Formation of Natural Egg Yolk Granule Stabilized Pickering High Internal Phase Emulsions by Means of NaCl Ionic Strength and pH Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EYGs
2.3. EYGs Characterization
2.3.1. Zeta Potential
2.3.2. Scanning Electron Microscope (SEM)
2.4. Preparation of HIPEs Stabilized by EYGs
2.5. Emulsion Characterization
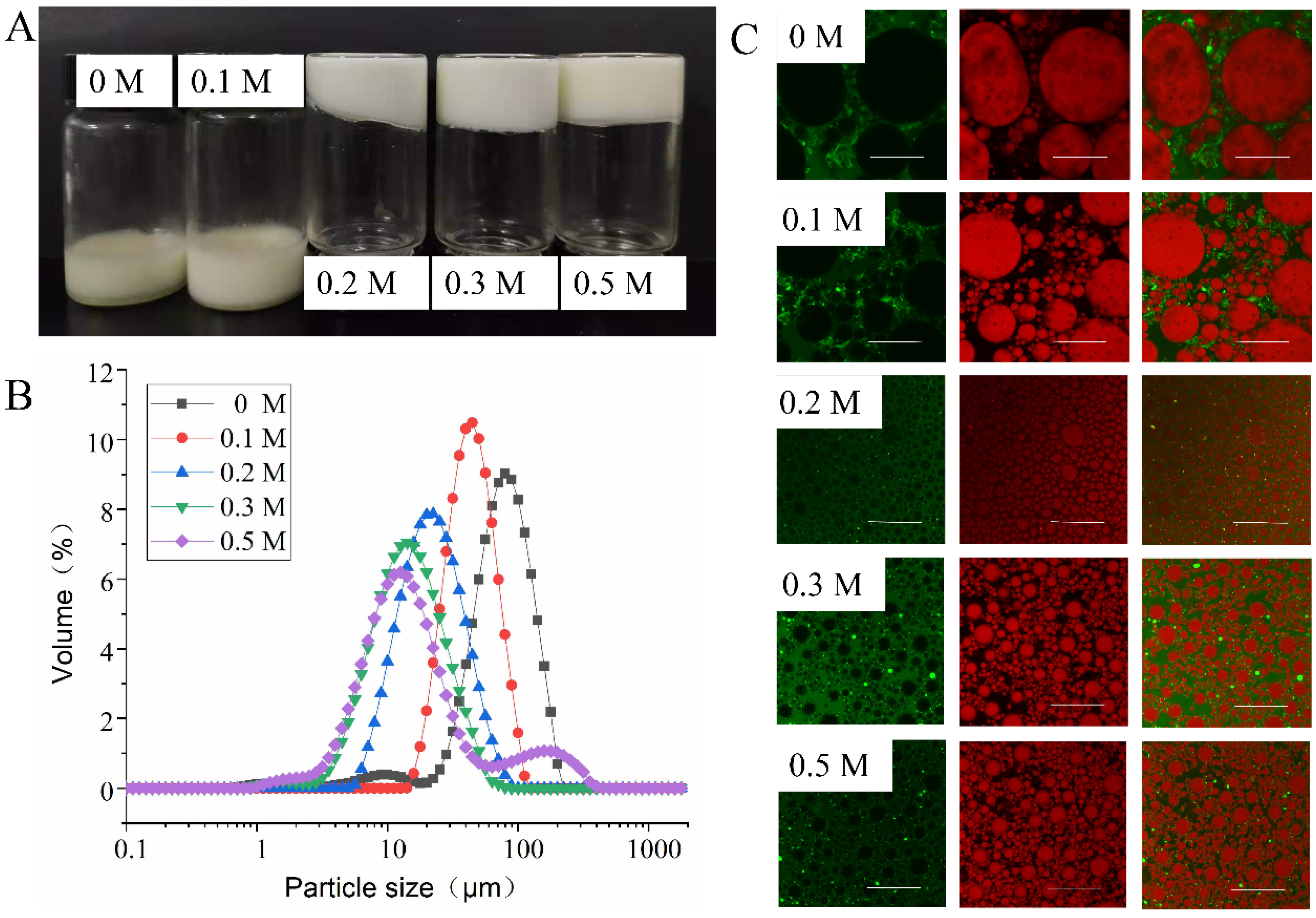
2.5.1. Particle Size Distribution of Emulsions
2.5.2. Confocal Laser Scanning Microscope (CLSM)
2.5.3. Optical Microscope
2.5.4. Rheological Properties of Emulsions
2.5.5. Storage Stability of Emulsions
2.6. Statistic Analysis
3. Results and Discussion
3.1. Zeta Potential of EYGs at Different NaCl Ionic Strength and pH
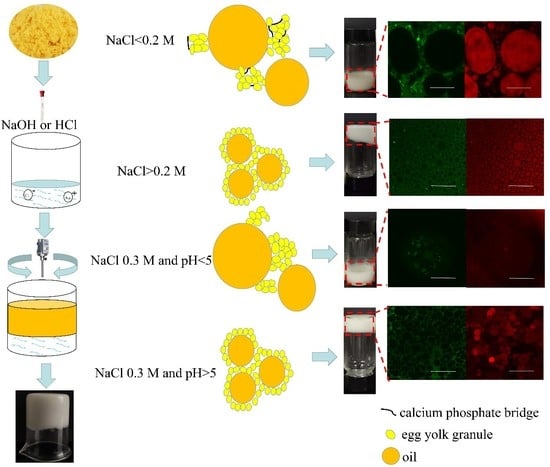
3.2. Aggregation States of EYGs under Different NaCl Ionic Strength and pH
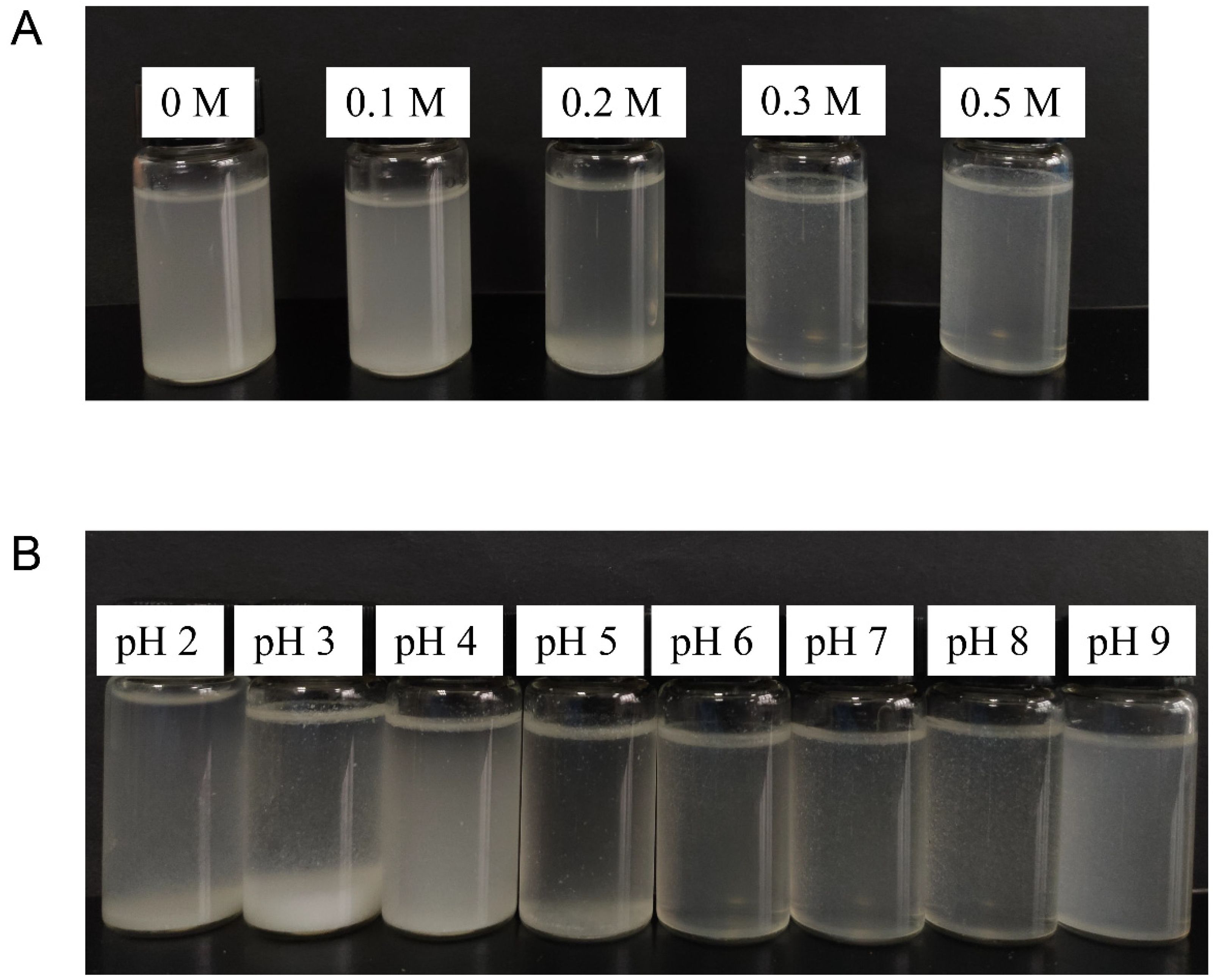
3.2.1. Visual Appearance of Suspension
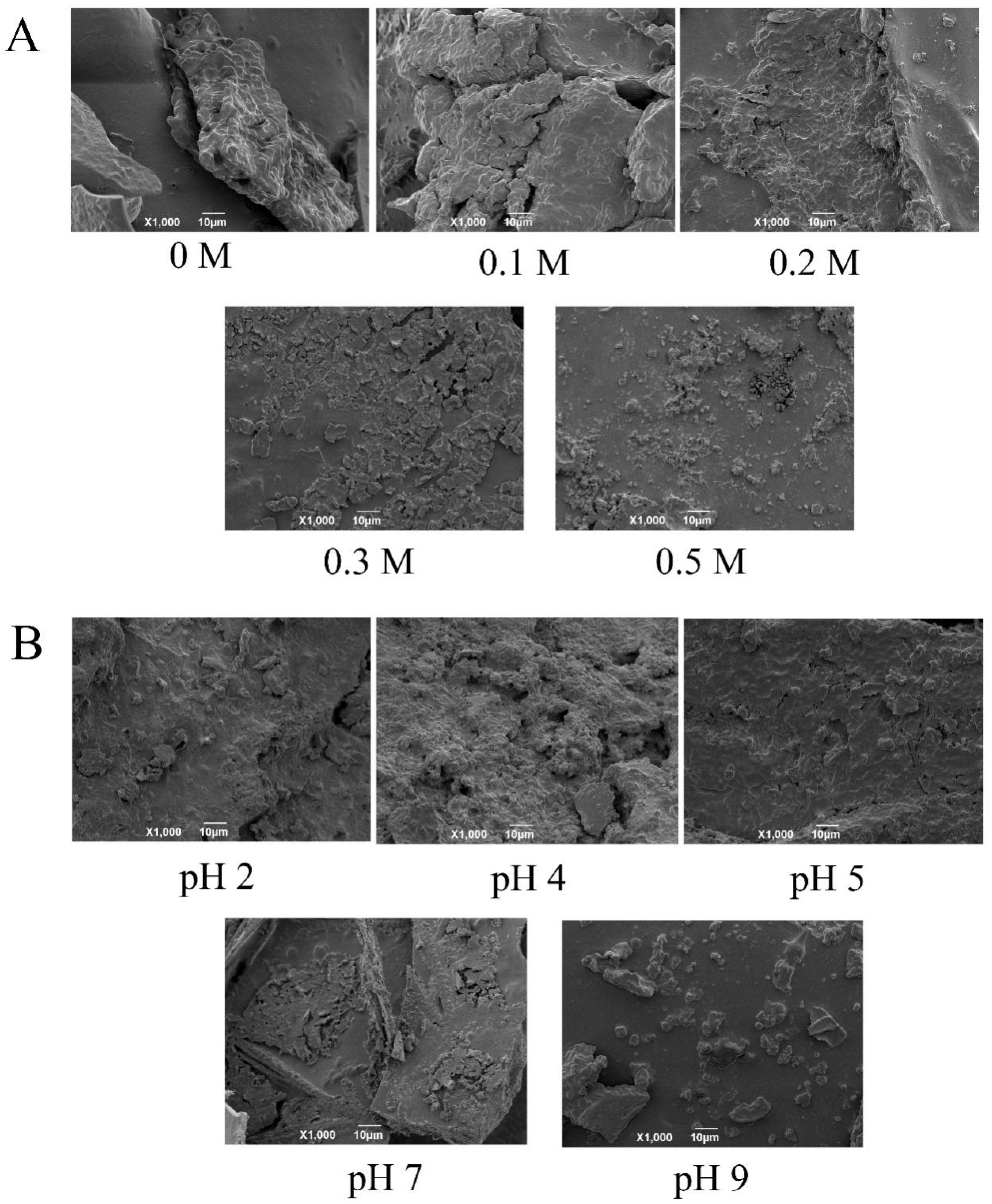
3.2.2. SEM of EYGs
3.3. Emulsion Characterization
3.3.1. Effect of NaCl Ionic Strength on Emulsion Properties
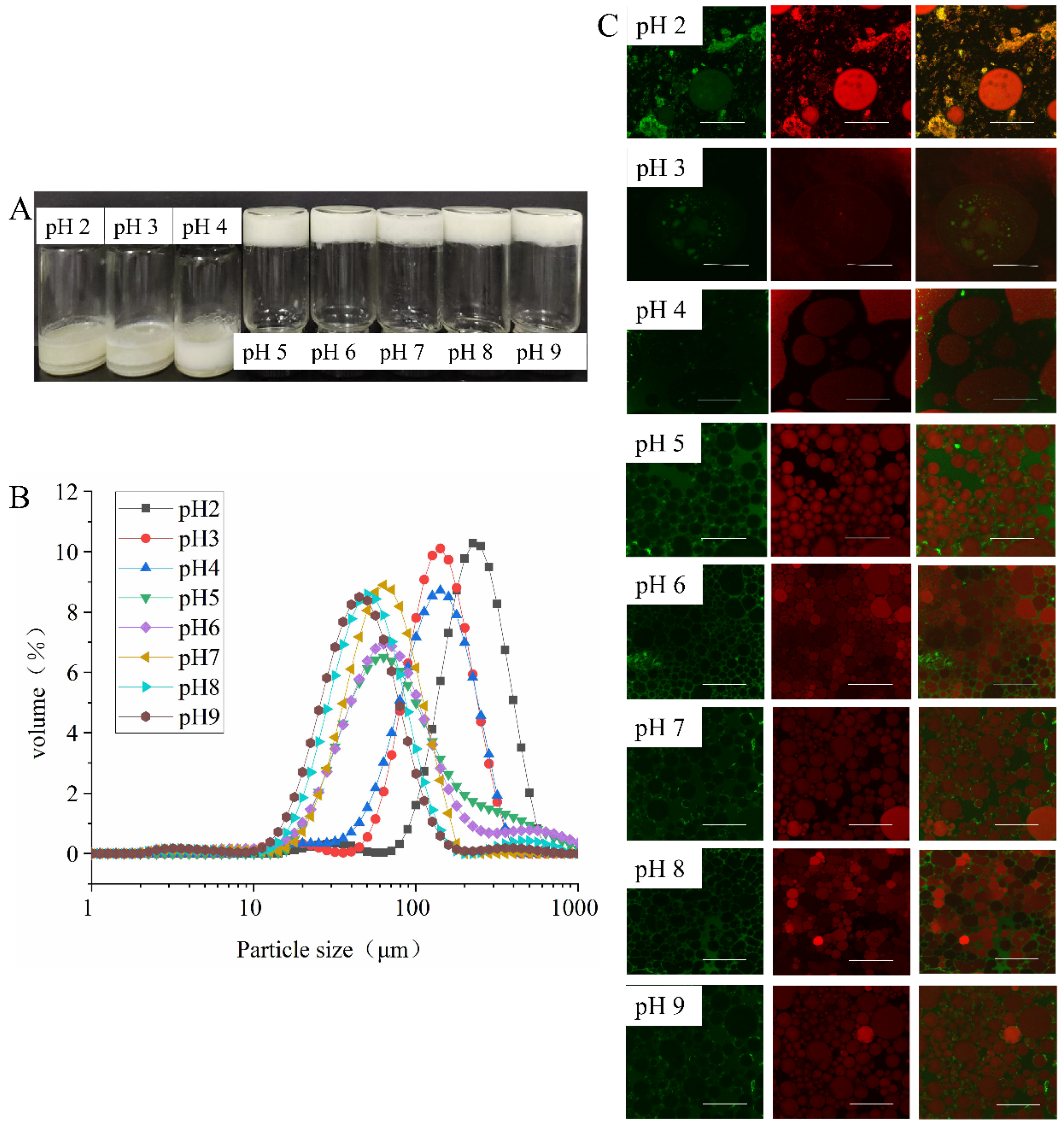
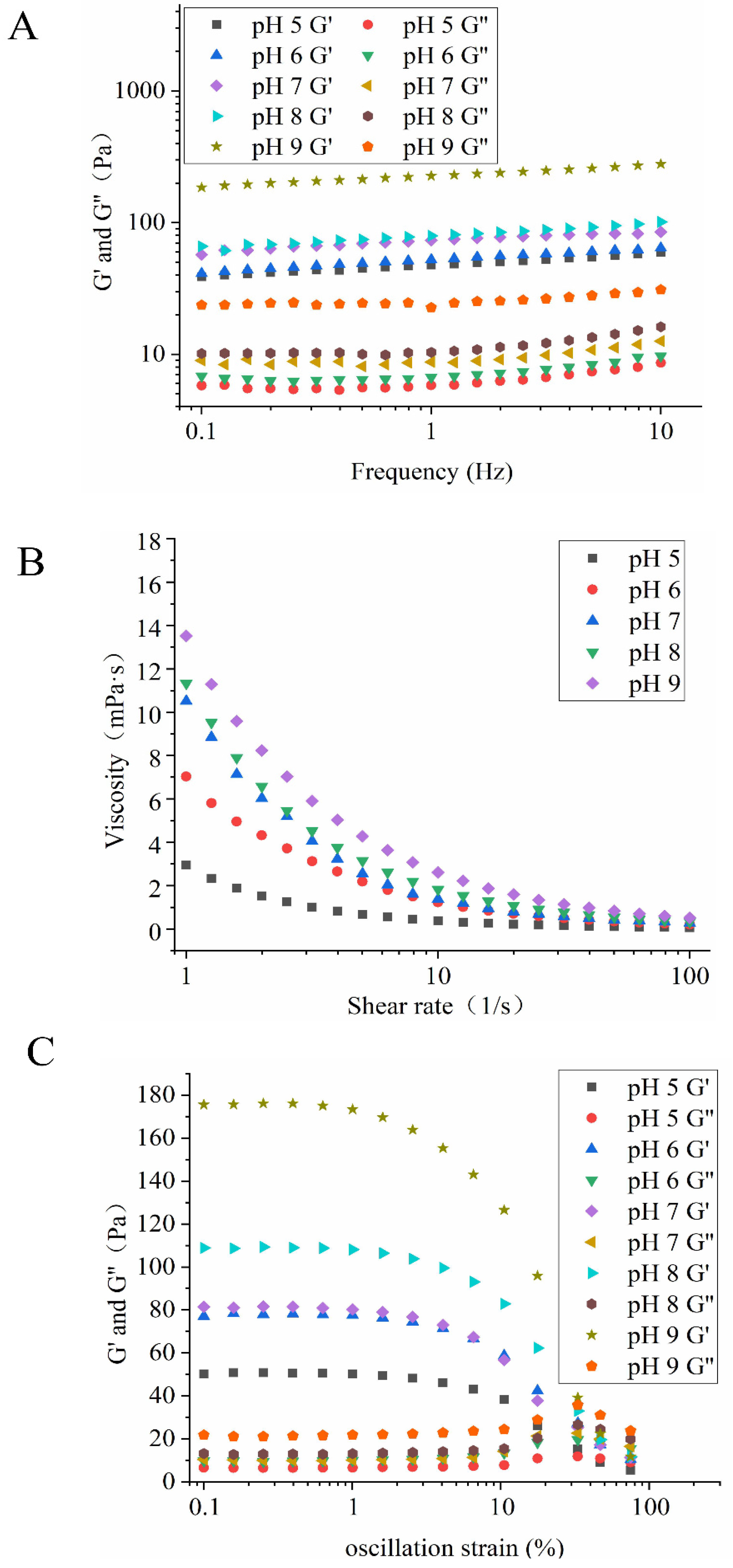
3.3.2. Effect of pH on Emulsion Properties
