Impact of the Plastein Reaction of Casein Hydrolysates in the Presence of Exogenous Amino Acids on Their Anti-Inflammatory Effect in the Lipopolysaccharide-Stimulated Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Plastein Reaction of Casein Hydrolysates
2.3. Cell Culture and Treatment
2.4. Assays of Protein and Free Amino Group Contents
2.5. Determination of Growth Proliferation and Macrophage Phagocytosis
2.6. Determination of LDH Release and ROS Level
2.7. Measurement of NO, PGE2, TNF-α, IL-6, TGF-β1, and IL-10 Secretion
2.8. Quantitative Real-Time PCR Analysis
2.9. Western Blot Analysis
2.10. Immunofluorescent Analysis
2.11. Statistical Analysis
3. Results
3.1. Chemical Features of the Modifiers and Their Effects on Cell Growth and Phagocytosis
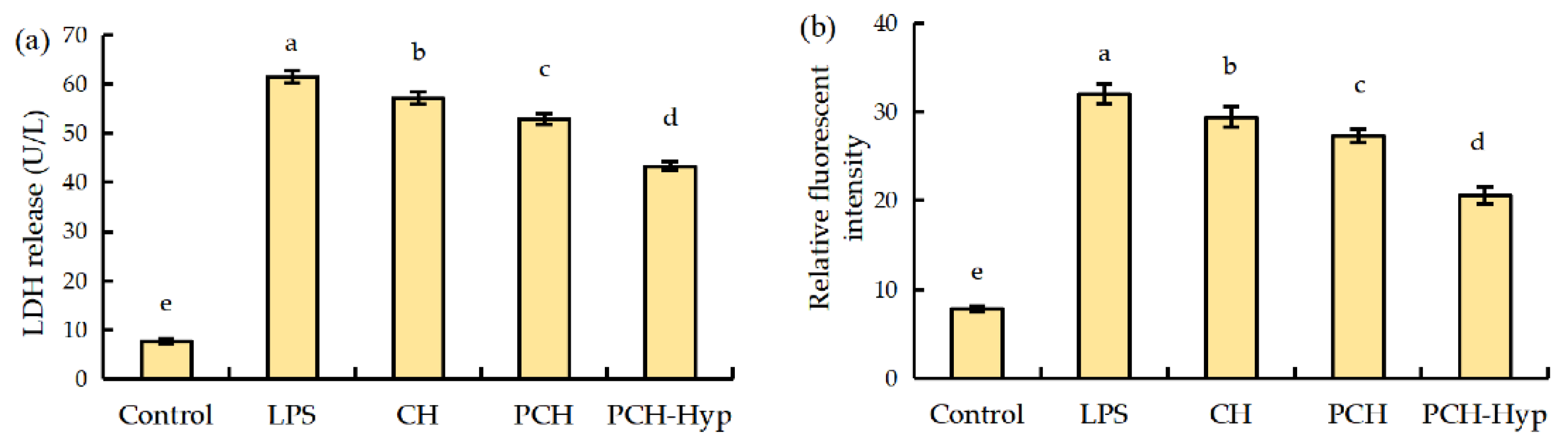
3.2. LDH Release and ROS Formation in Macrophages in Response to the LPS and Modifiers
3.3. Effect of the Modifiers on Inflammatory and Anti-Inflammatory Mediators in LPS-Injured Macrophages
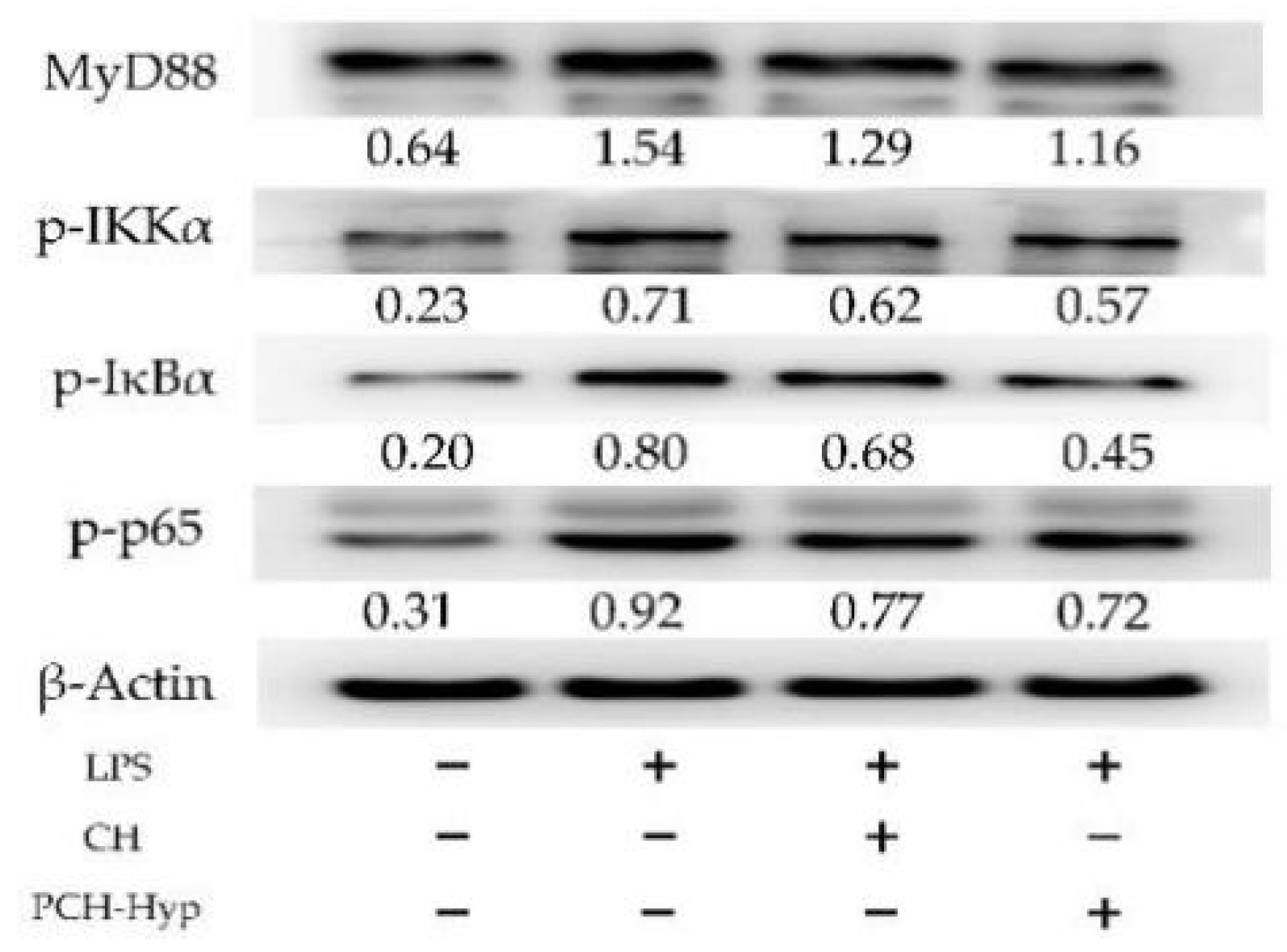
3.4. Effect of the Modifiers on Genes and Proteins Involved in the NF-κB Signaling Pathway
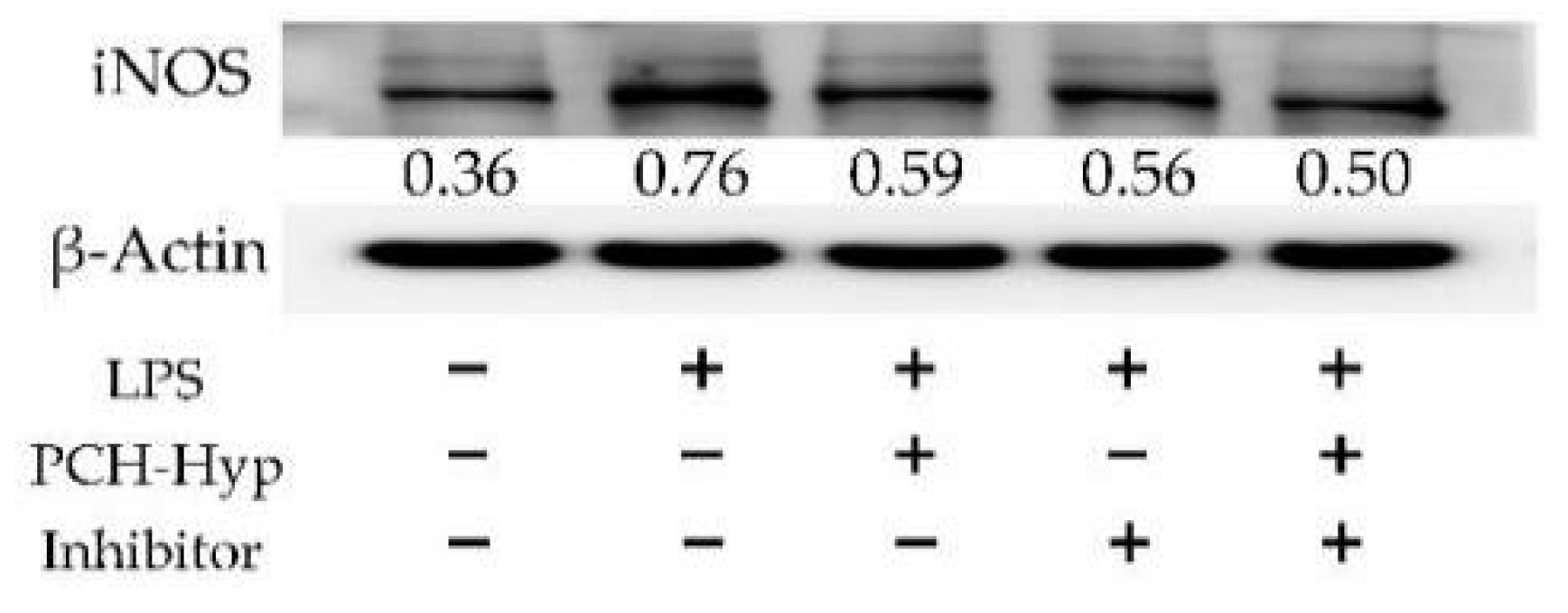
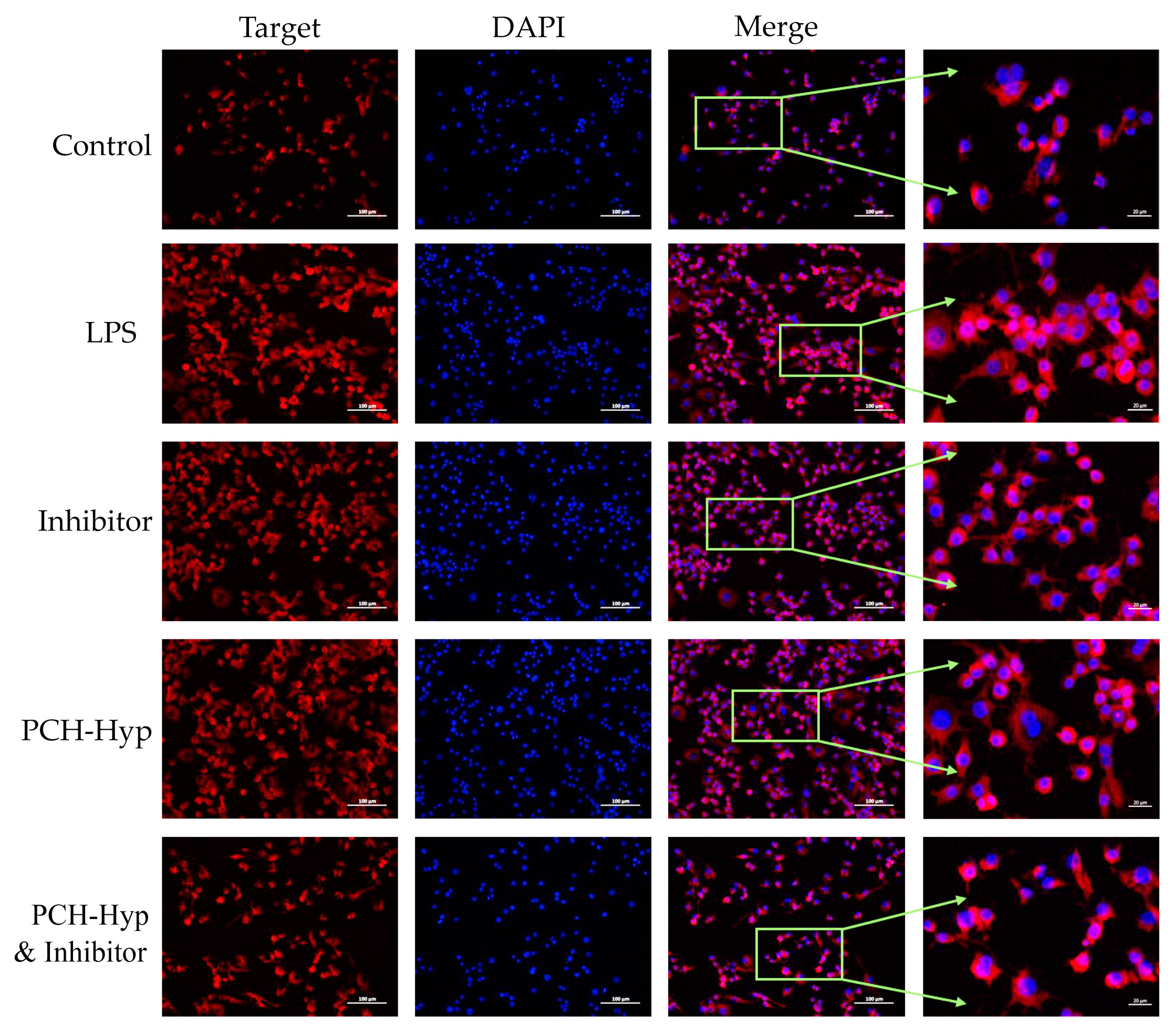
3.5. Inhibition of the Modifiers on the NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udenigwe, C.C.; Mohan, A.; Wu, S.H. Peptide aggregation during plastein reaction enhanced bile acid-binding capacity of enzymatic chicken meat hydrolysates. J. Food Biochem. 2015, 39, 344–348. [Google Scholar] [CrossRef]
- Sukarno; Marlia, L.; Yusnita, D.; Heryani; Santoso, J.; Nurhayati, T. Studies on protease from the digestive tract of tiger shrimp: Production of fish protein concentrate through plastein reaction. Fish. Sci. 2002, 68, 1335–1338. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.C.; Feng, Z.B.; Liu, C.H. A statistical experimental design to plastein synthesis with the mixture of soy protein isolate (SPI) hydrolysate and whey protein isolate (WPI) hydrolysate. Adv. Mater. Res. 2011, 236–238, 2773–2779. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, W.; Feng, Z.B.; Li, D.M. Aggregation of whey protein hydrolysate using alcalase 2.4 L. PLoS ONE 2014, 9, e109439. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Wu, S.H.; Drummond, K.; Gong, M. Revisiting the prospects of plastein: Thermal and simulated gastric stability in relation to the antioxidative capacity of casein plastein. J. Agric. Food Chem. 2014, 62, 130–135. [Google Scholar] [CrossRef]
