Analysis of Microbial Community Diversity on the Epidermis of Wine Grapes in Manasi’s Vineyard, Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Grape Sampling
2.3. Determination of Physicochemical Properties
2.4. Microbial Diversity Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Characteristics
3.1.1. General Physiochemical Indicators
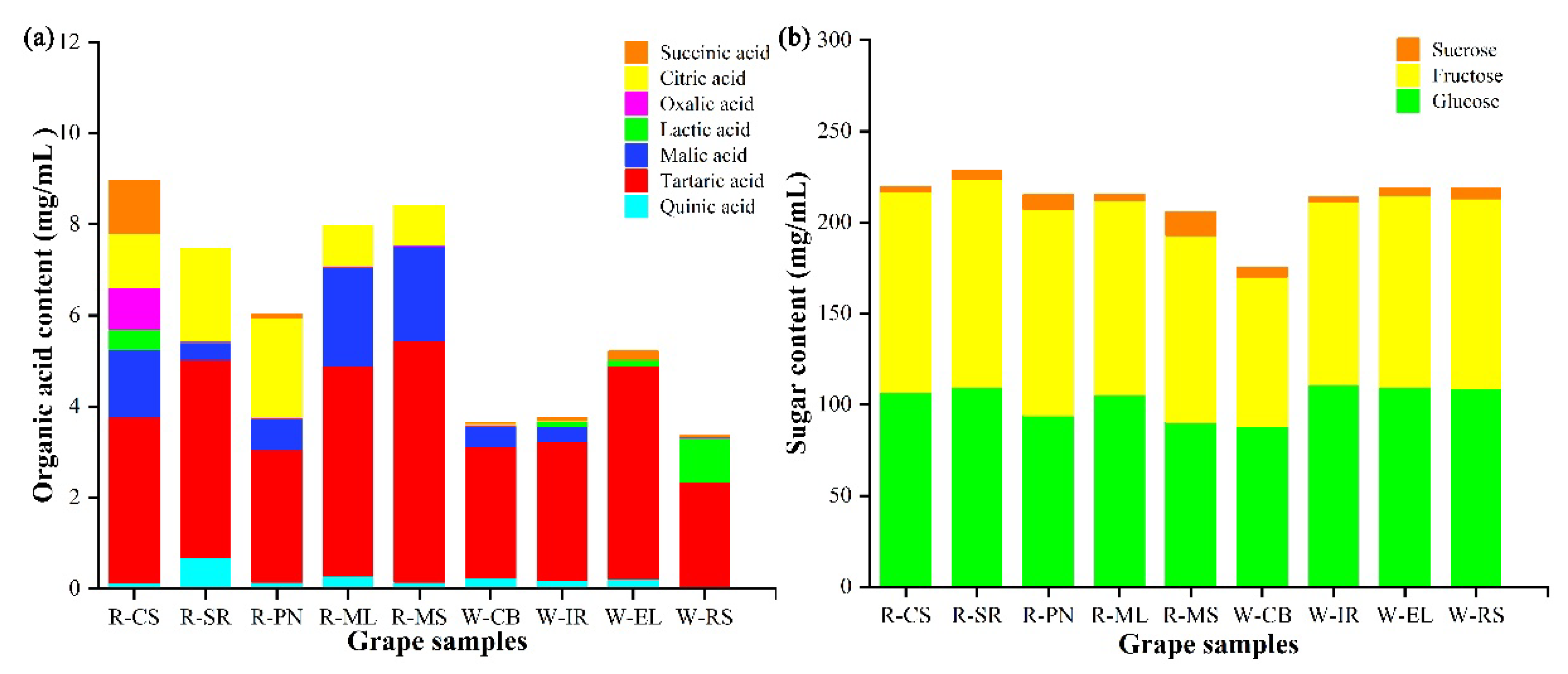
3.1.2. The Compositions and Contents of Sugar and Organic Acids
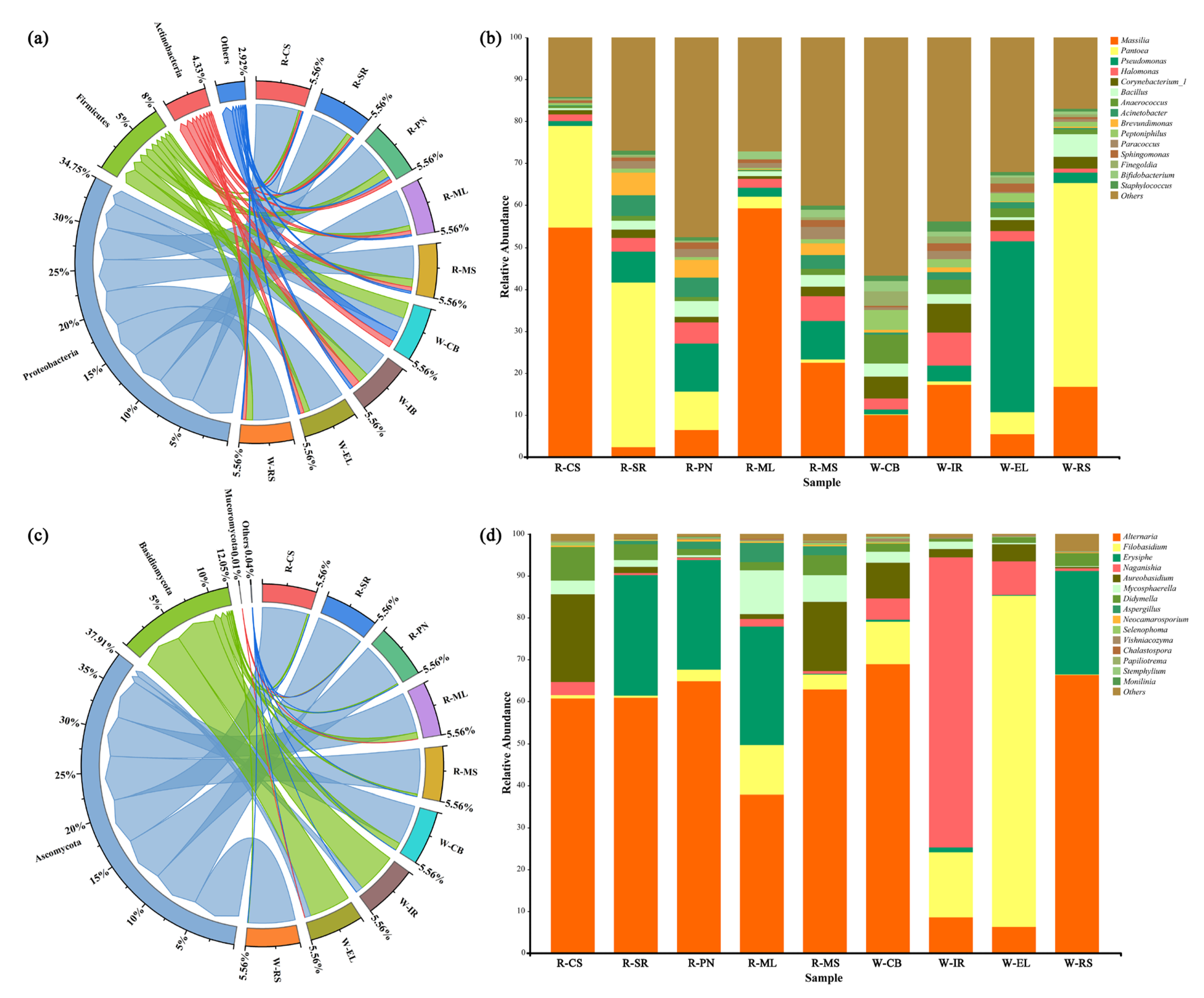
3.2. Microbial Succession and Interactions
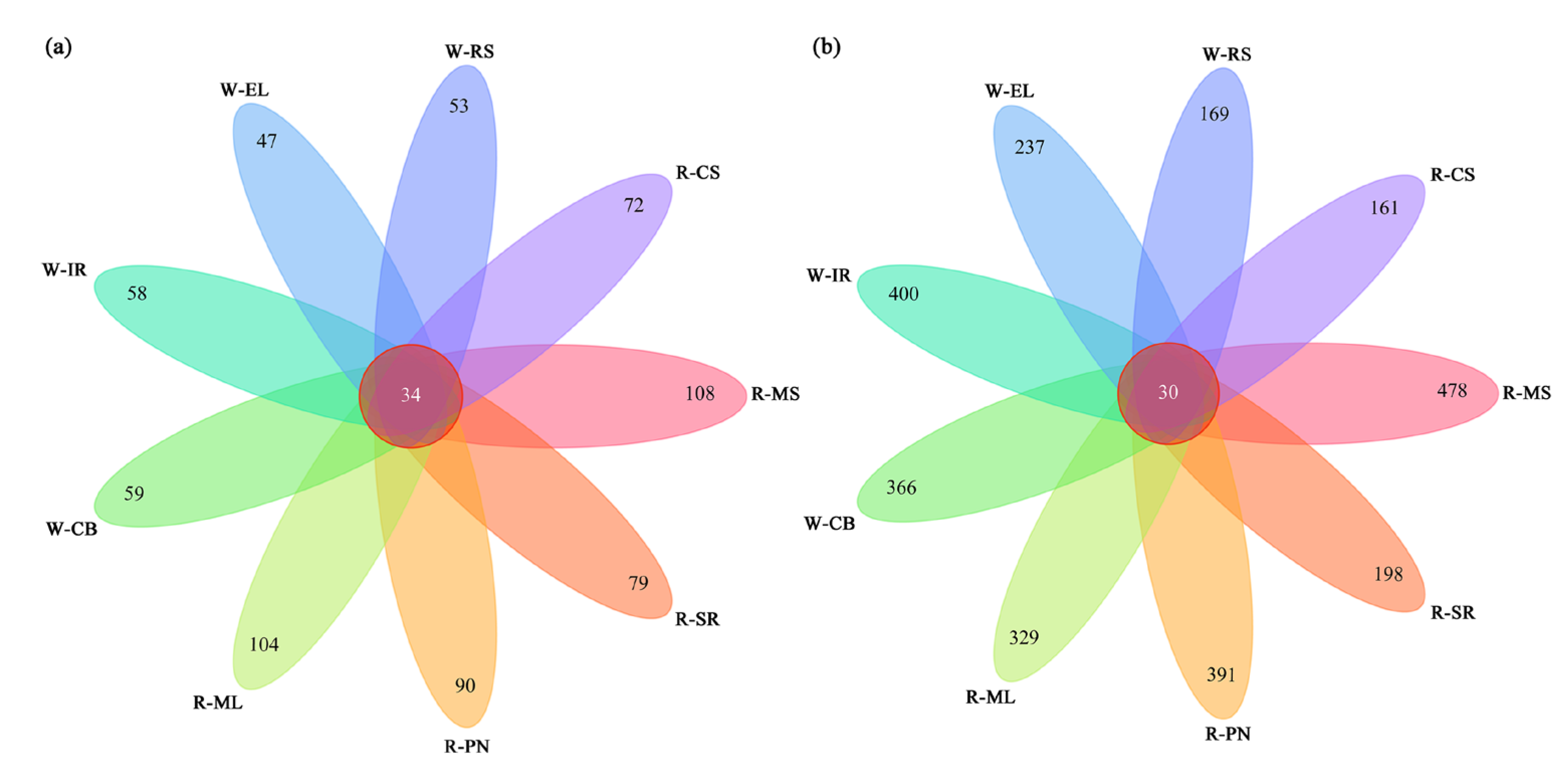
3.2.1. Sequencing Quality Assessment
3.2.2. Sequencing Quality Assessment
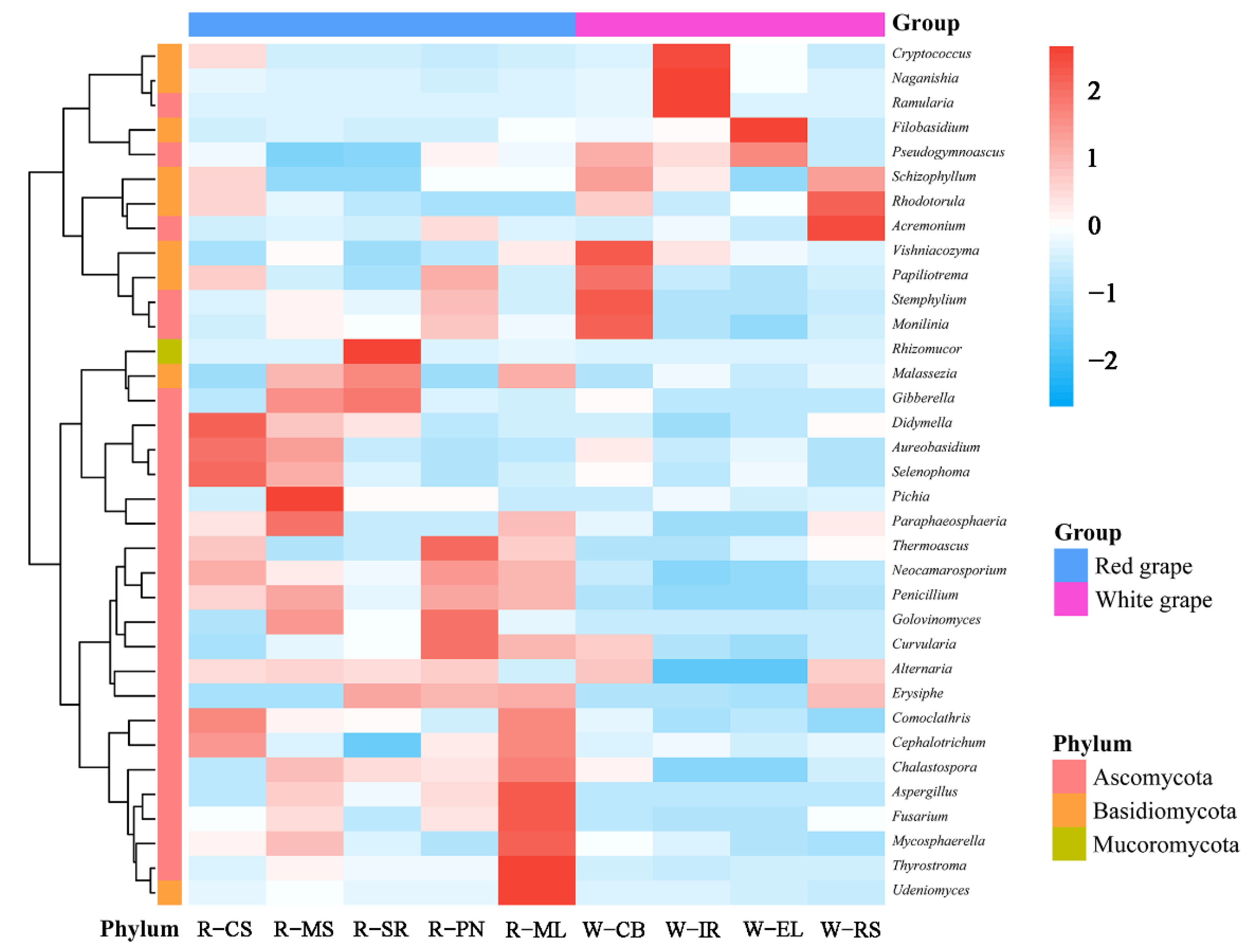
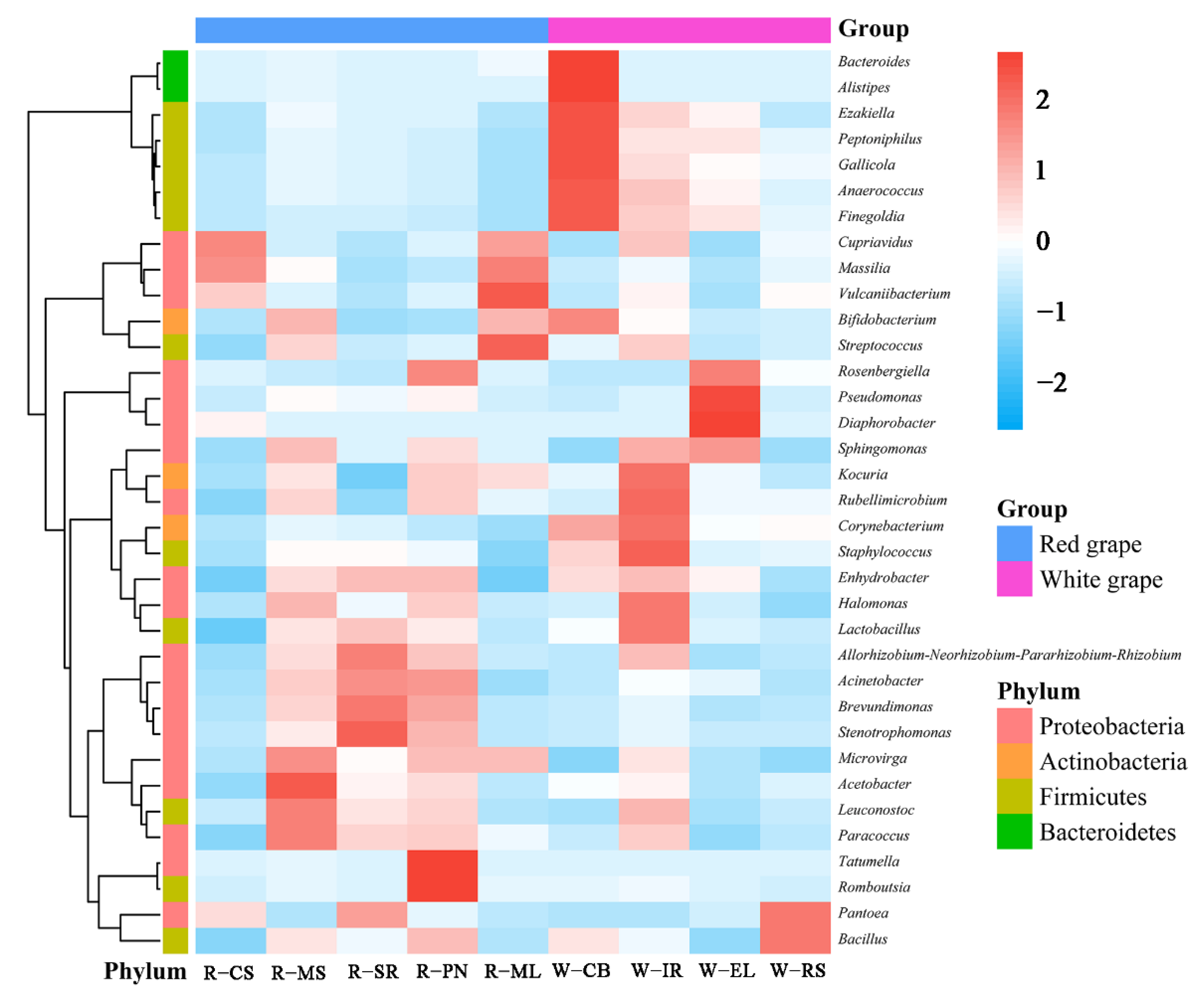
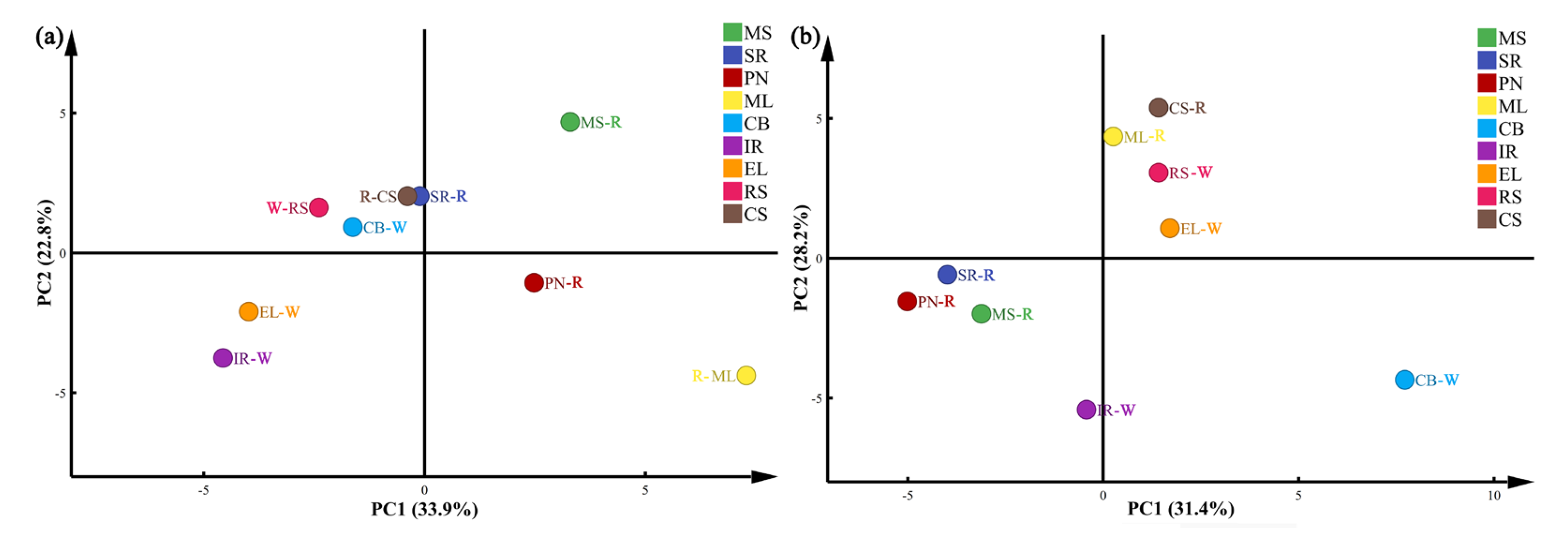
3.2.3. Cluster Analysis
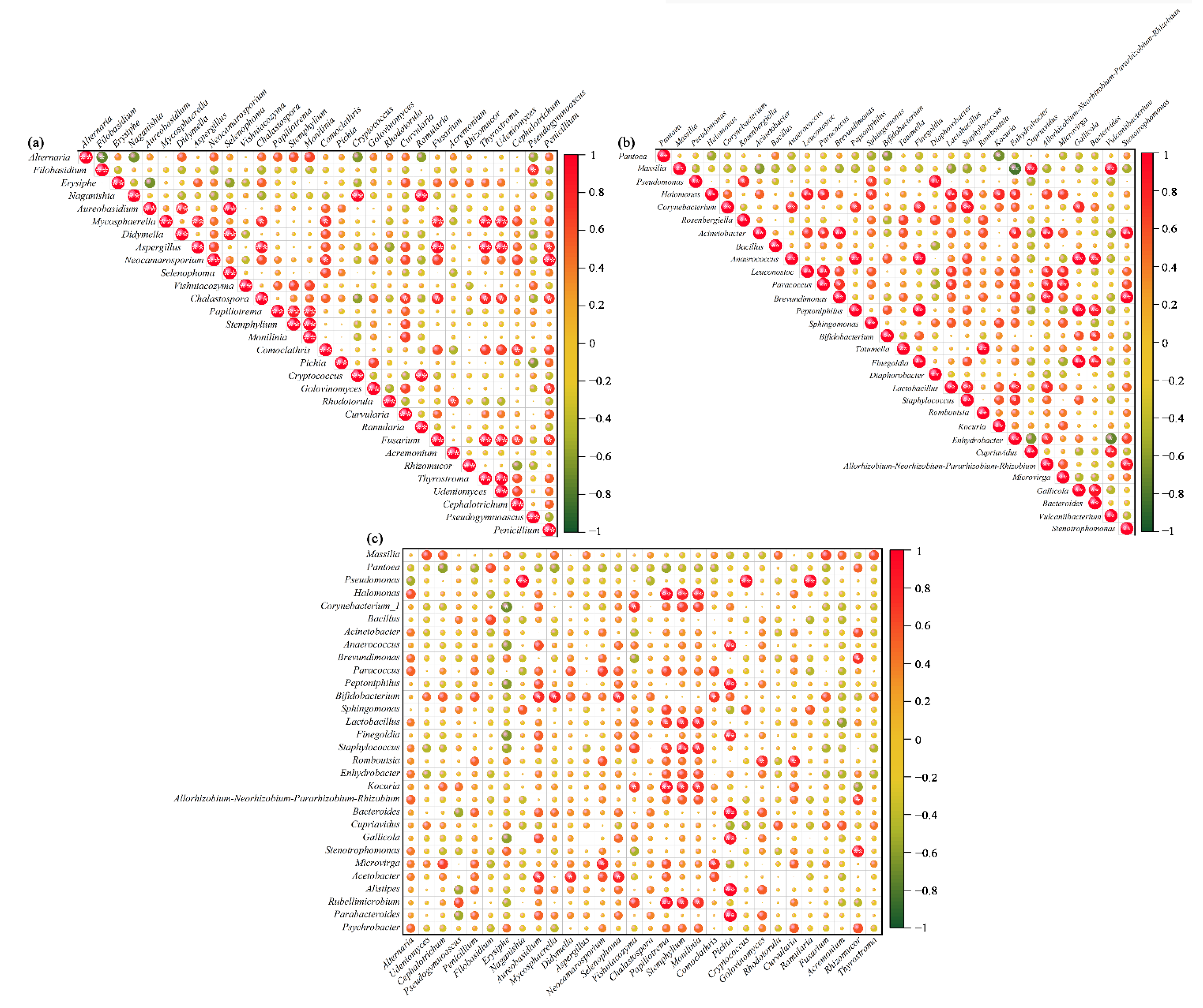
3.3. Co-Occurrence Analyses for Relationships between Different Microbes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, A.G. (Ed.) Managing Wine Quality: Viticulture and Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Nguyen, T.; Fluss, L.; Madej, R.; Ginther, C.; Leighton, T. The distribution of mutagenic activity in red, rose and white wines. Mutat. Res. Genet. Toxicol. 1989, 223, 205–212. [Google Scholar] [CrossRef]
- Portillo, M.d.C.; Franquès, J.; Araque, I.; Reguant, C.; Bordons, A. Bacterial diversity of Grenache and Carignan grape surface from different vineyards at Priorat wine region (Catalonia, Spain). Int. J. Food Microbiol. 2016, 219, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kerridge, G.H.; Antcliff, A.J. Wine Grape Varieties; Csiro Publishing: Clayton, Australia, 1999. [Google Scholar]
