Palmatine Inhibits the Pathogenicity of Aeromonas hydrophila by Reducing Aerolysin Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Regents
2.2. Determination of MICs
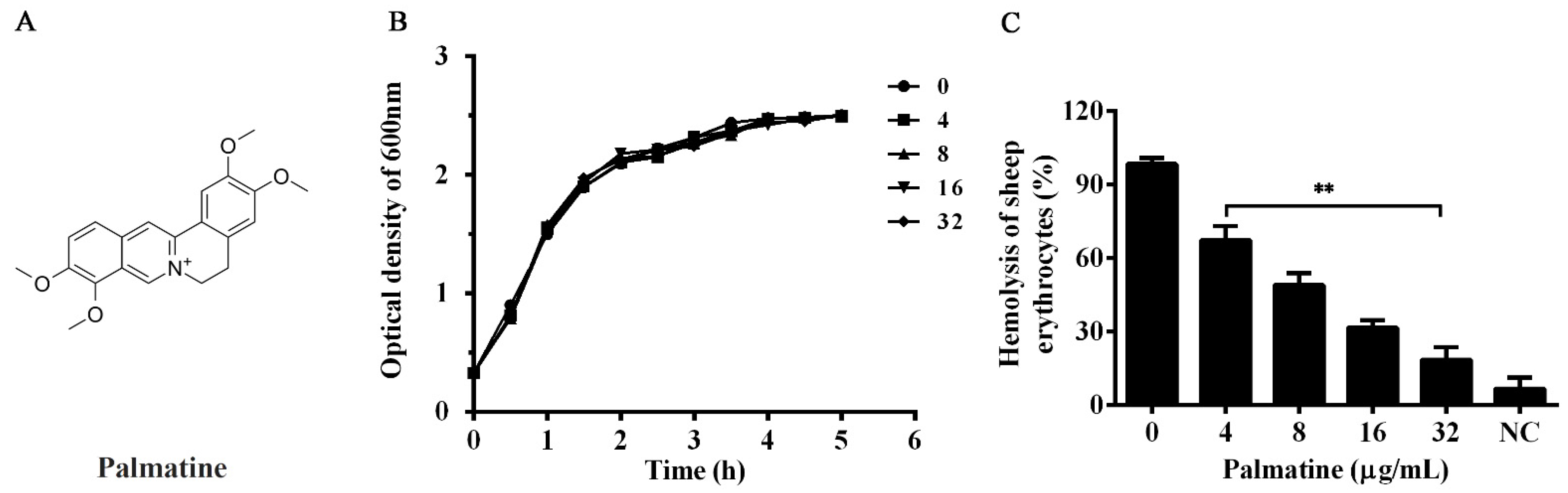
2.3. Growth Curves
2.4. Hemolytic Assay
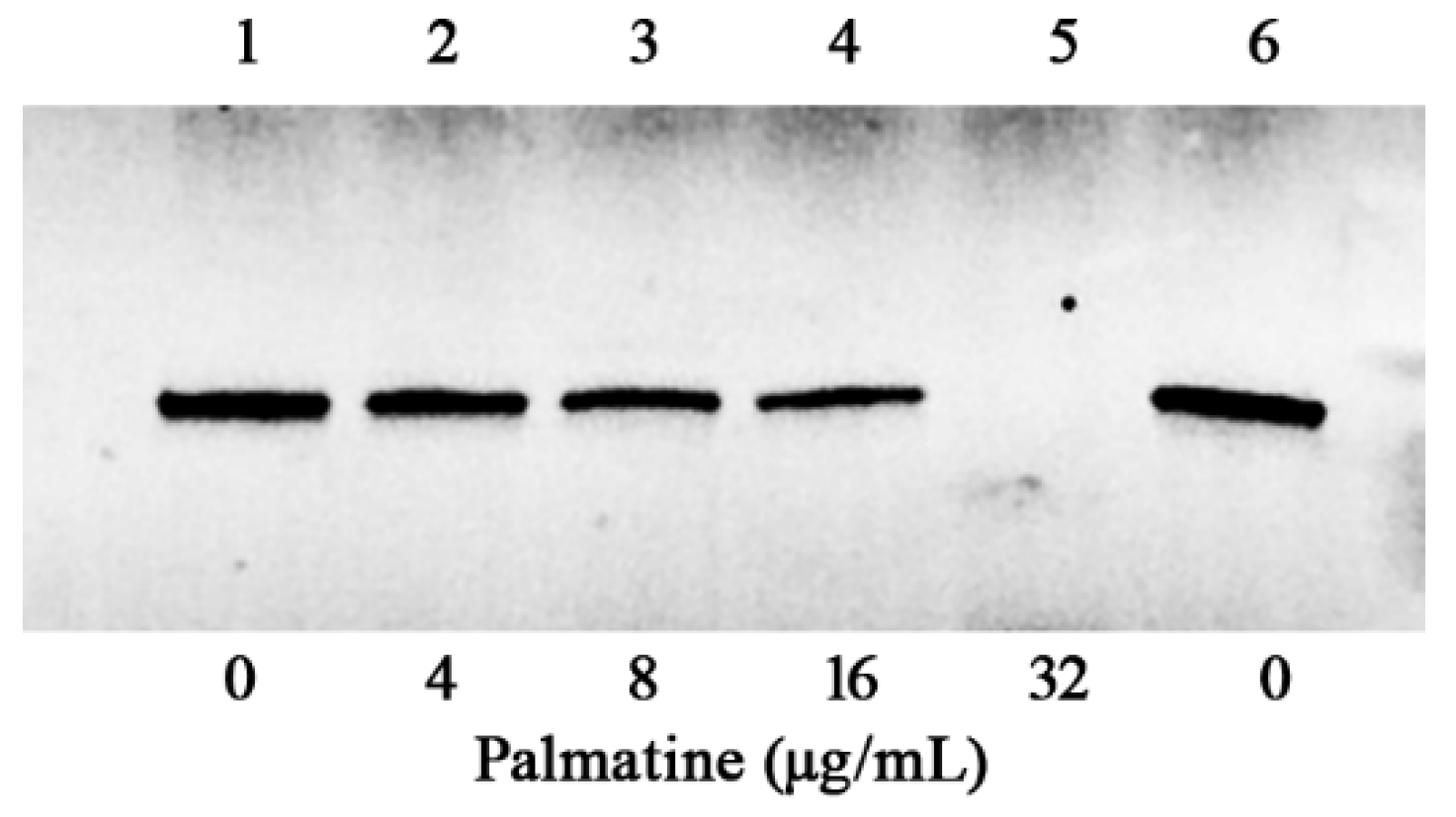
2.5. Western Blotting Assay
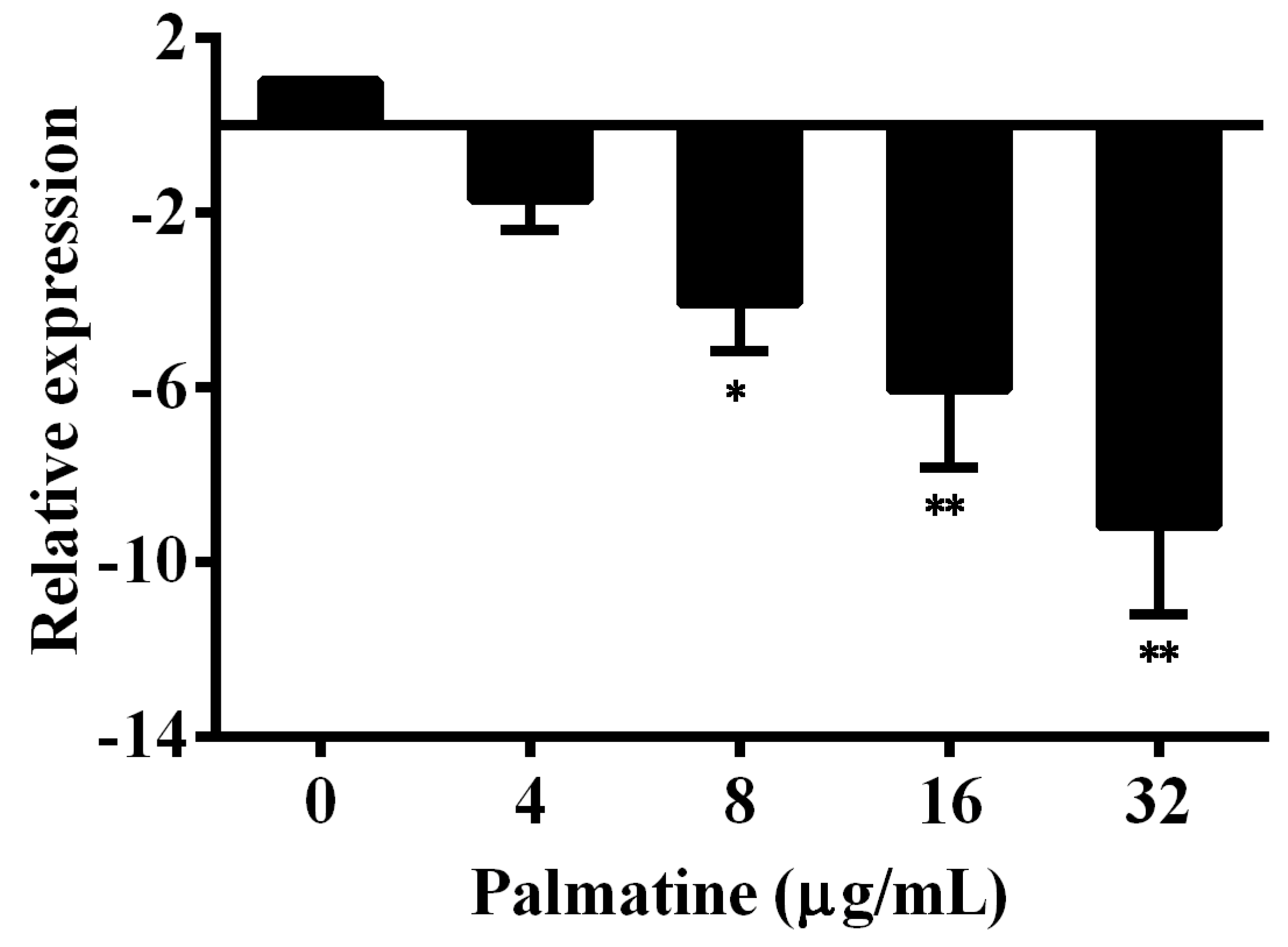
2.6. qPCR
2.7. Cytotoxicity Assay
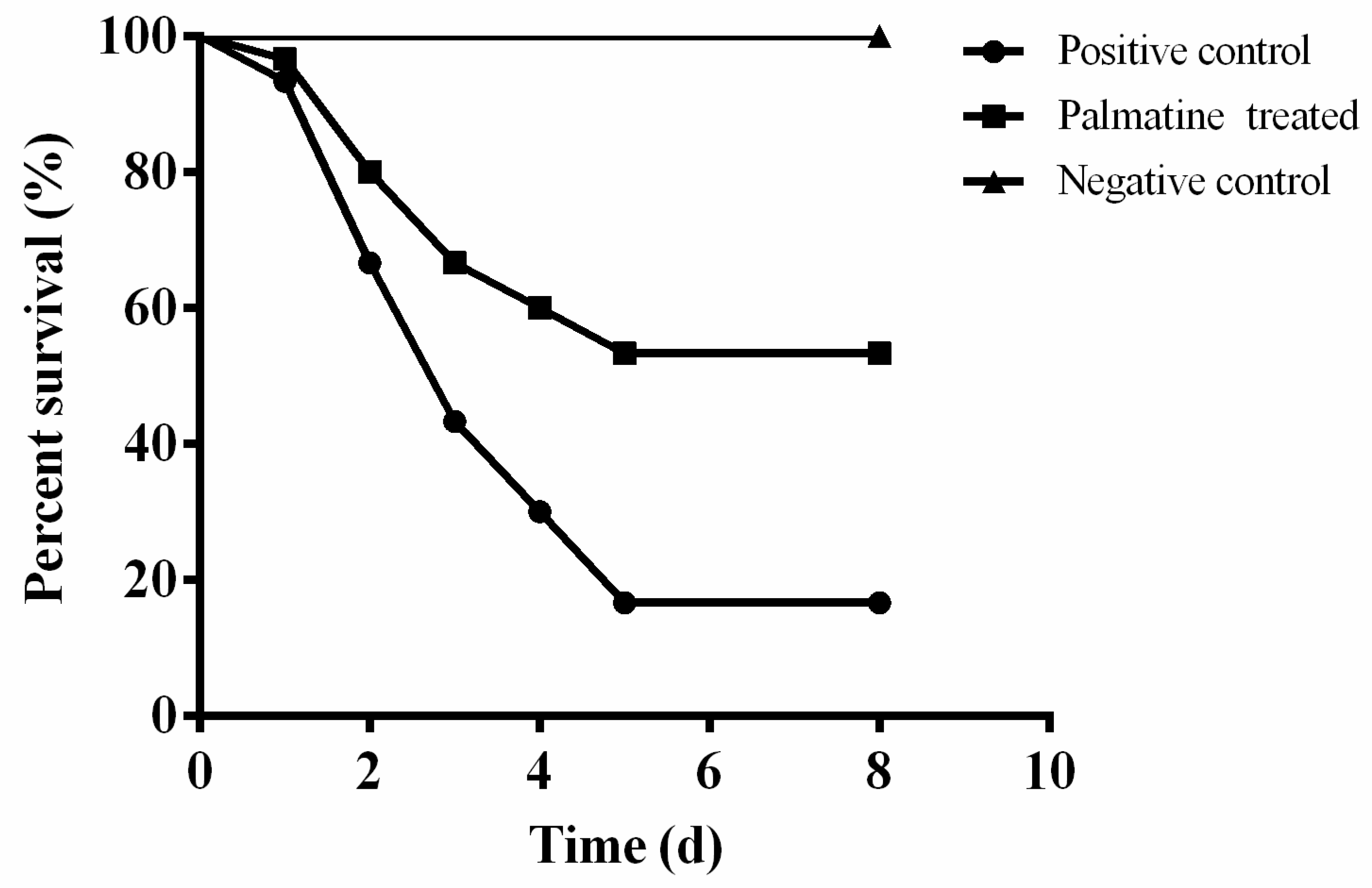
2.8. Animal Studies
2.9. Statistical Analysis
3. Results
3.1. Palmatine Had No Role on the Growth of A. hydrophila
3.2. Palmatine Decreased Hemolysis of A. hydrophila Induced by Aerolysin
3.3. Quantitative Real-Time PCR Results
3.4. Palmatine Reduced Aerolysin-Mediated A549 Cell Injury
3.5. Results of In Vivo Study
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Norman, R.A.; Crumlish, M.; Stetkiewicz, S. The importance of fisheries and aquaculture production for nutrition and food security. Rev. Sci. Tech. 2019, 38, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.; Keller-Costa, T.; Sanches-Fernandes, G.M.M.; Louvado, A.; Gomes, N.C.M.; Costa, R. Bacteriome structure, function, and probiotics in fish larviculture: The good, the bad, and the gaps. Annu. Rev. Anim. Biosci. 2021, 9, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Barger, P.C.; Liles, M.R.; Beck, B.H.; Newton, J.C. Differential production and secretion of potentially toxigenic extracellular proteins from hypervirulent Aeromonas hydrophila under biofilm and planktonic culture. BMC Microbiol. 2021, 21, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Shang, N.; Li, P. Synergistic inhibition of plantaricin E/F and lactic acid against Aeromonas hydrophila LPL-1 reveals the novel potential of class IIb bacteriocin. Front. Microbiol. 2022, 13, 774184. [Google Scholar] [CrossRef]