- Li, Q.; Fu, Y.; Zhang, L.T.; Otte, J.; Lametsch, R. Plastein from hydrolysates of porcine hemoglobin and meat using Alcalase and papain. Food Chem. 2020, 320, 126654. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Rajendran, S.R.C.K. Old products, new applications? Considering the multiple bioactivities of plastein in peptide-based functional food design. Curr. Opin. Food Sci. 2016, 8, 8–13. [Google Scholar] [CrossRef]
- Jiang, S.S.; Zhao, Y.H.; Shen, Q.Q.; Zhu, X.J.; Dong, S.Y.; Liu, Z.Y.; Wu, H.H.; Zeng, M.Y. Modification of ACE-inhibitory peptides from Acaudinamolpadioidea using the plastein reaction and examination of its mechanism. Food Biosci. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Gao, D.D.; Guo, P.H.; Cao, X.; Ge, L.L.; Ma, H.X.; Cheng, H.; Ke, Y.Q.; Chen, S.E.; Ding, G.T.; Feng, R.F.; et al. Improvement of chicken plasma protein hydrolysate angiotensin I-converting enzyme inhibitory activity by optimizing plastein reaction. Food Sci. Nutr. 2020, 8, 2798–2808. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhao, X.H. In vitro calcium-chelating and platelet anti-aggregation activities of soy protein hydrolysate modified by the alcalase-catalyzed plastein reaction. J. Food Biochem. 2014, 38, 374–380. [Google Scholar] [CrossRef]
- Zhao, X.H.; Fu, Y.; Yue, N. In vitro cytoprotection of modified casein hydrolysates by plastein reaction on rat hepatocyte cells. CYTA-J. Food 2014, 12, 40–47. [Google Scholar] [CrossRef]
- Bo, L.Y.; Pang, J.N.; Song, C.L.; Li, T.J. Effect of the plastein reaction in presence of extrinsic amino acids on the protective activity of casein hydrolysate against ethanol-induced damage in HHL-5 cells. Foods 2019, 8, 112. [Google Scholar]
- Zhao, X.H.; Li, Y.Y. An approach to improve ACE-inhibitory activity of casein hydrolysates with plastein reaction catalyzed by Alcalase. Eur. Food Res. Technol. 2009, 229, 795–805. [Google Scholar] [CrossRef]
- Qian, F.; Wang, Y.; Wen, Z.J.; Jiang, S.J.; Tuo, Y.F.; Mu, G.Q. Plastein reaction enhanced bile-acid binding capacity of soybean protein hydrolysates and whey protein hydrolysates. J. Food Sci. Technol. 2018, 55, 1021–1027. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural marine anti-inflammatory products. Mini-Reviews Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Dadar, M.; Shahali, Y.; Chakraborty, S.; Prasad, M.; Tahoori, F.; Tiwari, R.; Dhama, K. Antiinflammatory peptides: Current knowledge and promising prospects. Inflamm. Res. 2019, 68, 125–145. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar]
