Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cherries
2.2. Yeast
2.3. Experimental Design
2.3.1. Vinification Process
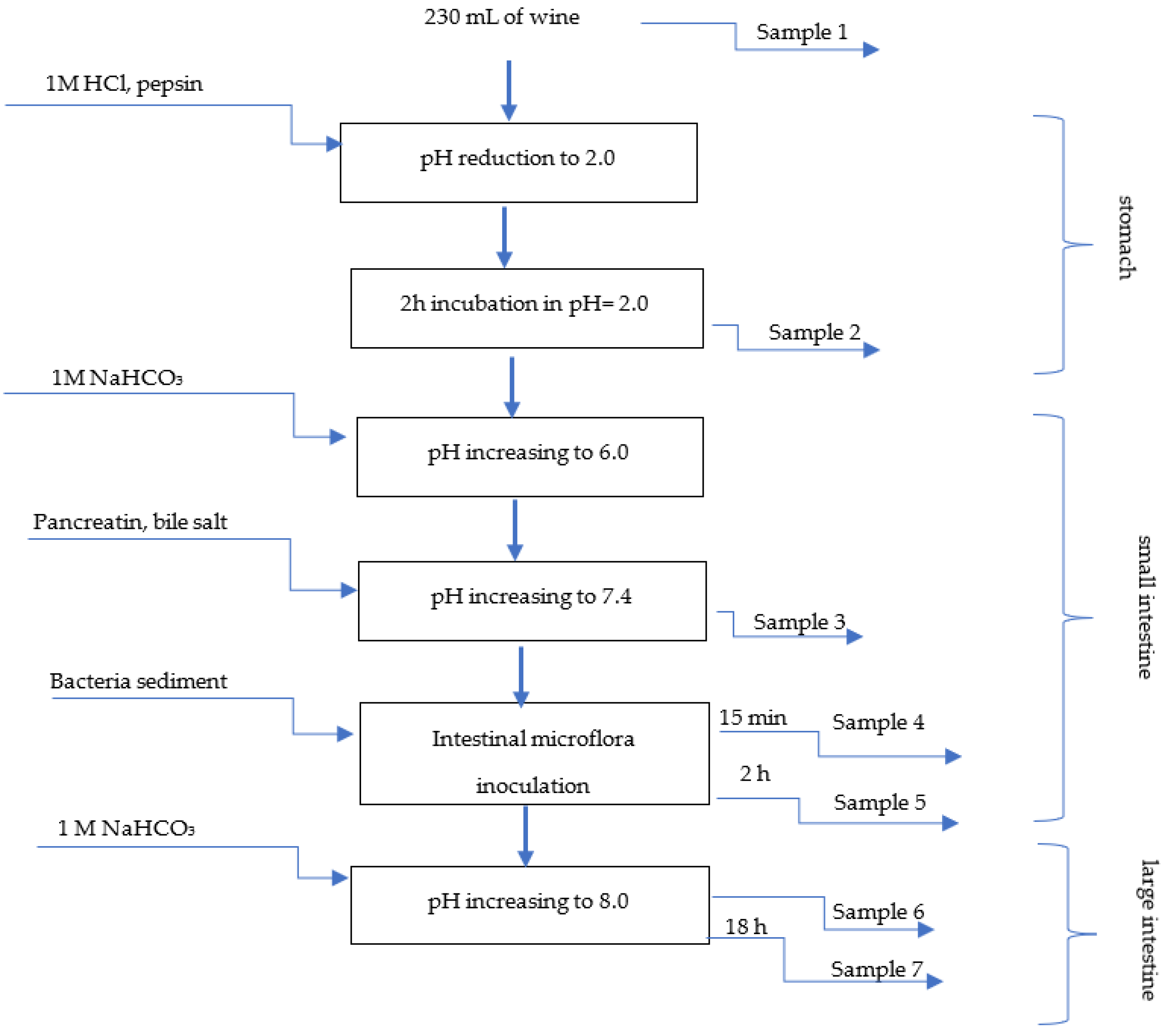
2.3.2. In Vitro Gastrointestinal Digestion Process
2.3.3. Determination of the Number of Bacteria
2.4. Analytical Methods
2.4.1. Standard Enological Parameters
2.4.2. The Total Polyphenols Content
2.4.3. The Antioxidative Activity
2.4.4. Alpha Glucosidase Inhibition Assay
2.5. Statistical Analysis
3. Results and Discussion
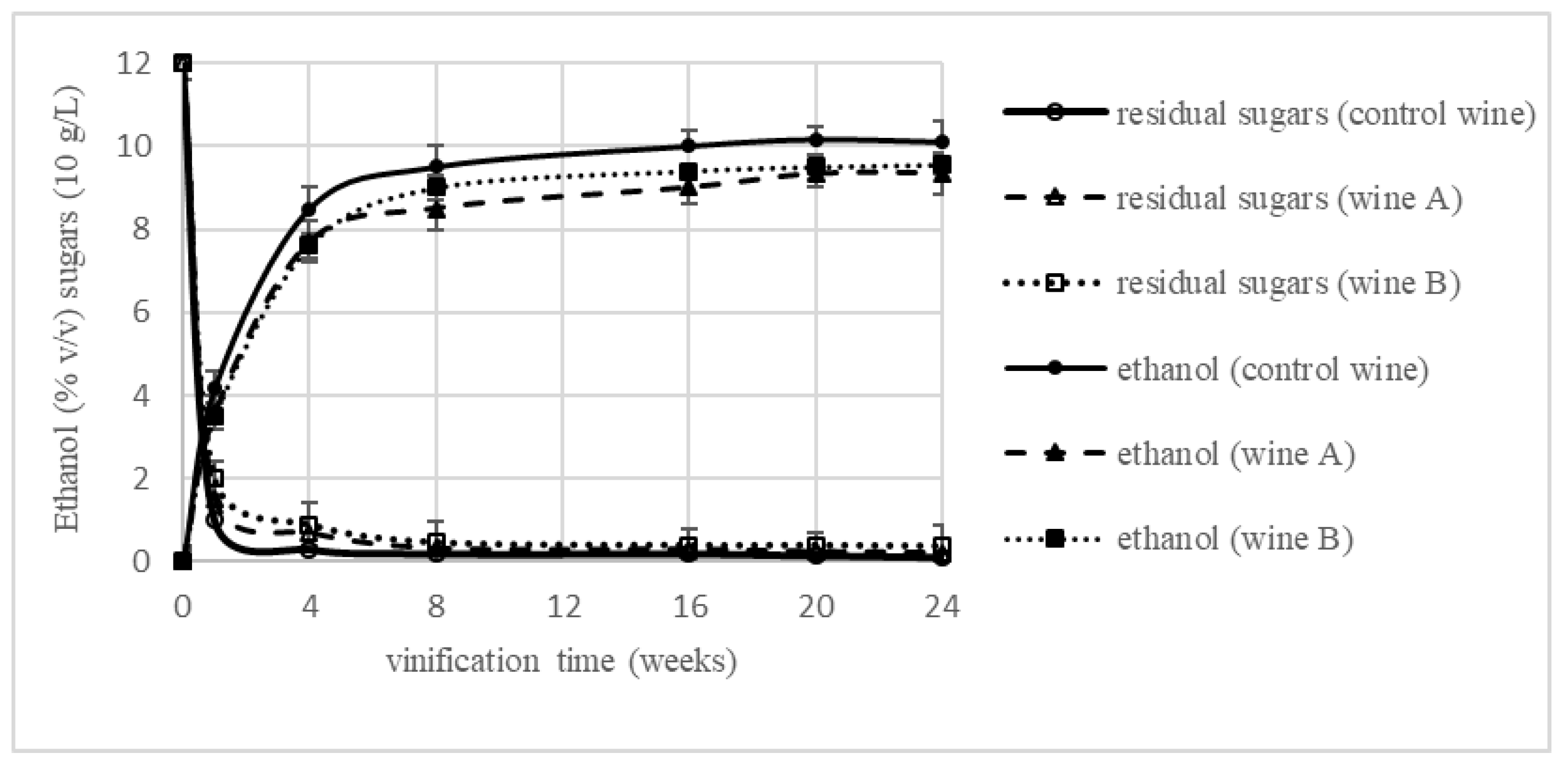
3.1. Changes of Vinification Parameters during Winemaking
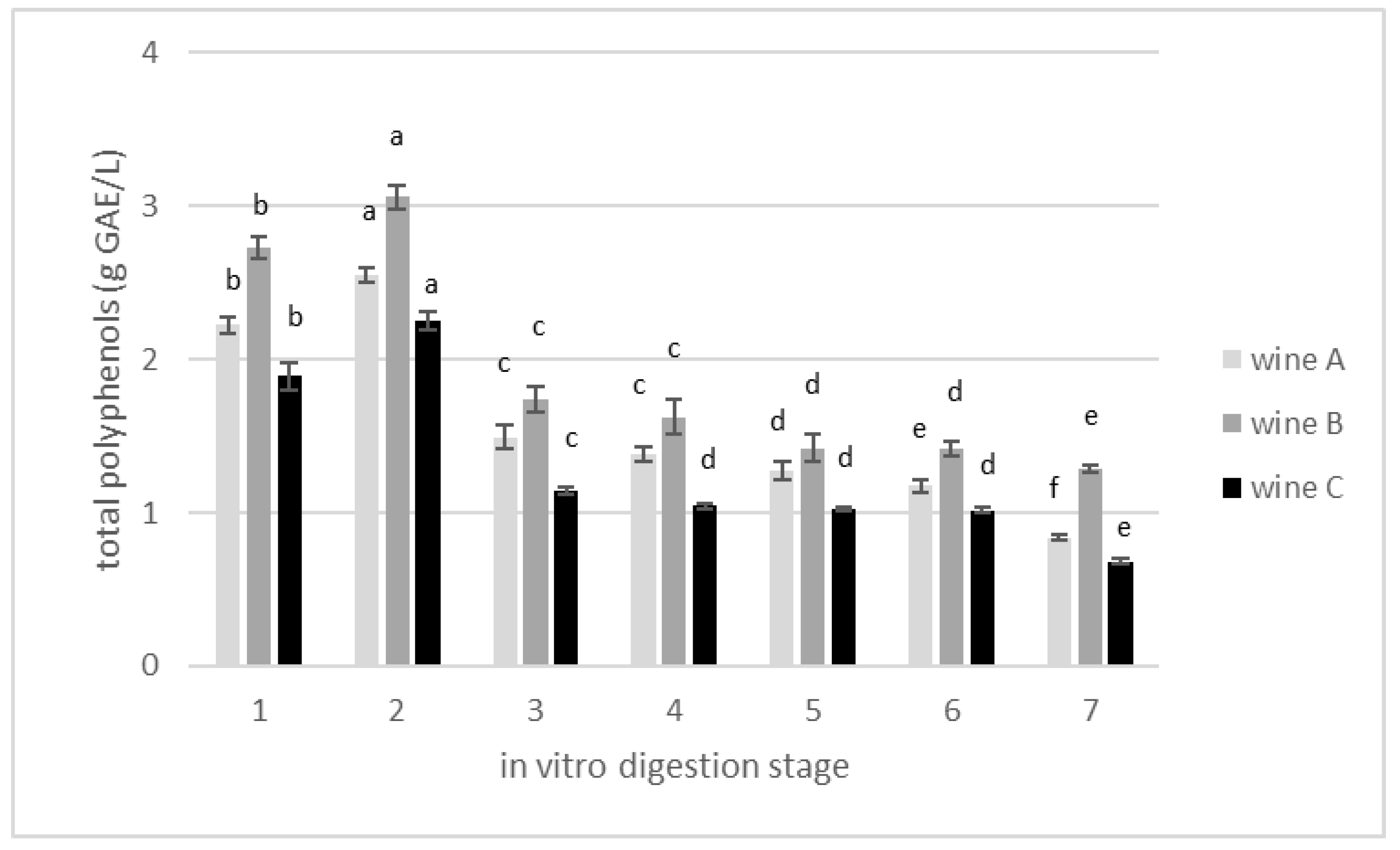
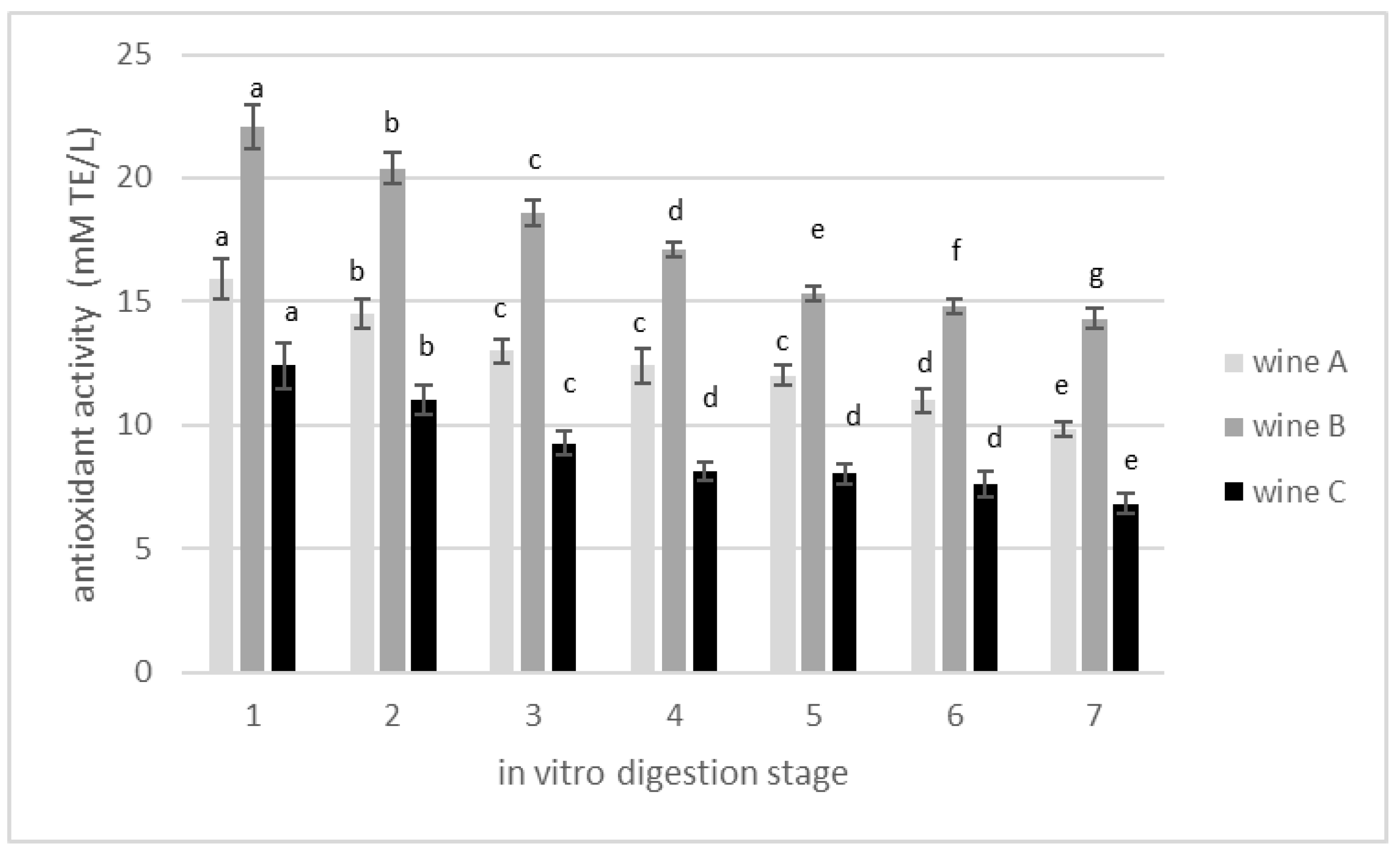
3.2. Changes in Total Polyphenolics Content during In Vitro Gastrointestinal Digestion
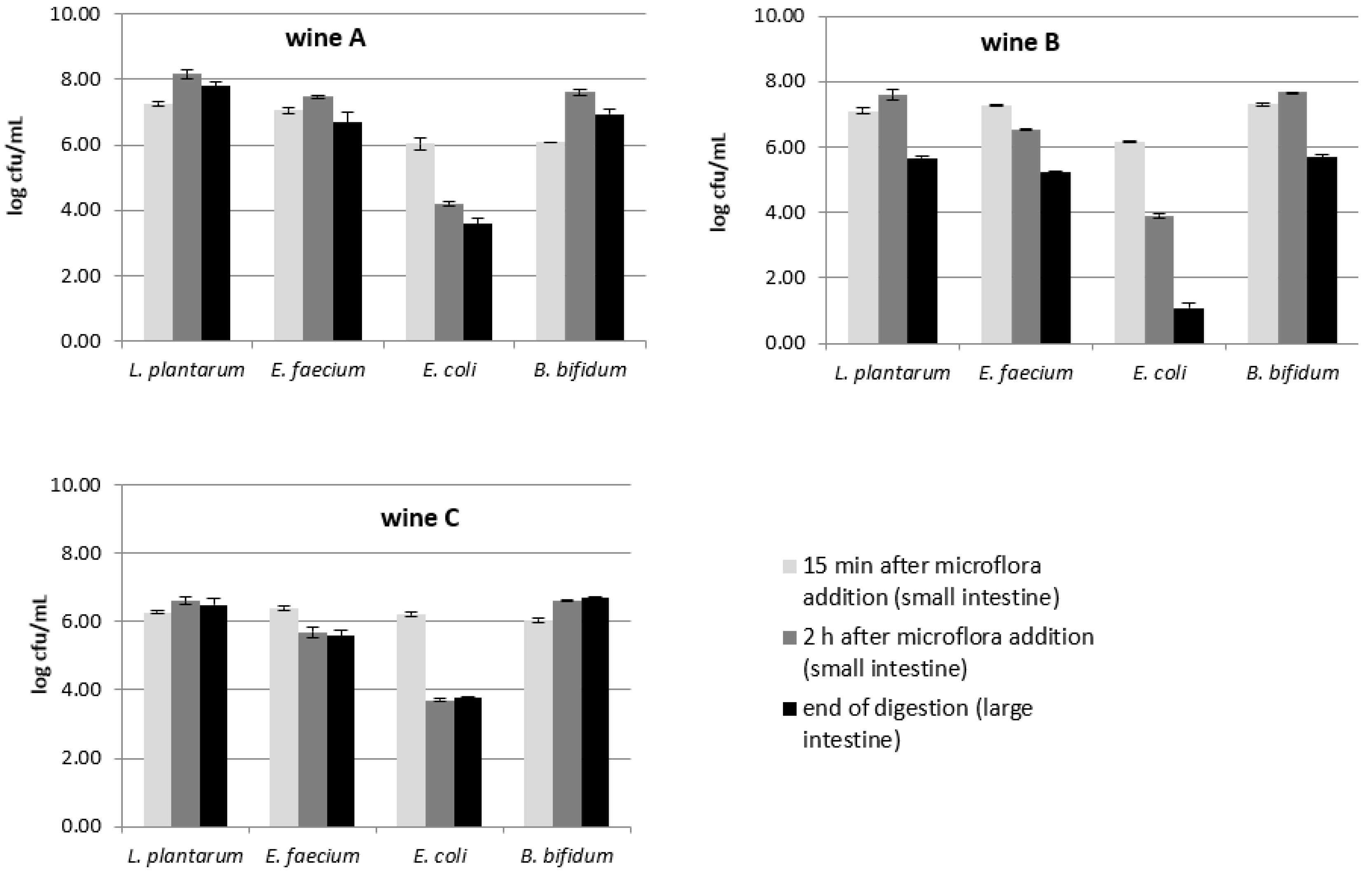
3.3. Changes in Intestinal Microflora during In Vitro Digestion
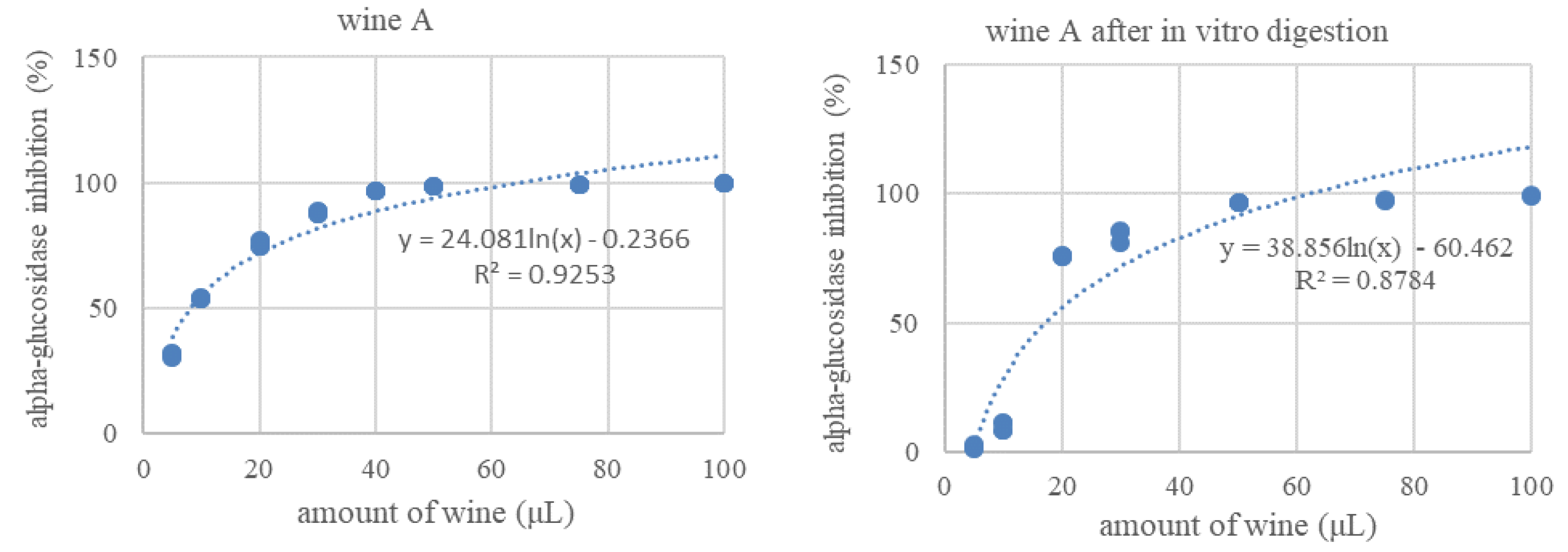
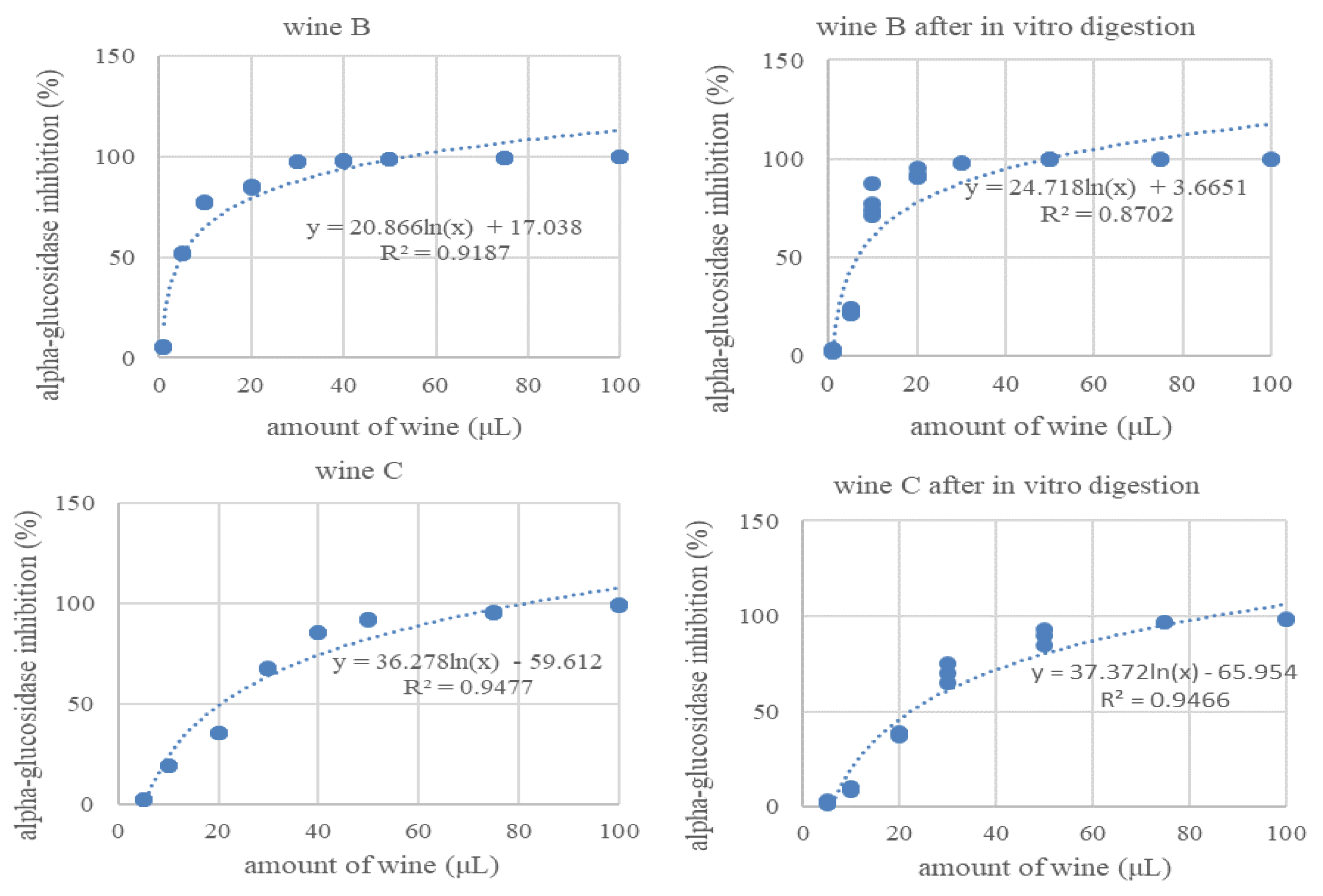
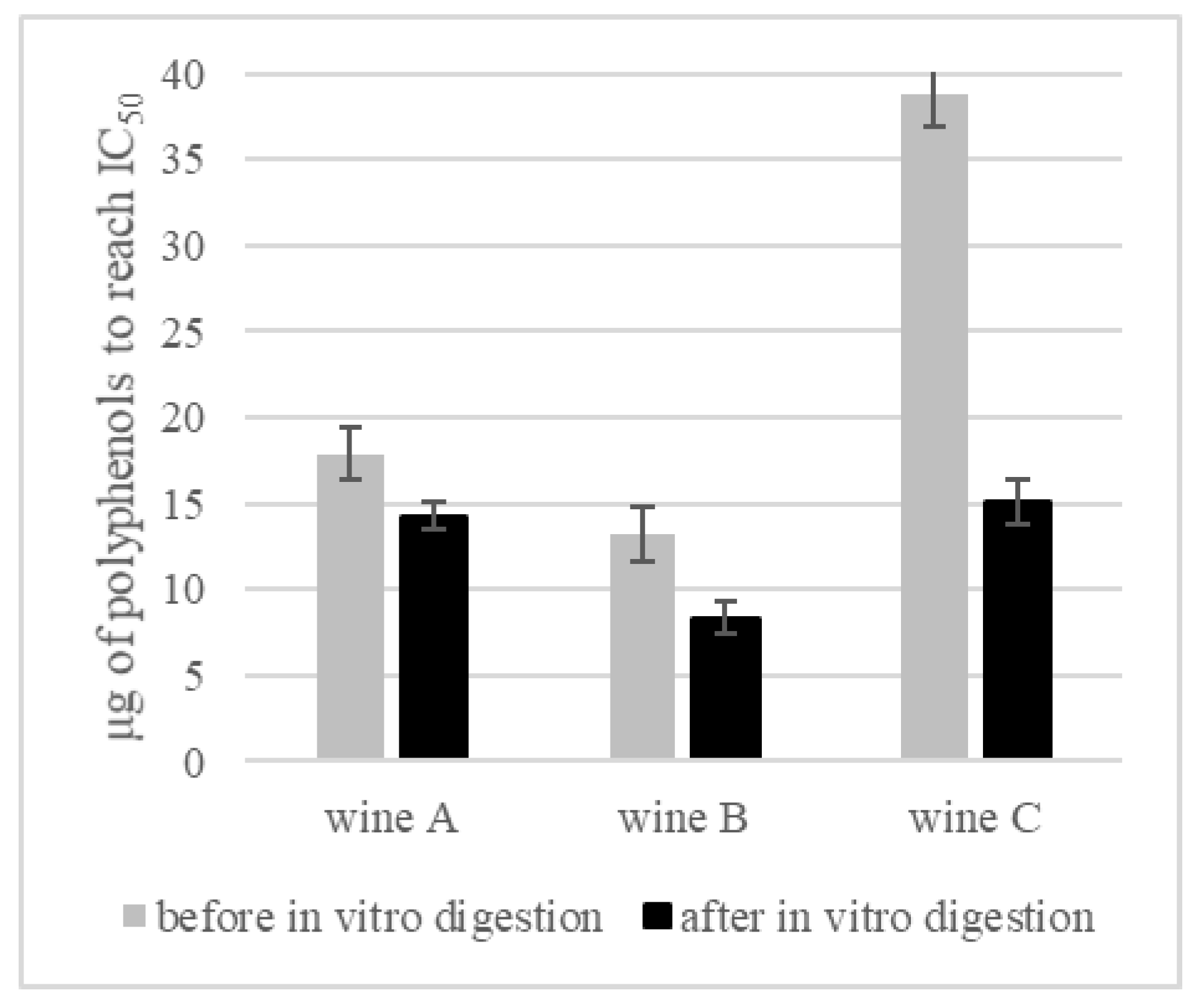
3.4. Inhibition of Alpha-Glucosidase Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Wichienchot, S.; Chakkaravarthi, S. Polyphenols from food processing byproducts and their microbiota–gut–brain axis-based health benefits. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 855–880. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Dai, J.; Wang, L.; Xu, Y.; Peng, X.; Xie, X.; Peng, C. Molecular mechanisms and applications of tea polyphenols: A narrative review. J. Food Biochem. 