- Rombough, L. The Grape Grower: A Guide to Organic Viticulture; Chelsea Green Publishing: Hartford, VT, USA, 2002. [Google Scholar]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.; Coe, M.; Grose, C.; Martin, D.; Greenwood, D.R. Metabolomics Approach to Assess the Relative Contributions of the Volatile and Non-volatile Composition to Expert Quality Ratings of Pinot Noir Wine Quality. J. Agric. Food Chem. 2020, 68, 13380–13396. [Google Scholar] [CrossRef]
- Sun, D.; Qu, J.; Huang, Y.; Lu, J.; Yin, L. Analysis of microbial community diversity of muscadine grape skins. Food Res. Int. 2021, 145, 110417. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ma, W.; Wu, Y.; Wei, Y.; Zou, W.; Yan, Y.; Xue, J.; Tian, G.; Wang, L.; Wang, W.; Pan, H. Microbial diversity analysis of vineyards in the Xinjiang region using high-throughput sequencing. J. Inst. Brew. 2018, 124, 276–283. [Google Scholar] [CrossRef]
- Guo, L.; Luo, Y.; Zhou, Y.; Bianba, C.; Guo, H.; Zhao, Y.; Fu, H. Exploring microbial dynamics associated with flavours production during highland barley wine fermentation. Food Res. Int. 2020, 130, 108971. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Wu, Y.; Yan, Y.-Z.; Zou, W.; Xue, J.; Ma, W.-R.; Wang, W.; Tian, G.; Wang, L.-Y. High-throughput sequencing of microbial community diversity in soil, grapes, leaves, grape juice and wine of grapevine from China. PLoS ONE 2018, 13, e0193097. [Google Scholar] [CrossRef]