- Shim, D.W.; Han, J.W.; Sun, X.; Jang, C.H.; Koppula, S.; Kim, T.J.; Kang, T.B.; Lee, K.H. Lysimachia clethroides Duby extract attenuates inflammatory response in Raw 264.7 macrophages stimulated with lipopolysaccharide and in acute lung injury mouse model. J. Ethnopharmacol. 2013, 150, 1007–1015. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, Y.H.; Zhang, L.; Yu, H.X.; Xu, Z.; You, H.X.; Cheng, Y.H. Rice protein hydrolysates (RPHs) inhibit the LPS-stimulated inflammatory response and phagocytosis in RAW264.7 macrophages by regulating the NF-κB signaling pathway. RSC Adv. 2016, 6, 71295–71304. [Google Scholar] [CrossRef]
- Heo, S.Y.; Ko, S.C.; Jung, W.K. The pepsinolytic hydrolysate from Johniusbelengerii frame inhibited LPS-stimulated production of pro-inflammatory mediators via the inactivating of JNK and NF-κB pathways in RAW 264.7 macrophages. Fish. Aquat. Sci. 2018, 21, 14. [Google Scholar] [CrossRef]
- Wang, N.G.; Geng, C.P.; Sun, H.Y.; Wang, X.; Li, F.M.; Liu, X.C. Hesperetin ameliorates lipopolysaccharide-induced acute lung injury in mice through regulating the TLR4–MyD88–NF-κB signaling pathway. Arch. Pharm. Res. 2019, 42, 1063–1070. [Google Scholar] [CrossRef]
- Diao, J.J.; Chi, Z.P.; Guo, Z.W.; Zhang, L.P. Mung Bean Protein Hydrolysate Modulates the Immune Response Through NF-κB Pathway in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Food Sci. 2019, 84, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculusnudus) in lipopolysaccharide-induced RAW264.7 macrophages. Food Funct. 2020, 11, 552–560. [Google Scholar] [CrossRef]
- Xu, J.L.; Pang, J.N.; Chen, F.F.; Li, T.J.; Zhao, X.H. Actividadantihipertensiva de plasteínaderivada de hidrolizados de caseínaenratas con hipertensiónespontánea. CYTA-J. Food 2017, 15, 105–109. [Google Scholar] [CrossRef]
- Wang, S.; Fu, Y.; Zhao, X.H. The cooperative effect of genistein and protein hydrolysates on the proliferation and survival of osteoblastic cells (hFOB 1.19). Molecules 2016, 21, 1489. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.H. The growth proliferation, apoptotic prevention, and differentiation induction of the gelatin hydrolysates from three sources to human fetal osteoblasts (hFOB 1.19 cells). Molecules 2018, 23, 1287. [Google Scholar] [CrossRef]