2021, 45, e13910. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Zhang, J.; Xu, D.; Li, Y.; Liu, Y.; Zhang, X.; Zhang, R.; Wu, Z.; Weng, P. Antimicrobial Effect of Tea Polyphenols against Foodborne Pathogens: A Review. J. Food Prot. 2021, 84, 1801–1808. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wan, X.; Zhan, J.; Ho, C.T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Butt, M.S.; Ahmad, R.S.; Sultan, M.T.; Qayyum, M.M.; Naz, A. Green tea and anticancer perspectives: Updates from last decade. Crit. Rev. Food Sci. Nutr. 2015, 55, 792–805. [Google Scholar] [CrossRef]
- Pisani, M.; Astolfi, P.; Sabbatini, S.; Carloni, P. Antioxidant Activity Level, Bioactive Compounds, Colour and Spectroscopic Analysis (UV-Vis and FT-IR) of Flavoured Drinks Made with Wine and Sour Cherries (Prunuscerasus Var. austera). Foods 2021, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Fang, L.; Niu, Y.; Yu, H. Effect of cultivar and variety on phenolic compounds and antioxidant activity of cherry wine. Food Chem. 2015, 186, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Pantelic, M.; Dabic, D.; Matijasevic, S.; Davidovic, S.; Dojcinovic, B.; Milojkovic-Opsenica, D.; Tesic, Z.; Natic, M. Chemical Characterization of Fruit Wine Made from Oblacinska Sour Cherry. Sci. World J. 2014, 2014, 454797. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds and polyphenols in cherry wines. Food Chem. 2011, 127, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Flores-Félix, J.D.; Costa, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Hepatoprotective Effects of Sweet Cherry Extracts (cv. Saco). Foods 2021, 10, 2623. [Google Scholar] [CrossRef]
- Silva, V.; Pereira, S.; Vilela, A.; Bacelar, E.; Guedes, F.; Ribeiro, C.; Silva, A.P.; Gonçalves, B. Preliminary Insights in Sensory Profile of Sweet Cherries. Foods 2021, 10, 612. [Google Scholar] [CrossRef]
- Cakar, U.; Petrovic, A.; Pejin, B.; Cakar, M.; Zivkovic, M.; Vajs, V.; Dordevic, D. Fruit as a substrate for a wine: A case study of selected berry and drupe fruit wines. Sci. Hortic. 2019, 244, 42–49. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Jia, C.; Yu, H.; Fang, L.; Xu, C. Characterization of taste-active compounds of various cherry wines and their correlation with sensory attributes. J. Chromatogr. B 2012, 902, 55–60. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 28119. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Harkonen, H.; Fabritius, M.; Poutanen, K. Development of in vitro enzymatic digestion method for removal of starch and protein and assessment of its performance using rye and wheat breads. J. Cereal Sci. 1999, 29, 139–152. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Terreres, F.; Barberan, T. In vitro availibility of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int. J. Food Sci. Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef]