- Morgan, H.H.; du Toit, M.; Setati, M.E. The Grapevine and Wine Microbiome: Insights from High-Throughput Amplicon Sequencing. Front. Microbiol. 2017, 8, 820. [Google Scholar] [CrossRef]
- Rivas, G.A.; Semorile, L.; Delfederico, L. Microbial diversity of the soil, rhizosphere and wine from an emerging wine-producing region of Argentina. LWT 2022, 153, 112429. [Google Scholar] [CrossRef]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 856–873. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Gao, F.; Chen, J.; Xiao, J.; Cheng, W.; Zheng, X.; Wang, B.; Shi, X. Microbial community composition on grape surface controlled by geographical factors of different wine regions in Xinjiang, China. Food Res. Int. 2019, 122, 348–360. [Google Scholar] [CrossRef]
- Ma, Y.; Li, T.; Xu, X.; Ji, Y.; Jiang, X.; Shi, X.; Wang, B. Investigation of Volatile Compounds, Microbial Succession, and Their Relation During Spontaneous Fermentation of Petit Manseng. Front. Microbiol. 2021, 12, 717387. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Zhong, Q.; Zhuang, X.; Bai, Z. Microbial Community Analyses Associated with Nine Varieties of Wine Grape Carposphere Based on High-Throughput Sequencing. Microorganisms 2019, 7, 668. [Google Scholar] [CrossRef]