- Napis, S.; Rusul, G. Detection of aerolysin and hemolysin genes in Aeromonas spp. isolated from environmental and shellfish sources by polymerase chain reaction. ASEAN Food J. 2007, 14, 115–122. [Google Scholar]
- Wang, Q.C.; Ji, W.; Xu, Z. Current use and development of fish vaccines in China. Fish Shellfish Immun. 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Khor, W.C.; Puah, S.M.; Koh, T.H.; Tan, J.A.M.A.; Puthucheary, S.D.; Chua, K.H. Comparison of clinical isolates of Aeromonas from Singapore and Malaysia with regard to molecular identification, virulence, and antimicrobial profiles. Microb. Drug Resist. 2018, 24, 469–478. [Google Scholar] [CrossRef]
- Stratev, D.; Odeyemi, O.A. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: A mini-review. J. Infect. Public Health 2016, 9, 535–544. [Google Scholar] [CrossRef]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef]
- Muhlen, S.; Dersch, P. Anti-virulence strategies to target bacterial infections. Curr. Top. Microbiol. 2016, 398, 147–183. [Google Scholar] [CrossRef]
- Fivaz, M.; Abrami, L.; Tsitrin, Y.; van der Goot, F.G. Aerolysin from Aeromonas hydrophila and related toxins. Curr. Top. Microbiol. Immunol. 2001, 257, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Huhle, B.; Hof, H.; Bergbauer, H.; Goebel, W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect. Immun. 1987, 55, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, I.; Peeters, J.G.; Blom, A.E.; Theile, C.S.; Li, Z.; Spooner, E.; Ploegh, H.L.; Guimaraes, C.P. Site-specific chemoenzymatic labeling of aerolysin enables the identification of new aerolysin receptors. PLoS ONE 2014, 9, e109883. [Google Scholar] [CrossRef]
- Iacovache, I.; Degiacomi, M.T.; Pernot, L.; Ho, S.; Schiltz, M.; Dal Peraro, M.; van der Goot, F.G. Dual chaperone role of the C-terminal propeptide in folding and oligomerization of the pore-forming toxin aerolysin. PLoS Pathog. 2011, 7, e1002135. [Google Scholar] [CrossRef]