- Wang, M.C.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Cao, L.; Liu, J.; Zeng, X.X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fu, Y.; Zhao, X.H. Effects of Maillard-type caseinate glycation on the preventive action of caseinate digests in acrylamide-induced intestinal barrier dysfunction in IEC-6 cells. RSC Adv. 2018, 8, 38036–38046. [Google Scholar] [CrossRef]
- Wu, S.J.; Chen, Y.W.; Wang, C.Y.; Shyu, Y.T. Anti-inflammatory properties of high pressure-assisted extracts of Grifolafrondosa in lipopolysaccharide-activated RAW 264.7 macrophages. Int. J. Food Sci. Technol. 2017, 52, 671–678. [Google Scholar] [CrossRef]
- Shou, J.Q.; Kong, X.Z.; Wang, X.Y.; Tang, Y.; Wang, C.M.; Wang, M.; Zhang, L.F.; Liu, Y.C.; Fei, C.Z.; Xue, F.Q.; et al. Tizoxanide Inhibits Inflammation in LPS-Activated RAW264.7 Macrophages via the Suppression of NF-κB and MAPK Activation. Inflammation 2019, 42, 1336–1349. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Tsopmo, A. Reactivity of peptides within the food matrix. J. Food Biochem. 2019, 43, e12489. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Voorspoels, S.; Jacobs, G.; Matthijs, B.; Possemiers, S.; Van der Saag, H.; Van Camp, J.; Lucey, A. Clinical aspects of egg bioactive peptide research: A review. Int. J. Food Sci. Technol. 2019, 54, 1967–1975. [Google Scholar] [CrossRef]
- Katayama, S.; Nakamura, S. Emerging roles of bioactive peptides on brain health promotion. Int. J. Food Sci. Technol. 2019, 54, 1949–1955. [Google Scholar] [CrossRef]
- Chen, X.X.; Zheng, X.T.; Zhang, M.; Yin, H.F.; Jiang, K.F.; Wu, H.C.; Dai, A.L.; Yang, S.S. Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-κB signaling pathway. Inflamm. Res. 2018, 67, 903–911. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. Chemical features of the oligochitosan-glycated caseinate digest and its enhanced protection on barrier function of the acrylamide-injured IEC-6 cells. Food Chem. 2019, 290, 246–254. [Google Scholar] [CrossRef]
- Li, J.P.; Liu, Z.Y.; Zhao, Y.H.; Zhu, X.J.; Yu, R.L.; Dong, S.Y.; Wu, H.H. Novel natural angiotensin converting enzyme (ACE)-inhibitory peptides derived from sea cucumber-modified hydrolysates by adding exogenous proline and a study of their structure-activity relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef]