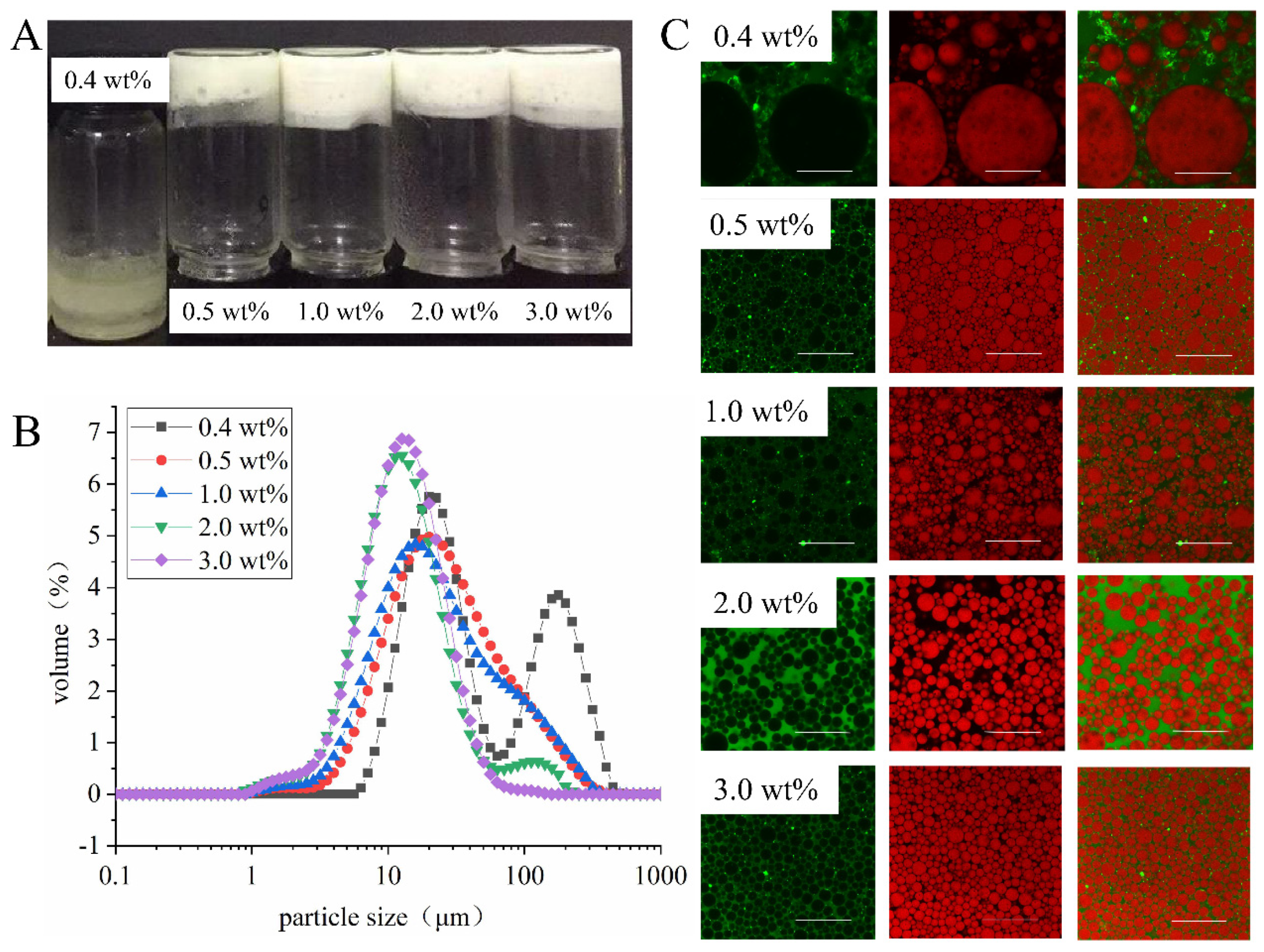
3.3.3. Effect of Concentration of EYGs on Emulsion Properties
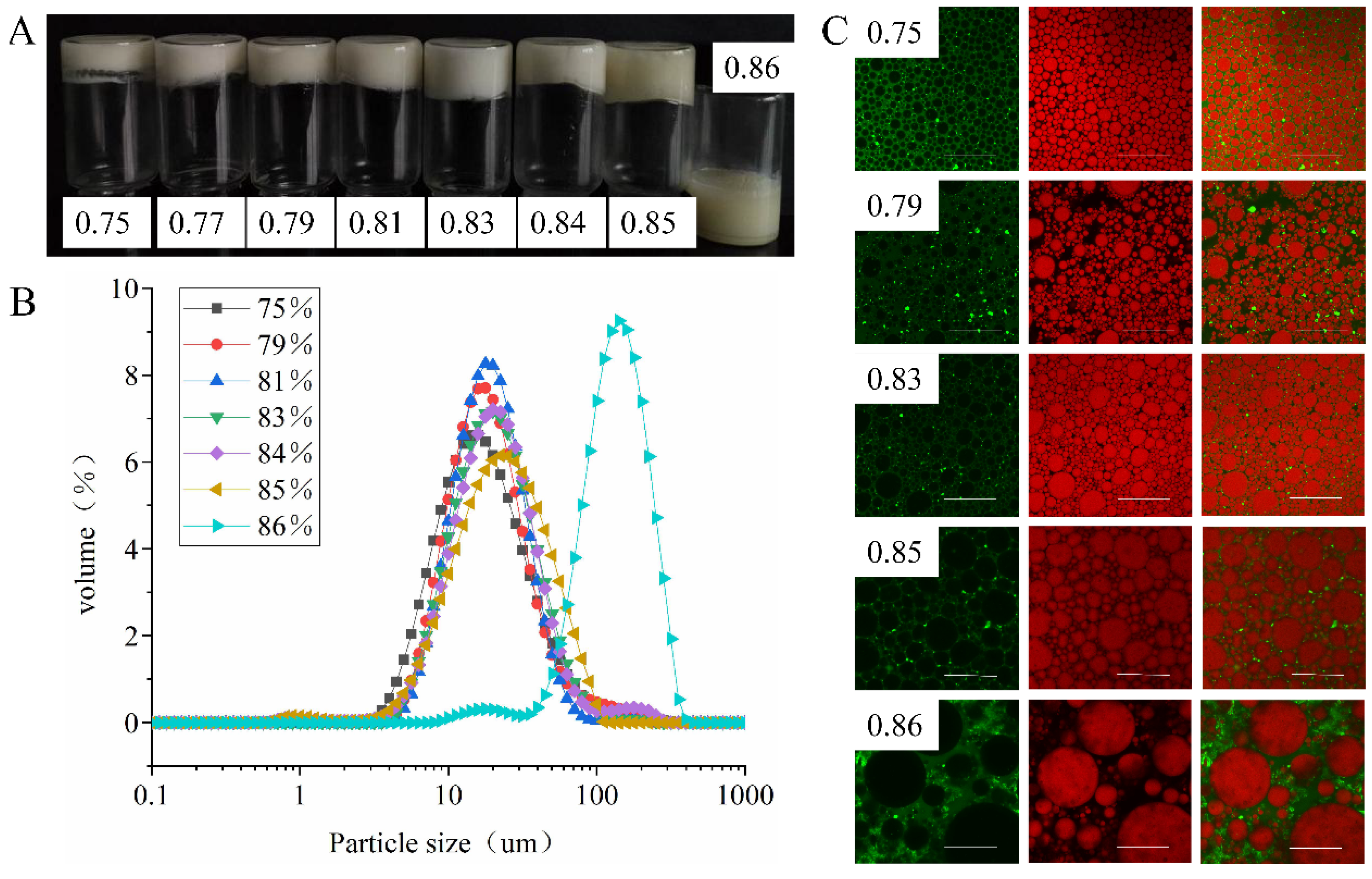
3.3.4. Effect of φ on Emulsion Properties
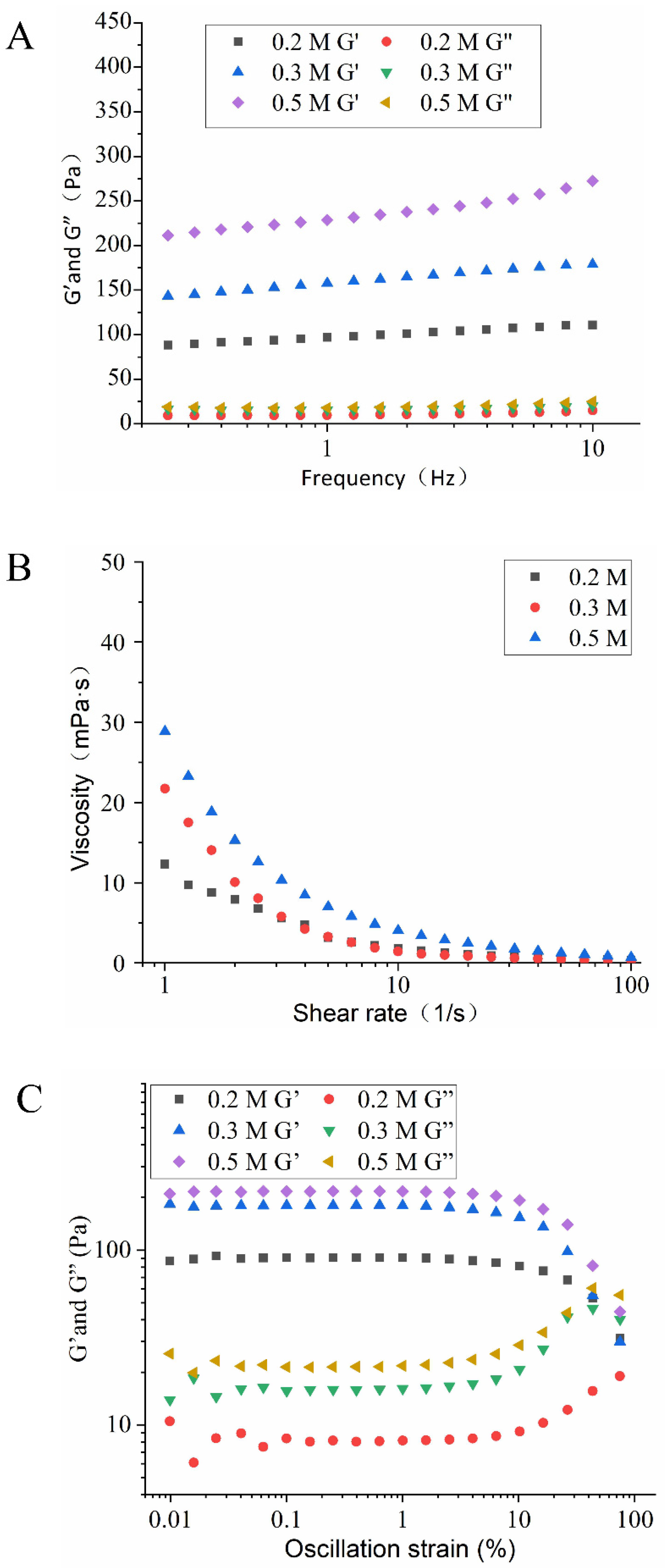
3.3.5. Rheological Properties of HIPEs
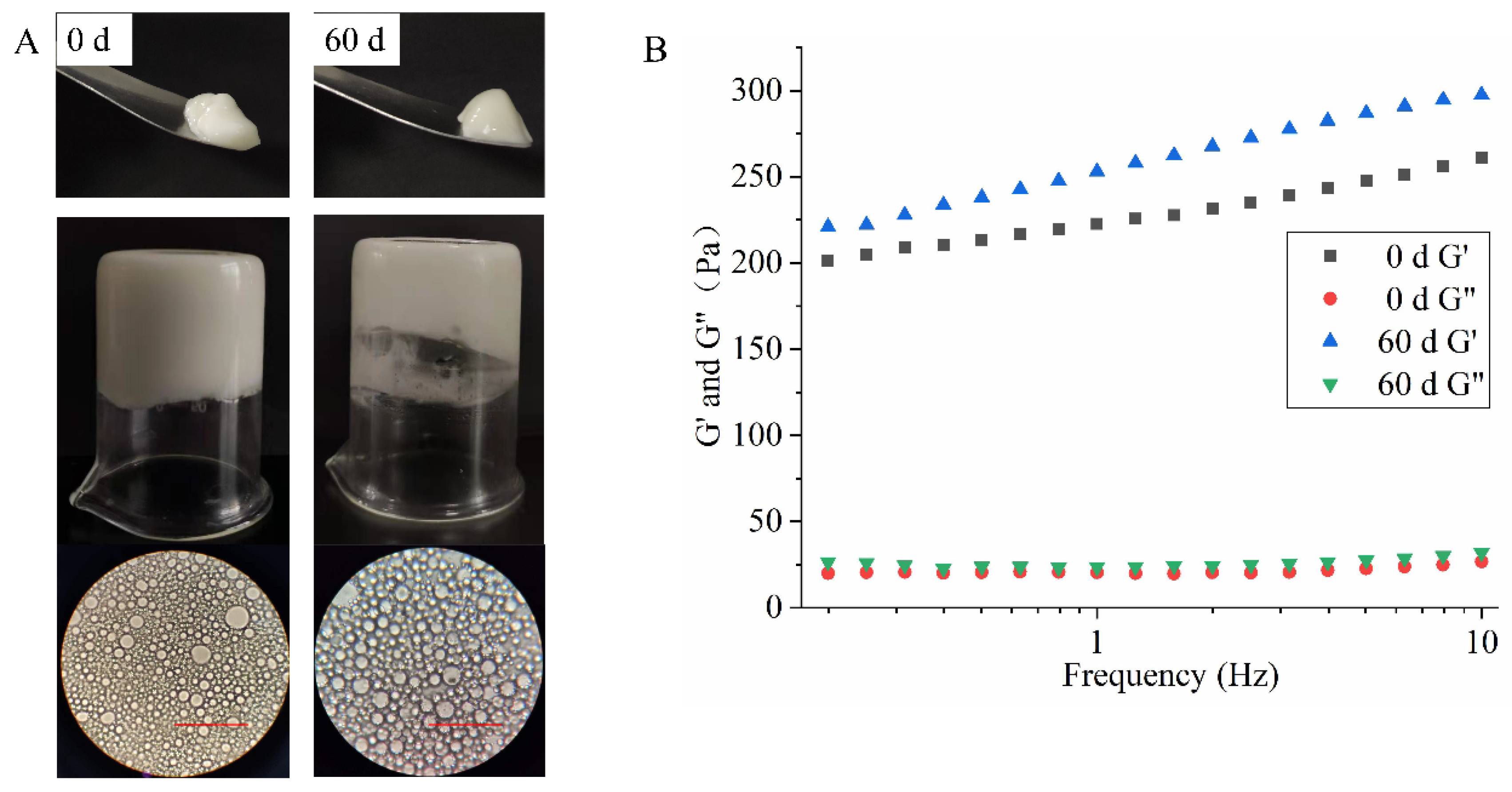
3.3.6. Storage Stability of HIPEs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Gu, L.; Su, Y.; Chang, C.; Dong, S.; Tang, X.; Yang, Y.; Li, J. Formation of egg yolk-modified starch complex and its stabilization effect on high internal phase emulsions. Carbohydr. Polym. 2020, 247, 116726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Z.; Huang, X.N.; Wu, Z.l.; Yin, S.W.; Zhu, J.h.; Tang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; Guo, J.; Wu, L.Y.; Tang, C.H.; Yang, X.Q. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Tan, H.; Sun, G.; Lin, W.; Mu, C.; Ngai, T. Gelatin Particle-Stabilized High Internal Phase Emulsions as Nutraceutical Containers. ACS Appl. Mater. Interfaces 2014, 6, 13977–13984. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, L.; Tian, S.; Wen, H.; Gou, X.; Ngai, T. Gelatin Particle-Stabilized High-Internal Phase Emulsions for Use in Oral Delivery