- van der Goot, F.G.; Pattus, F.; Parker, M.; Buckley, J.T. The pore-forming toxin aerolysin: From the soluble form to the transmembrane channel. Toxicology 1994, 87, 19–28. [Google Scholar] [CrossRef]
- Zhang, D.; Pridgeon, J.W.; Klesius, P.H. Expression and activity of recombinant proaerolysin derived from Aeromonas hydrophila cultured from diseased channel catfish. Vet. Microbiol. 2013, 165, 478–482. [Google Scholar] [CrossRef]
- Iacovache, I.; De Carlo, S.; Cirauqui, N.; Dal Peraro, M.; van der Goot, F.G.; Zuber, B. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat. Commun. 2016, 7, 12062. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Valladao, G.M.R.; Gallani, S.U.; Pilarski, F. Phytotherapy as an alternative for treating fish disease. J. Vet. Pharm. 2015, 38, 417–428. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.E.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and antibacterial functionality of essential oils: An alternative approach for sustainable aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Song, J.W.; Zhong, L.; Liao, Y.M.; Liu, L.N.; Li, X.F. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Lin, L.; Xie, Y.; Li, D.; Xiao, M.; Zhang, Y.; Cheung, S.C.K.; Shaw, P.C.; Yang, X.; Chan, P.K.S.; et al. Blood-glucose-lowering effect of Coptidis rhizoma extracts from different origins via gut microbiota modulation in db/db mice. Front. Pharm. 2021, 12, 684358. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Y.; Guo, X.X.; Zeng, Q.X.; Wei, W.; You, X.F.; Pang, J.; Wang, Y.X.; Song, D.Q. Synthesis and structure-activity relationship of palmatine derivatives as a novel class of antibacterial agents against Helicobacter pylori. Molecules 2020, 25, 1352. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI: Berwyn, PA, USA, 2015. [Google Scholar]