- Knarreborg, A.; Simon, M.A.; Engberg, R.M.; Jensen, B.B.; Tannock, G.W. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 2002, 68, 5918–5924. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis of Wine and Musts; International Organisation of Vine and Wine: Paris, France, 2009. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Czapska-Pietrzak, I.; Studzinska-Sroka, E.; Bylka, W. Evaluation of antidiabetic activity of extract obtained from selected plant materials. Postep. Fitoter. 2019, 20, 167–174. (In Polish) [Google Scholar]
- Johnson, M.H.; Lucius, A.; Meyer, T.; Gonzales de Mejia, E. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and β-glucosidase by highbush blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pérez, M.D.; Muñiz, P.; González-Sanjosé, M.L. Contribution of anthocyanin fraction to the antioxidant properties of wine. Food Chem. Toxicol. 2008, 46, 2815–2822. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]
- Czyzowska, A.; Pogorzelski, E. Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part I. Total pylyphenols and phenolic acids. Eur. Food Res. Technol. 2002, 214, 148–154. [Google Scholar] [CrossRef]
- Fernandez-Pachón, M.S.; Villano, D.; Garcia-Parrilla, M.C.; Troncoso, A.M. Antioxidant Activity of Wines and Relation with Their Polyphenolic Composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G.H. Degradation of malic and tartaric acids by high density cell suspensions of wine yeasts. Food Microbiol. 1995, 12, 65–71. [Google Scholar] [CrossRef]