Systems: Protection Effect and in Vitro Digestion Study. J. Agric. Food Chem. 2017, 65, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.M. Casein nanogels as effective stabilizers for Pickering high internal phase emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123662. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, A. Novel Pickering High Internal Phase Emulsion Stabilized by Food Waste-Hen Egg Chalaza. Foods 2021, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Guo, B.; Hu, X.; Wu, J.; Chen, R.; Dai, T.; Liu, Y.; Luo, S.; Liu, C. Soluble starch/whey protein isolate complex-stabilized high internal phase emulsion: Interaction and stability. Food Hydrocoll. 2021, 111, 106377. [Google Scholar] [CrossRef]
- Xu, Y.T.; Tang, C.H.; Liu, T.X.; Liu, R. Ovalbumin as an Outstanding Pickering Nanostabilizer for High Internal Phase Emulsions. J. Agric. Food Chem. 2018, 66, 8795–8804. [Google Scholar] [CrossRef]
- Li, Q.; Tang, S.; Mourad, F.K.; Zou, W.; Lu, L.; Cai, Z. Emulsifying stability of enzymatically hydrolyzed egg yolk granules and structural analysis. Food Hydrocoll. 2020, 101, 105521. [Google Scholar] [CrossRef]
- Motta-Romero, H.; Zhang, Z.; An Tien, N.; Schlegel, V.; Zhang, Y. Isolation of Egg Yolk Granules as Low-Cholesterol Emulsifying Agent in Mayonnaise. J. Food Sci. 2017, 82, 1588–1593. [Google Scholar] [CrossRef]
- Causeret, D.; Matringe, E.; Lorient, D. Ionic-strength and pH effects on composition and microstructure of yolk granules. J. Food Sci. 1991, 56, 1532–1536. [Google Scholar] [CrossRef]
- Anton, M. Egg yolk: Structures, functionalities and processes. J. Sci. Food Agric. 2013, 93, 2871–2880. [Google Scholar] [CrossRef]
- Wang, A.; Xiao, Z.; Wang, J.; Li, G.; Wang, L. Fabrication and characterization of emulsion stabilized by table egg-yolk granules at different pH levels. J. Sci. Food Agric. 2020, 100, 1470–1478. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Xia, M.; Zeng, Q.; Cai, Z. Intelligent colorimetric film incorporated with anthocyanins-loaded ovalbumin-propylene glycol alginate nanocomplexes as a stable pH indicator of monitoring pork freshness. Food Chem. 2021, 368, 130825. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Mourad, F.K.; Zhang, X.; Ahn, D.U.; Cai, Z.; Jin, Y. Phase separation behavior and characterization of ovalbumin and propylene glycol alginate complex coacervates. Food Hydrocoll. 2020, 108, 105978. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, Q.; Zhou, X.; Tang, C.; Chen, Y.; Cai, Z. Changes in protein structure and physicochemical properties of egg white by super critical carbon dioxide treatment. J. Food Eng. 2020, 284, 110076. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Chen, X.; McClements, D.J.; Zou, L.; Liu, W. Pickering-stabilized emulsion gels fabricated from wheat protein nanoparticles: Effect of pH, NaCl and oil content. J. Dispers. Sci. Technol. 2018, 39, 826–835. [Google Scholar] [CrossRef]
- Gmach, O.; Bertsch, A.; Bilke-Krause, C.; Kulozik, U. Impact of oil type and pH value on oil-in-water emulsions stabilized by egg yolk granules. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123788. [Google Scholar] [CrossRef]
- Anton, M.; Gandemer, G. Composition, Solubility and Emulsifying Properties of Granules and Plasma of Egg Yolk. J. Food Sci. Technol. 2010, 62, 484–487. [Google Scholar] [CrossRef]
- Geng, F.; Xie, Y.; Wang, Y.; Wang, J. Depolymerization of chicken egg yolk granules induced by high-intensity ultrasound. Food Chem. 2021, 354, 129580. [Google Scholar] [CrossRef]
- Li, T.; Su, H.; Zhu, J.; Fu, Y. Janus effects of NaCl on structure of egg yolk granules. Food Chem. 2022, 371, 131077. [Google Scholar] [CrossRef] [PubMed]
- Laca, A.; Paredes, B.; Rendueles, M.; Diaz, M. Egg yolk granules: Separation, characteristics and applications in food industry. LWT-Food Sci. Technol. 2014, 59, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Singh, V.; Behrens, S.H. Image Charge Effects on the Formation of Pickering Emulsions. J. Phys. Chem. 2012, 3, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- Li, M.A.; Lz, A.; Mc, B.; Wei, L. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: Gliadin nanoparticles/gum Arabic. Food Hydrocoll. 2020, 100, 105381. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mine, Y. Characterization of oil-in-water emulsions stabilized by hen’s egg yolk granule. Food Hydrocoll. 1998, 12, 203–210. [Google Scholar] [CrossRef]
- Denmat, M.L.; Anton, M.; Beaumal, V. Characterisation of emulsion properties and of interface composition in O/W emulsions prepared with hen egg yolk, plasma and granules. Food Hydrocoll. 2000, 14, 539–549. [Google Scholar] [CrossRef]