- Xu, X.; Miao, Y.; Wang, H.; Ye, P.; Li, T.; Li, C.; Zhao, R.; Wang, B.; Shi, X. A Snapshot of Microbial Succession and Volatile Compound Dynamics in Flat Peach Wine During Spontaneous Fermentation. Front. Microbiol. 2022, 13, 919047. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef]
- Ryan, J.; Hutchings, S.C.; Fang, Z.; Bandara, N.; Gamlath, S.; Ajlouni, S.; Ranadheera, C.S. Microbial, physico-chemical and sensory characteristics of mango juice-enriched probiotic dairy drinks. Int. J. Dairy Technol. 2020, 73, 182–190. [Google Scholar] [CrossRef]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Novikova, L.Y.; Naumova, L.G. Dependence of Fresh Grapes and Wine Taste Scores on the Origin of Varieties and Weather Conditions of the Harvest Year in the Northern Zone of Industrial Viticulture in Russia. Agronomy 2020, 10, 1613. [Google Scholar] [CrossRef]
- Soyer, Y.; Koca, N.; Karadeniz, F. Organic acid profile of Turkish white grapes and grape juices. J. Food Compos. Anal. 2003, 16, 629–636. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic Acid Metabolism and the Impact of Fermentation Practices on Wine Acidity: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- Lima, M.d.S.; da Conceição Prudêncio Dutra, M.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem. 2015, 188, 384–392. [Google Scholar] [CrossRef]
- Haggerty, L.L. Ripening Profile of Grape Berry Acids and Sugars in University of Minnesota Wine Grape Cultivars, Select Vitis Vinifera, and other hybrid cultivars. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, August 2013. [Google Scholar]
