| 2,3,6-Me3-Glc | →4)-D-Glcp-(1→ | 71, 87, 99, 117, 129, 142, 159, 233 | 4.1 | |
| 2,3,4-Me3-Man | →6)-D-Manp-(1→ | 71, 87, 99, 118, 129, 159, 161, 189, 233 | 2.6 | |
| 2,3,4-Me3-Gal | →6)-D-Galp-(1→ | 59, 71, 87, 99, 117, 129, 159, 189 | 1.0 | |
| 2,6-Me2-Man | →3,4)-D-Manp-(1→ | 87, 117, 129, 160, 172, 185, 203 | 1.4 | |
| 2,4-Me2-Man | →3,6)-D-Manp-(1→ | 59, 87, 101, 117, 129, 189, 233 | 2.3 | |
| GL1-E2 | 2,3,4,6-Me4-Man | D-Manp-(1→ | 71, 87, 102, 117, 129, 145, 162, 205 | 4.4 |
| 2,3,6-Me3-Glc | →4)-D-Glcp-(1→ | 71, 87, 99, 117, 129, 142, 159, 233 | 3.2 | |
| 2,3,6-Me3-Man | →4)-D-Manp-(1→ | 71, 87, 102, 117, 129, 143, 162, 189, 233 | 1.2 | |
| 2,3,4-Me3-Man | →6)-D-Manp-(1→ | 71, 87, 99, 118, 129, 159, 161, 189, 233 | 1.0 | |
| 2,3,4-Me3-Gal | →6)-D-Galp-(1→ | 59, 71, 87, 99, 117, 129, 159, 189 | 1.0 | |
| 2,6-Me2-Man | →3,4)-D-Manp-(1→ | 87, 117, 129, 160, 172, 185, 203 | 2.2 | |
| 2,4-Me2-Man | →3,6)-D-Manp-(1→ | 59, 87, 101, 117, 129, 189, 233 | 2.1 |
| Fractions | Residues | Sugar Linkages | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|---|---|
| GL1-E1 | A | →4)-α-D-Glcp-(1→ | 5.41 | 3.64 | 3.72 | 3.91 | 3.69 | 3.81 |
| 100.01 | 73.44 | 73.76 | 78.23 | 73.27 | 60.73 | |||
| B | →3,4)-α-D-Manp-(1→ | 5.31 | 4.15 | 3.82 | 4.01 | 3.65 | 3.59 | |
| 100.59 | 69.69 | 79.18 | 78.23 | 72.93 | 60.90 | |||
| C | T-α-D-Glcp-(1→ | 5.17 | 4.10 | 3.91 | 3.78 | 3.48 | 4.00 | |
| 102.39 | 68.84 | 73.39 | 71.00 | 71.49 | 60.69 | |||
| D | →3,6)-α-D-Manp-(1→ | 5.08 | 4.10 | 3.88 | 3.69 | 3.76 | 3.72 | |
| 102.35 | 68.82 | 78.33 | 73.76 | 71.72 | 70.32 | |||
| E | →6)-α-D-Manp-(1→ | 5.00 | 3.62 | 3.98 | 3.80 | 3.89 | 3.66 | |
| 98.52 | 71.18 | 73.42 | 73.49 | 71.11 | 69.87 | |||
| F | →6)-α-D-Galp-(1→ | 4.93 | 4.02 | 3.85 | 3.91 | 3.68 | 3.81 | |
| 100.37 | 70.37 | 70.96 | 70.22 | 70.68 | 70.54 | |||
| GL1-E2 | A | →4)-α-D-Glcp-(1→ | 5.41 | 3.64 | 3.72 | 3.91 | 3.69 | 3.81 |
| 100.01 | 73.44 | 73.76 | 78.23 | 73.27 | 60.73 | |||
| B | →3,4)-α-D-Manp-(1→ | 5.31 | 4.15 | 3.82 | 4.01 | 3.65 | 3.59 | |
| 100.59 | 69.69 | 79.18 | 78.23 | 70.93 | 60.90 | |||
| G | T-α-D-Manp-(1→ | 5.16 | 4.11 | 3.94 | 3.67 | 3.79 | 3.72 | |
| 102.42 | 68.55 | 73.66 | 73.22 | 71.49 | 61.15 | |||
| D | →3,6)-α-D-Manp-(1→ | 5.08 | 4.10 | 3.88 | 3.69 | 3.76 | 3.72 | |
| 102.35 | 68.82 | 78.33 | 73.76 | 71.72 | 70.32 | |||
| E | →6)-α-D-Manp-(1→ | 5.00 | 3.62 | 3.98 | 3.80 | 3.89 | 3.66 | |
| 98.52 | 71.18 | 73.42 | 73.49 | 71.11 | 69.87 | |||
| F | →6)-α-D-Galp-(1→ | 4.93 | 4.02 | 3.85 | 3.91 | 3.68 | 3.81 | |
| 100.37 | 70.37 | 70.96 | 70.22 | 70.68 | 70.54 | |||
| H | →4)-β-D-Manp-(1→ | 4.64 | 3.85 | 3.69 | 3.92 | 3.77 | 3.61 | |
| 102.65 | 70.99 | 73.66 | 78.22 | 71.38 | 61.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tian, J.; Zhang, X.; Tang, N.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods 2022, 11, 3330. https://doi.org/10.3390/foods11213330
Wang X, Tian J, Zhang X, Tang N, Rui X, Zhang Q, Dong M, Li W. Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods. 2022; 11(21):3330. https://doi.org/10.3390/foods11213330
Chicago/Turabian StyleWang, Xiaomeng, Juanjuan Tian, Xueliang Zhang, Nanyu Tang, Xin Rui, Qiuqin Zhang, Mingsheng Dong, and Wei Li. 2022. "Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains" Foods 11, no. 21: 3330. https://doi.org/10.3390/foods11213330
APA StyleWang, X., Tian, J., Zhang, X., Tang, N., Rui, X., Zhang, Q., Dong, M., & Li, W. (2022). Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods, 11(21), 3330. https://doi.org/10.3390/foods11213330