- Zhao, X.H.; Song, J.T. Evaluation of antioxidant properties in vitro of plastein-reaction-stressed soybean protein hydrolysate. Int. J. Food Prop. 2014, 17, 152–162. [Google Scholar] [CrossRef]
- Ma, C.M.; Li, T.J.; Zhao, X.H. Pepsin-catalyzed plastein reaction with tryptophan increases the in vitro activity of lactoferrin hydrolysates with BGC-823 cells. Food Biosci. 2019, 28, 109–115. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Huo, J.X.; Zhong, S.; Zhu, J.X.; Li, Y.G.; Li, X.J. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.G.; Yu, S.Y.; Li, C.E.; Kang, S.M. Protective effect of acacetin in human periodontal ligament cells via regulation of autophagy and inflammation. Pharmazie 2020, 75, 436–439. [Google Scholar] [CrossRef]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.K.; Kapila, S.; Kapila, R. Buffalo Milk Casein Derived Decapeptide (YQEPVLGPVR) Having Bifunctional Anti-inflammatory and Antioxidative Features Under Cellular Milieu. Int. J. Pept. Res. Ther. 2019, 25, 623–633. [Google Scholar] [CrossRef]
- Iskandar, M.M.; Dauletbaev, N.; Kubow, S.; Mawji, N.; Lands, L.C. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. Br. J. Nutr. 2013, 110, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; de Mejia, E.G. Protein hydrolysates from β-Conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation in vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Zhou, F.; Shen, C.; Wang, H.X.; Xiao, Y.D. LBP reduces the inflammatory injury of kidney in septic rat and regulates the keap1-Nrf2/ARE signaling pathway. Acta Cir. Bras. 2019, 34, e20190010000003. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Park, H.J.; Jeong, Y.Y.; Han, S.; Shin, J.H.; Lee, S.J.; Kang, M.J.; Sung, N.J.; Kang, D. Aged red garlic extract suppresses nitric oxide production in lipopolysaccharide-treated RAW 264.7 macrophages through inhibition of NF-κB. J. Med. Food 2015, 18, 439–445. [Google Scholar] [CrossRef]
- Tsou, Y.A.; Tung, Y.T.; Wu, T.F.; Chang, G.R.L.; Chen, H.C.; Lin, C.D.; Lai, C.H.; Chen, H.L.; Chen, C.M. Lactoferrin interacts with SPLUNC1 to attenuate lipopolysaccharide-induced inflammation of human nasal epithelial cells via down-regulated MEK1/2-MAPK signaling. Biochem. Cell Biol. 2017, 95, 394–399. [Google Scholar] [CrossRef]
- Kim, S.H.; Bang, J.; Son, C.N.; Baek, W.K.; Kim, J.M. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-κB signaling pathway. Korean J. Intern. Med. 2018, 33, 612–621. [Google Scholar] [CrossRef]