- Dai, Z.; Wu, H.; Baldazzi, V.; van Leeuwen, C.; Bertin, N.; Gautier, H.; Wu, B.; Duchêne, E.; Gomès, E.; Delrot, S.; et al. Inter-Species Comparative Analysis of Components of Soluble Sugar Concentration in Fleshy Fruits. Front. Plant Sci. 2016, 7, 649. [Google Scholar]
- Liu, H.-F.; Wu, B.-H.; Fan, P.-G.; Li, S.-H.; Li, L.-S. Sugar and acid concentrations in 98 grape cultivars analyzed by principal component analysis. J. Sci. Food Agric. 2006, 86, 1526–1536. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Xu, X.; Shi, X.; Wang, B. Succession of Fungal Communities at Different Developmental Stages of Cabernet Sauvignon Grapes From an Organic Vineyard in Xinjiang. Front. Microbiol. 2021, 12, 718261. [Google Scholar] [CrossRef]
- Radita, R.; Suwanto, A.; Kurosawa, N.; Wahyudi, A.T.; Rusmana, I. Firmicutes is the predominant bacteria in tempeh. Int. Food Res. J. 2018, 25, 2313–2320. [Google Scholar]
- Mezzasalma, V.; Sandionigi, A.; Guzzetti, L.; Galimberti, A.; Grando, M.S.; Tardaguila, J.; Labra, M. Geographical and Cultivar Features Differentiate Grape Microbiota in Northern Italy and Spain Vineyards. Front. Microbiol. 2018, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens Sarah, M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Lauga, B.; Miot-Sertier, C.; Mercier, A.; Lonvaud, A.; Soulas, M.-L.; Soulas, G.; Masneuf-Pomarède, I. Characterization of Epiphytic Bacterial Communities from Grapes, Leaves, Bark and Soil of Grapevine Plants Grown, and Their Relations. PLoS ONE 2013, 8, e73013. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef]