- Radler, F. Yeasts-metabolism of organic acids. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 165–182. [Google Scholar]
- Tamargo, A.; Cueva, C.; Silva, M.; Molinero, N.; Miralles, B.; Bartolome, B.; Moreno-Arribas, M.V. Gastrointestinal co-digestion of wine polyphenols with glucose/whey proteins affects their bioaccessibility and impact on colonic microbiota. Food Res. Int. 2022, 155, 111010. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of simulated digestion on the phenolic components of red grapes and their corresponding wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Yoo, K.M.; Al-Farsi, M.; Lee, H.; Yoon, H.; Lee, C.Y. Antiproliferative effects of cherry juice and wine in Chinese hamster lung fibroblast cells and their phenolic constituents and antioxidant activities. Food Chem. 2010, 123, 734–740. [Google Scholar] [CrossRef]
- Celepa, E.; Charehsaz, M.; Akyüz, S.; Türköz Acar, E.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. Impacts of processing conditions on digestive recovery of polyphenolic compounds and stability of the antioxidant activity of green tea infusion during in vitro gastrointestinal digestion. LWT 2018, 89, 648–656. [Google Scholar] [CrossRef]
- Record, I.R.; Lane, J.M. Simulated intestinal digestion of green and black teas. Food Chem. 2001, 73, 481–486. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine—Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E. Phenolic aids—Their properties, occurrence in plant material, absorption and metabolism. Bromatol. Chem. Toksykol. 2006, 39, 103–110. (In Polish) [Google Scholar]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- Barroso, E.; Van De Wiele, T.; Jiménez-Girón, A.; Muñoz-González, I.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Lactobacillus plantarum IFPL935 impacts colonic metabolism in a simulator of the human gut microbiota during feeding with red wine polyphenols. Appl. Microbiol. Biotechnol. 2014, 98, 6805–6815. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simoes, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-Amylase and Glucoamylase by Tannins Extracted from Cocoa, Pomegranates, Cranberries, and Grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C.; Ferranti, P.; Scarafoni, A.; Iametti, S. Grape skin phenolics as inhibitors of mammalian α-glucosidase and α-amylase—effect of food matrix and processing on efficacy. Food Funct. 2016, 7, 1655–1663. [Google Scholar] [CrossRef]
- Oliveira, A.; Pintado, M. Stability of polyphenols and carotenoids in strawberry and peach yoghurt throughout in vitro gastrointestinal digestion. Food Funct. 2015, 6, 3444–3453. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jeong, Y.K.; Wang, M.H.; Lee, W.Y.; Rhee, H.I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.C.; Sun, S.; Zhu, W.; Zhou, K. α-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Foods 2016, 26, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Shin, J.G.; Jang, H.D. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009, 117, 35–41. [Google Scholar] [CrossRef]
- Chen, S.; Yong, T.; Xiao, C.; Su, J.; Zhang, Y.; Jiao, C.; Xie, Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods 2018, 43, 196–205. [Google Scholar] [CrossRef]

| Ingredient | Variant of Wine | ||
|---|---|---|---|
| Wine A (“with Green Tea Infusion 7 g/L”) | Wine B (“with Concentrated Green Tea Infusion 21 g/L”) | Wine C (“Control Wine”) | |
| Cherries | 12 ± 0.1 kg | 12 ± 0.1 kg | 12 ± 0.1 kg |
| Sugar (sucrose) | 1.4 ± 0.01 kg (beginning) 1.4 ± 0.01 kg (after 1 week) | 1.4 ± 0.01 kg (beginning) 1.4 ± 0.01 kg (after 1 week) | 1.4 ± 0.01 kg (beginning) 1.4 ± 0.01 kg (after 1 week) |
| Water | not applied | not applied | 10 ± 0.1 L |
| Green tea infusion | 10 ± 0.1 L * | 10 ± 0.1 L ** | not applied |
| K2S2O5 | 50 ± 0.01 mg/L | 50 ± 0.01 mg/L | 50 ± 0.01 mg/L |
| nutrition | 0.25 ± 0.01 g/L | 0.25 ± 0.01 g/L | 0.25 ± 0.01 g/L |
| Parameters | Wine A | Wine B | Wine C | |||
|---|---|---|---|---|---|---|
| Must | Wine | Must | Wine | Must | Wine | |
| Ethanol (% v/v) | n.d. | 10.97 ± 0.66 a | n.d. | 10.54 ± 0.71 a | n.d. | 11.25 ± 0.83 a |
| Reducing sugars (g/L) | 121.64 ± 3.34 a | 6.93 ± 0.33 c | 123.48 ± 2.96 a | 5.86 ± 0.54 b | 121.26 ± 1.68 a | 1.31 ± 0.29 a |