- Xu, Y.T.; Liu, T.X.; Tang, C.H. Novel pickering high internal phase emulsion gels stabilized solely by soy beta-conglycinin. Food Hydrocoll. 2019, 88, 21–30. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, J.; Rousseau, D.; Tang, C.H. Chitosan-stabilized emulsion gels via pH-induced droplet flocculation. Food Hydrocoll. 2020, 105, 105811. [Google Scholar] [CrossRef]
- Zamani, S.; Malchione, N.; Selig, M.J.; Abbaspourrad, A. Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels. Food Funct. 2018, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, J.; Wan, Z.-L.; Liu, Y.-Y.; Ruan, Q.-J.; Yang, X.-Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Zhu, Y.; Xu, S.; Liu, X. Influence of ionic strength on gel-like Pickering emulsions stabilized by self-assembled colloidal nanoparticles containing lysozyme. Colloid Polym. Sci. 2020, 298, 1249–1262. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, X.; Zhou, W.; Zhang, L.; Liu, F.; Li, J.; Peng, S.; Cao, Y.; Li, Y.; Li, R.; et al. Fabrication and stability of Pickering emulsions using moringa seed residue protein: Effect of pH and ionic strength. Int. J. Food Sci. Tech. 2021, 56, 3484–3494. [Google Scholar] [CrossRef]