- Singh, P.; Santoni, S.; This, P.; Péros, J.-P. Genotype-Environment Interaction Shapes the Microbial Assemblage in Grapevine’s Phyllosphere and Carposphere: An NGS Approach. Microorganisms 2018, 6, 96. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Sabate, J.; Cano, J.; Esteve-Zarzoso, B.; Guillamón, J.M. Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol. Res. 2002, 157, 267–274. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A. Unraveling the Enzymatic Basis of Wine “Flavorome”: A Phylo-Functional Study of Wine Related Yeast Species. Front. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Shi, W.-K.; Wang, J.; Chen, F.-S.; Zhang, X.-Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 108477. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yi, H.; Wang, B.; Xiao, J.; Zhou, X.; Xu, J.; Jiang, L.; Shi, X. Microbial community composition and its role in volatile compound formation during the spontaneous fermentation of ice wine made from Vidal grapes. Process Biochem. 2020, 92, 365–377. [Google Scholar] [CrossRef]
- Chang, C.-F.; Huang, S.-Y.; Lee, C.-F. Vishniacozyma changhuana sp. nov., and Vishniacozyma taiwanica sp. nov., two novel yeast species isolated from mangrove forests in Taiwan. Int. J. Syst. Evol. Microbiol. 2021, 71, 004703. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Vishniacozyma ellesmerensis sp. nov., a psychrophilic yeast isolated from a retreating glacier in the Canadian High Arctic. Int. J. Syst. Evol. Microbiol. 2019, 69, 696–700. [Google Scholar] [CrossRef]