| pH | 3.69 ± 0.06 a | 3.77 ± 0.08 a | 3.71 ± 0.08 a | 3.79 ± 0.09 a | 3.64 ± 0.04 a | 3.74 ± 0.09 a |
| Titratable acidity (g/L) * | 8.24 ± 0.11 b | 7.79 ± 0.08 b | 8.39 ± 0.11 b | 7.95 ± 0.11 b | 8.09 ± 0.12 a | 7.68 ± 0.07 a |
| Volatile acidity (g/L) ** | n.d. | 0.26 ± 0.01 b | n.d. | 0.17 ± 0.01 a | n.d. | 0.31 ± 0.02 c |
| Total polyphenols (g GEA/L) *** | 2.97 ± 0.08 b | 2.22 ± 0.03 b | 3.47 ± 0.12 c | 2.73 ± 0.07 c | 2.43 ± 0.04 a | 1.89 ± 0.02 a |
| Antioxidant activity (mM TE/L) **** | 17.04 ± 0.65 b | 15.92 ± 0.51 b | 23.71 ± 0.44 c | 22.07 ± 0.66 c | 14.78 ± 0.61 a | 12.39 ± 0.47 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasik-Kurdyś, M.; Gumienna, M.; Górna, B.; Adzahan, N.M. Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion. Foods 2022, 11, 3298. https://doi.org/10.3390/foods11203298
Lasik-Kurdyś M, Gumienna M, Górna B, Adzahan NM. Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion. Foods. 2022; 11(20):3298. https://doi.org/10.3390/foods11203298
Chicago/Turabian StyleLasik-Kurdyś, Małgorzata, Małgorzata Gumienna, Barbara Górna, and Noranizan Mohd Adzahan. 2022. "Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion" Foods 11, no. 20: 3298. https://doi.org/10.3390/foods11203298
APA StyleLasik-Kurdyś, M., Gumienna, M., Górna, B., & Adzahan, N. M. (2022). Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion. Foods, 11(20), 3298. https://doi.org/10.3390/foods11203298