- Delamare, A.P.L.; Costa, S.O.P.; Da Silveira, M.M.; Echeverrigaray, S. Growth of Aeromonas species on increasing concentrations of sodium chloride. Lett. Appl. Microbiol. 2000, 30, 57–60. [Google Scholar] [CrossRef]
- Handfield, M.; Simard, P.; Couillard, M.; Letarte, R. Aeromonas hydrophila isolated from food and drinking water: Hemagglutination, hemolysis, and cytotoxicity for a human intestinal cell line (HT-29). Appl. Environ. Microbiol. 1996, 62, 3459–3461. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.S.; Liu, Y.T.; Zhou, S.; Yang, Y.B.; Xu, N.; Yang, Q.H.; Ai, X.H. Resveratrol influences the pathogenesis of Aeromonas hydrophila by inhibiting production of aerolysin and biofilm. Food Control 2021, 126, 108803. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ran, C.; Qin, C.B.; Xie, M.X.; Zhang, J.X.; Li, J.; Xie, Y.D.; Wang, Y.B.; Li, S.N.; Liu, L.H.; Fu, X.Z.; et al. Aeromonas veronii and aerolysin are important for the pathogenesis of motile aeromonad septicemia in cyprinid fish. Environ. Microbiol. 2018, 20, 3442–3456. [Google Scholar] [CrossRef]
- Howard, S.P.; Buckley, J.T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry 1982, 21, 1662–1667. [Google Scholar] [CrossRef]
- Nelson, K.L.; Raja, S.M.; Buckley, J.T. The glycosylphosphatidylinositol-anchored surface glycoprotein Thy-1 is a receptor for the channel-forming toxin aerolysin. J. Biol. Chem. 1997, 272, 12170–12174. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Fivaz, M.; Glauser, P.E.; Sugimoto, N.; Zurzolo, C.; van der Goot, F.G. Sensitivity of polarized epithelial cells to the pore-forming toxin aerolysin. Infect. Immun. 2003, 71, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018, 2018, 465–468. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of antibiotic resistance in important Gram-positive and Gram-negative pathogens and novel antibiotic solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Yin, Z.; Wu, Y.S.; Cai, T.Z. Studies on the taxonomy of pathogenic bacteria of the bacterial hemorrhagic septicemia in cultured fishes in freshwater. Acta Hydrobiol. Sin. 1993, 17, 259–266. [Google Scholar]
- Zhu, D.L.; Li, A.H.; Wang, J.G.; Li, M.; Cai, T.Z.; Hu, J. The correlation between the distribution pattern of virulence genes and the virulence of Aeromonas hydrophila strains. Acta Sci. Nat. Univ. Sunyatseni 2006, 45, 82–85. [Google Scholar] [CrossRef]
- Dong, J.; Ding, H.; Liu, Y.T.; Yang, Q.H.; Xu, N.; Yang, Y.B.; Ai, X.H. Magnolol protects channel catfish from Aeromonas hydrophila infection via inhibiting the expression of aerolysin. Vet. Microbiol. 2017, 211, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Fujinami, C.; Kuroda, T.; Takeuchi, Y.; Miyoshi, S.; Arimoto, S.; Negishi, T.; Okamoto, K. Indolo[3,2-b]quinoline derivatives suppressed the hemolytic activity of beta-pore forming toxins, aerolysin-like hemolysin produced by Aeromonas sobria and alpha-hemolysin produced by Staphylococcus aureus. Biol. Pharm. Bull. 2016, 39, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.C.; Zhang, M.; Luo, H.Y. Identification and antimicrobial activity of two alkaloids from traditional Chinese medicinal plant Tinospora capillipes. Ind. Crop. Prod. 2012, 37, 298–302. [Google Scholar] [CrossRef]
- Xiao, C.W.; Ji, Q.A.; Wei, Q.; Liu, Y.; Bao, G.L. Antifungal activity of berberine hydrochloride and palmatine hydrochloride against Microsporum canis -induced dermatitis in rabbits and underlying mechanism. BMC Complement. Altern. Med. 2015, 15, 177. [Google Scholar] [CrossRef]