- Félix, C.R.; Andrade, D.A.; Almeida, J.H.; Navarro, H.M.C.; Fell, J.W.; Landell, M.F. Vishniacozyma alagoana sp. nov. a tremellomycetes yeast associated with plants from dry and rainfall tropical forests. Int. J. Syst. Evol. Microbiol. 2020, 70, 3449–3454. [Google Scholar] [CrossRef]
- Megías, E.; Megías, M.; Ollero Francisco, J.; Hungria, M. Draft Genome Sequence of Pantoea ananatis Strain AMG521, a Rice Plant Growth-Promoting Bacterial Endophyte Isolated from the Guadalquivir Marshes in Southern Spain. Genome Announc. 2016, 4, e01681-15. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Xu, Z.; Walker, M.E.; Zhang, J.; Gardner, J.M.; Sumby, K.M.; Jiranek, V. Exploring the diversity of bacteriophage specific to Oenococcus oeni and Lactobacillus spp. and their role in wine production. Appl. Microbiol. Biotechnol. 2021, 105, 8575–8592. [Google Scholar] [CrossRef]
- Leveau, J.; Tech, J. Grapevine microbiomics: Bacterial diversity on grape leaves and berries revealed by high-throughput sequence analysis of 16S rRNA amplicons. In Proceedings of the International Symposium on Biological Control of Postharvest Diseases: Challenges and Opportunities 905, Leesburg, VA, USA, 25 October 2010; pp. 31–42. [Google Scholar]