- Wang, Z.X.; Liang, M.C.; Li, H.; Cai, L.; Yang, L. Rice protein exerts anti-inflammatory effect in growing and adult rats via suppressing NF-κB pathway. Int. J. Mol. Sci. 2019, 20, 6164. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, H.J. Immunomodulatory effects of fermented platycodon grandiflorum extract through NF-κB signaling in raw 264.7 cells. Nutr. Res. Pract. 2020, 14, 453–462. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Theil, P.K.; Larsen, L.B.; Purup, S. Effect of milk hydrolysates on inflammation markers and drug-induced transcriptional alterations in cell-based models. J. Anim. Sci. 2012, 90, 403–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Sullivan, S.M.; O’Callaghan, Y.C.; O’Keeffe, M.B.; FitzGerald, R.J.; O’Brien, N.M. Immunomodulatory activity of 5 kDa permeate fractions of casein hydrolysates generated using a range of enzymes in Jurkat T cells and RAW264.7 macrophages. Int. Dairy J. 2019, 91, 9–17. [Google Scholar] [CrossRef]
- Li, T.G.; Cheng, X.; Du, M.; Chen, B.; Mao, X.Y. Upregulation of heme oxygenase-1 mediates the anti-inflammatory activity of casein glycomacropeptide (GMP) hydrolysates in LPS-stimulated macrophages. Food Funct. 2017, 8, 2475–2484. [Google Scholar] [CrossRef]
| Genes | Primer Sequences (5′-3′) | Lengths of Output (bp) |
|---|---|---|
| iNOS | Forward: 5′-ACT CAG CCA AGC CCT CAC CTA C-3′ Reverse: 5′-TCC AAT CTC TGC CTA TCC GTC TCG-3′ | 111 |
| IL-6 | Forward: 5′-AGA CAG CCA CCA CAC TGG AGA TAG-3′ Reverse: 5′-CCT GCC TCC TGT TGA TGT GAA GTC-3′ | 149 |
| TNF-α | Forward: 5′-GCC TCT TCT CAT TCC TGC TTG TGG-3′ Reverse: 5′-GTG GTT TGT GAG TGT GAG GGT CTG-3′ | 149 |
| IL-1β | Forward: 5′-TCG CAG CAG CAC ATC AAC AAG AG-3′ Reverse: 5′-AGG TCC ACG GGA AAG ACA CAG G-3′ | 97 |
| COX-2 | Forward: 5′-GGT GCC TGG TCT GAT GAT GTA TGC-3′ Reverse: 5′-GGA TGC TCC TGC TTG AGT ATG TCG-3′ | 81 |
| IL-10 | Forward: 5′-TCCCTGGGTGAGAAGCTGAAGAC-3′ Reverse: 5′-CACCTGCTCCACTGCCTTGC-3′ | 96 |
| TGF-β1 | Forward: 5′-ACCGCAACAACGCCATCTATGAG-3′ Reverse: 5′-GGCACTGCTTCCCGAATGTCTG-3′ | 91 |
| TLR4 | Forward: 5′-CCGCTTTCACCTCTGCCTTCAC-3′ Reverse: 5′-ACCACAATAACCTTCCGGCTCTTG-3′ | 105 |
| β-actin | Forward: 5′-CGCAAAGACCTGTATGCCAAT-3′ Reverse: 5′-GGGCTGTGATCTCCTTCTGC-3′ | 174 |
| miR-181a | Forward: 5′-CGAACATTCAACGCTGTCG-3′ Reverse: 5′-AGTGCAGGGTCCGAGGTATT-3′ | 60 |
| miR-30d | Forward: 5′-GCGTGTAAACATCCCCGAC-3′ Reverse: 5′-AGTGCAGGGTCCGAGGTATT-3′ | 60 |