- Wang, P.; Hu, L.F.; Hao, Z.H. Palmatine is a plasmid-mediated quinolone resistance (PMQR) inhibitor that restores the activity of ciprofloxacin against QnrS and AAC (6′)-Ib-cr-producing Escherichia coli. Infect. Drug Resist. 2020, 13, 749–759. [Google Scholar] [CrossRef]
- Nitulescu, G.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Mihai, D.P.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Molecular docking and screening studies of new natural Sortase A inhibitors. Int. J. Mol. Sci. 2017, 18, 2217. [Google Scholar] [CrossRef]
- Aghayan, S.S.; Kalalian Mogadam, H.; Fazli, M.; Darban-Sarokhalil, D.; Khoramrooz, S.S.; Jabalameli, F.; Yaslianifard, S.; Mirzaii, M. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn Infections. J. Med. Biotechnol. 2017, 9, 2–7. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Yan, T.; Yang, Q.; Song, Y.; Cheng, B.; Zhou, S.; Liu, Y.; Ai, X. Palmatine Inhibits the Pathogenicity of Aeromonas hydrophila by Reducing Aerolysin Expression. Foods 2022, 11, 3250. https://doi.org/10.3390/foods11203250
Dong J, Yan T, Yang Q, Song Y, Cheng B, Zhou S, Liu Y, Ai X. Palmatine Inhibits the Pathogenicity of Aeromonas hydrophila by Reducing Aerolysin Expression. Foods. 2022; 11(20):3250. https://doi.org/10.3390/foods11203250
Chicago/Turabian StyleDong, Jing, Tianhui Yan, Qiuhong Yang, Yi Song, Bo Cheng, Shun Zhou, Yongtao Liu, and Xiaohui Ai. 2022. "Palmatine Inhibits the Pathogenicity of Aeromonas hydrophila by Reducing Aerolysin Expression" Foods 11, no. 20: 3250. https://doi.org/10.3390/foods11203250
APA StyleDong, J., Yan, T., Yang, Q., Song, Y., Cheng, B., Zhou, S., Liu, Y., & Ai, X. (2022). Palmatine Inhibits the Pathogenicity of Aeromonas hydrophila by Reducing Aerolysin Expression. Foods, 11(20), 3250. https://doi.org/10.3390/foods11203250