- Ziegler, M.; Grupstra, C.G.B.; Barreto, M.M.; Eaton, M.; BaOmar, J.; Zubier, K.; Al-Sofyani, A.; Turki, A.J.; Ormond, R.; Voolstra, C.R. Coral bacterial community structure responds to environmental change in a host-specific manner. Nat. Commun. 2019, 10, 3092. [Google Scholar] [CrossRef]
- Mandakovic, D.; Rojas, C.; Maldonado, J.; Latorre, M.; Travisany, D.; Delage, E.; Bihouée, A.; Jean, G.; Díaz, F.P.; Fernández-Gómez, B.; et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci. Rep. 2018, 8, 5875. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Miao, Y.; Wang, H.; Du, J.; Wang, C.; Shi, X.; Wang, B. Analysis of Microbial Community Diversity on the Epidermis of Wine Grapes in Manasi’s Vineyard, Xinjiang. Foods 2022, 11, 3174. https://doi.org/10.3390/foods11203174
Xu X, Miao Y, Wang H, Du J, Wang C, Shi X, Wang B. Analysis of Microbial Community Diversity on the Epidermis of Wine Grapes in Manasi’s Vineyard, Xinjiang. Foods. 2022; 11(20):3174. https://doi.org/10.3390/foods11203174
Chicago/Turabian StyleXu, Xiaoyu, Yuanyuan Miao, Huan Wang, Juan Du, Chenqiang Wang, Xuewei Shi, and Bin Wang. 2022. "Analysis of Microbial Community Diversity on the Epidermis of Wine Grapes in Manasi’s Vineyard, Xinjiang" Foods 11, no. 20: 3174. https://doi.org/10.3390/foods11203174
APA StyleXu, X., Miao, Y., Wang, H., Du, J., Wang, C., Shi, X., & Wang, B. (2022). Analysis of Microbial Community Diversity on the Epidermis of Wine Grapes in Manasi’s Vineyard, Xinjiang. Foods, 11(20), 3174. https://doi.org/10.3390/foods11203174