- Li, R.; He, Q.; Guo, M.; Yuan, J.; Wu, Y.; Wang, S.; Rong, L.; Li, J. Universal and simple method for facile fabrication of sustainable high internal phase emulsions solely using meat protein particles with various pH values. Food Hydrocoll. 2020, 100, 105444. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, S.; Xia, M.; Zhang, X.; Liu, J.; Cai, Z. Formation of Natural Egg Yolk Granule Stabilized Pickering High Internal Phase Emulsions by Means of NaCl Ionic Strength and pH Change. Foods 2022, 11, 229. https://doi.org/10.3390/foods11020229
Mi S, Xia M, Zhang X, Liu J, Cai Z. Formation of Natural Egg Yolk Granule Stabilized Pickering High Internal Phase Emulsions by Means of NaCl Ionic Strength and pH Change. Foods. 2022; 11(2):229. https://doi.org/10.3390/foods11020229
Chicago/Turabian StyleMi, Sijie, Minquan Xia, Xinyue Zhang, Jihong Liu, and Zhaoxia Cai. 2022. "Formation of Natural Egg Yolk Granule Stabilized Pickering High Internal Phase Emulsions by Means of NaCl Ionic Strength and pH Change" Foods 11, no. 2: 229. https://doi.org/10.3390/foods11020229
APA StyleMi, S., Xia, M., Zhang, X., Liu, J., & Cai, Z. (2022). Formation of Natural Egg Yolk Granule Stabilized Pickering High Internal Phase Emulsions by Means of NaCl Ionic Strength and pH Change. Foods, 11(2), 229. https://doi.org/10.3390/foods11020229