| miR-155 | Forward: 5′-GCGCGTTAATGCTAATTGTGAT-3′ Reverse: 5′-AGTGCAGGGTCCGAGGTATT-3′ | 60 |
| miR-148a | Forward: 5′-GCGCGTCAGTGCACTACAGAA-3′ Reverse: 5′-AGTGCAGGGTCCGAGGTATT-3′ | 60 |
| U6 | Forward: 5′-AGGTATTCGCACTGGATACGAC-3′ Reverse: 5′-AGTGCAGGGTCCGAGGTATT-3′ | 60 |
| Sample | Classification | −NH2 Contents (mmol/g Protein) |
|---|---|---|
| CH | Casein hydrolysates | 1.160 ± 0.002 f |
| PCH | The modifier of CH | 0.935 ± 0.002 g |
| PCH-Gly | The modifier of CH and Gly | 1.433 ± 0.003 e |
| PCH-Pro | The modifier of CH and Pro | 1.451 ± 0.004 d |
| PCH-Hyp | The modifier of CH and Hyp | 1.456 ± 0.003 d |
| CH-Gly | The mixture of CH and Gly | 1.792 ± 0.006 c |
| CH-Pro | The mixture of CH and Pro | 1.807 ± 0.006 b |
| CH-Hyp | The mixture of CH and Hyp | 1.820 ± 0.005 a |
| Sample | Cell Viability at Different Dose Levels (μg/mL) | ||
|---|---|---|---|
| 25 | 50 | 100 | |
| CH | 102.5 ± 2.2 Ae | 110.2 ± 3.7 Bd | 111.7 ± 3.5 Bd |
| PCH | 111.8 ± 4.6 Aabcd | 115.2 ± 4.9 Abcd | 119.1 ± 1.8 Abc |
| PCH-Gly | 114.3 ± 6.3 Aab | 121.3 ± 5.5 Aab | 124.4 ± 2.6 Ab |
| PCH-Pro | 113.7 ± 3.9 Babc | 119.6 ± 4.2 ABabc | 122.9 ± 3.5 Ab |
| PCH-Hyp | 115.4 ± 5.6 Ba | 125.9 ± 4.1 Aa | 129.7 ± 2.2 Aa |
| CH-Gly | 103.9 ± 2.5 Bde | 112.5 ± 5.5 Abcd | 113.3 ± 4.2 Ad |
| CH-Pro | 105.6 ± 3.3 Acd | 112.1 ± 6.0 Acd | 112.7 ± 1.8 Ad |
| CH-Hyp | 106.4 ± 4.9 Bbcde | 113.7 ± 2.8 ABbcd | 114.5 ± 3.3 Acd |
| Sample | Dose Levels (μg/mL) | ||
|---|---|---|---|
| 25 | 50 | 100 | |
| CH | 2.01 ± 0.04 Aa | 1.96 ± 0.03 Aa | 1.95 ± 0.05 Aa |
| PCH | 1.77 ± 0.05 Ac | 1.74 ± 0.03 Ac | 1.72 ± 0.03 Ac |
| PCH-Gly | 1.73 ± 0.04 Ac | 1.61 ± 0.04 Bd | 1.61 ± 0.045 Bd |
| PCH-Pro | 1.75 ± 0.06 Ac | 1.71 ± 0.05 Ac | 1.70 ± 0.06 Ac |
| PCH-Hyp | 1.71 ± 0.04 Ac | 1.55 ± 0.02 Bd | 1.52 ± 0.04 Be |
| CH-Gly | 1.94 ± 0.05 Aab | 1.90 ± 0.05 Aab | 1.87 ± 0.03 Aab |
| CH-Pro | 1.93 ± 0.06 Aab | 1.92 ± 0.04 Acd | 1.90 ± 0.04 Aa |
| CH-Hyp | 1.87 ± 0.04 Ab | 1.86 ± 0.04 Ab | 1.81 ± 0.06 Ab |
| Mediator | The Cells with Different Treatments | ||||
|---|---|---|---|---|---|
| Control | LPS | CH | PCH | PCH-Hyp | |
| NO | 1.6 ± 0.3 e | 45.7 ± 1.0 a | 39.3 ± 0.6 b | 36.9 ± 0.8 c | 31.3 ± 0.9 d |
| PGE2 | 21.8 ± 2.9 e | 169.2 ± 3.3 a | 158.4 ± 3.2 b | 148.2 ± 2.2 c | 119.3 ± 2.8 d |
| TNF-α | 32.8 ± 1.2 e | 3945.8 ± 60.8 a | 3490.2 ± 71.3 b | 3266.7 ± 65.6 c | 2673.4 ± 49.3 d |
| IL-6 | 1.5 ± 0.1 e | 673.0 ± 14.8 a | 527.4 ± 15.3 b | 456.7 ± 15.9 c | 354.5 ± 9.2 d |
| IL-10 | 14.6 ± 2.1 e | 254.9 ± 9.4 d | 289.8 ± 6.2 c | 322.5 ± 8.0 b | 445.1 ± 8.9 a |
| TGF-β1 | 23.7 ± 4.5 d | 214.3 ± 5.2 c | 219.3 ± 4.8 c | 246.5 ± 6.7 b | 332.0 ± 8.1 a |
| Gene | The Cells with Different Treatments | ||||
|---|---|---|---|---|---|
| Control | LPS | CH | PCH | PCH-Hyp | |
| iNOS | 1.00 | 2.41 ± 0.04 | 2.29 ± 0.03 | 1.93 ± 0.16 | 1.71 ± 0.12 |
| IL-6 | 1.00 | 2.47 ± 0.05 | 2.28 ± 0.04 | 1.92 ± 0.28 | 1.84 ± 0.09 |
| TNF-α | 1.00 | 1.89 ± 0.08 | 1.81 ± 0.09 | 1.7 3± 0.14 | 1.68 ± 0.03 |
| IL-1β | 1.00 | 2.25 ± 0.13 | 2.19 ± 0.11 | 2.03 ± 0.07 | 1.80 ± 0.08 |
| COX-2 | 1.00 | 2.14 ± 0.03 | 1.97 ± 0.05 | 1.73 ± 0.04 | 1.67 ± 0.05 |
| TLR4 | 1.00 | 1.91 ± 0.02 | 1.86 ± 0.15 | 1.79 ± 0.13 | 1.73 ± 0.07 |
| IL-10 | 1.00 | 1.45 ± 0.02 | 1.53 ± 0.12 | 1.61 ± 0.19 | 1.68 ± 0.12 |
| TGF-β1 | 1.00 | 1.36 ± 0.06 | 1.38 ± 0.07 | 1.47 ± 0.02 | 1.51 ± 0.11 |
| miR-181a | 1.00 | 0.78 ± 0.04 | 1.43 ± 0.08 | 1.51 ± 0.06 | 1.75 ± 0.06 |
| miR-30d | 1.00 | 0.61 ± 0.05 | 0.67 ± 0.04 | 0.74 ± 0.04 | 0.77 ± 0.09 |
| miR-155 | 1.00 | 1.87 ± 0.06 | 1.80 ± 0.04 | 1.66 ± 0.04 | 1.59 ± 0.02 |
| miR-148a | 1.00 | 1.55 ± 0.19 | 1.51 ± 0.03 | 1.45 ± 0.07 | 1.37 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-J.; Zhao, X.-H. Impact of the Plastein Reaction of Casein Hydrolysates in the Presence of Exogenous Amino Acids on Their Anti-Inflammatory Effect in the Lipopolysaccharide-Stimulated Macrophages. Foods 2022, 11, 196. https://doi.org/10.3390/foods11020196
Shi Y-J, Zhao X-H. Impact of the Plastein Reaction of Casein Hydrolysates in the Presence of Exogenous Amino Acids on Their Anti-Inflammatory Effect in the Lipopolysaccharide-Stimulated Macrophages. Foods. 2022; 11(2):196. https://doi.org/10.3390/foods11020196
Chicago/Turabian StyleShi, Yun-Jiao, and Xin-Huai Zhao. 2022. "Impact of the Plastein Reaction of Casein Hydrolysates in the Presence of Exogenous Amino Acids on Their Anti-Inflammatory Effect in the Lipopolysaccharide-Stimulated Macrophages" Foods 11, no. 2: 196. https://doi.org/10.3390/foods11020196
APA StyleShi, Y.-J., & Zhao, X.-H. (2022). Impact of the Plastein Reaction of Casein Hydrolysates in the Presence of Exogenous Amino Acids on Their Anti-Inflammatory Effect in the Lipopolysaccharide-Stimulated Macrophages. Foods, 11(2), 196. https://doi.org/10.3390/foods11020196







