Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Preparation of Fungal Spore Suspensions
2.3. Preparation of LAB Fermentation Cell-Free Supernatant (CFS)
2.4. Screening of LAB Strains for Antifungal Properties In Vitro
2.4.1. Preliminary Screening
2.4.2. Rescreening
2.5. Identification of ZZUA493 Strain
2.5.1. Morphological, Physiological and Biochemical Tests of LAB
2.5.2. Drawing of Growth Curve of LAB
2.5.3. 16S rRNA Gene Sequencing
2.6. Studies on the Antifungal Activity and Properties of Antifungal Substances of ZZUA493
2.6.1. Antifungal Activity of ZZUA493 CFS
2.6.2. Protease Treatment
2.6.3. Hydrogen Peroxide Removal
2.6.4. Acid Exclusion
2.6.5. Heat Treatment
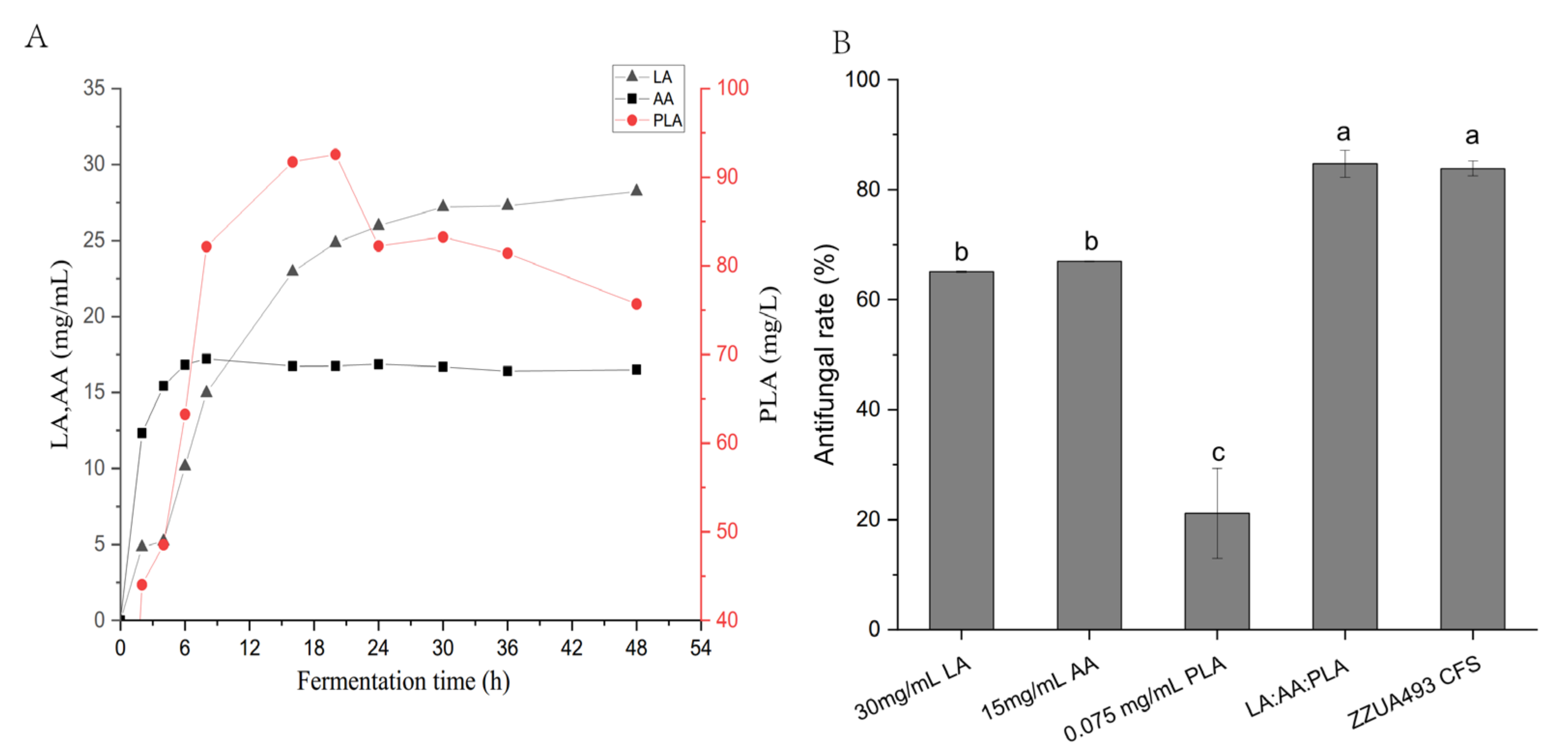
2.6.6. Determination of Organic Acid Content of ZZUA493 CFS
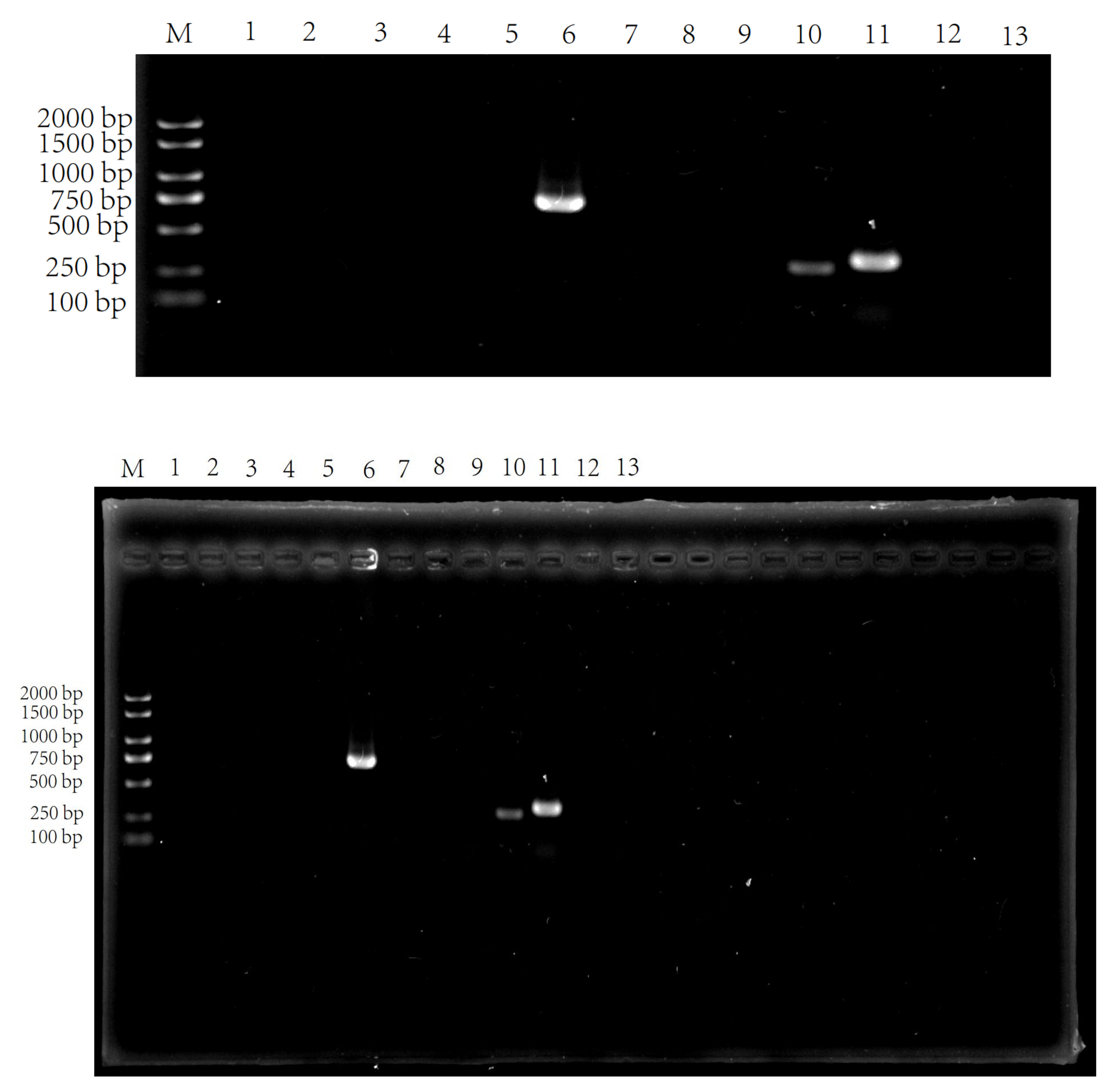
2.7. Cloning of Genes Associated with the Related of Bacteriocin of ZZUA493
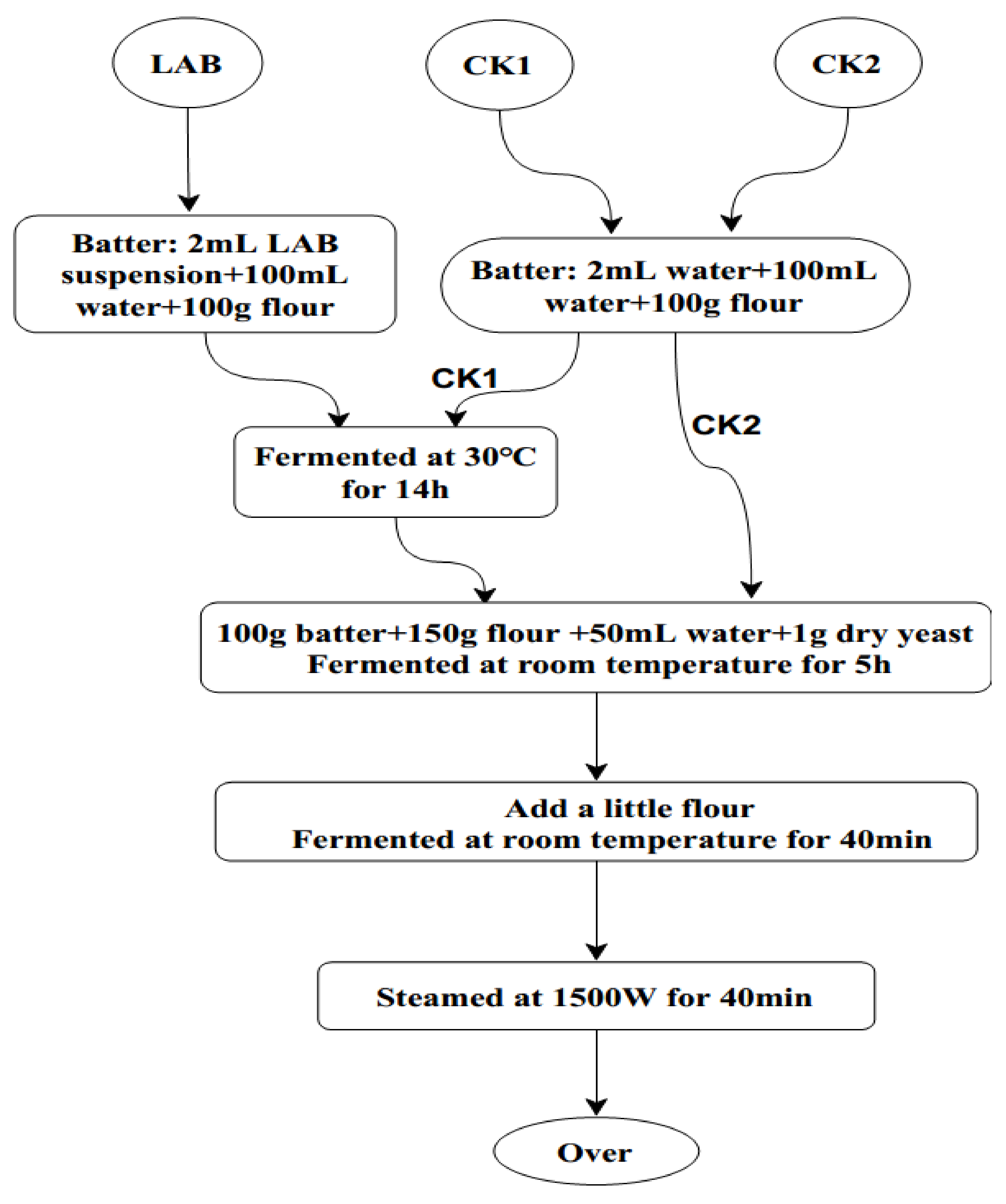
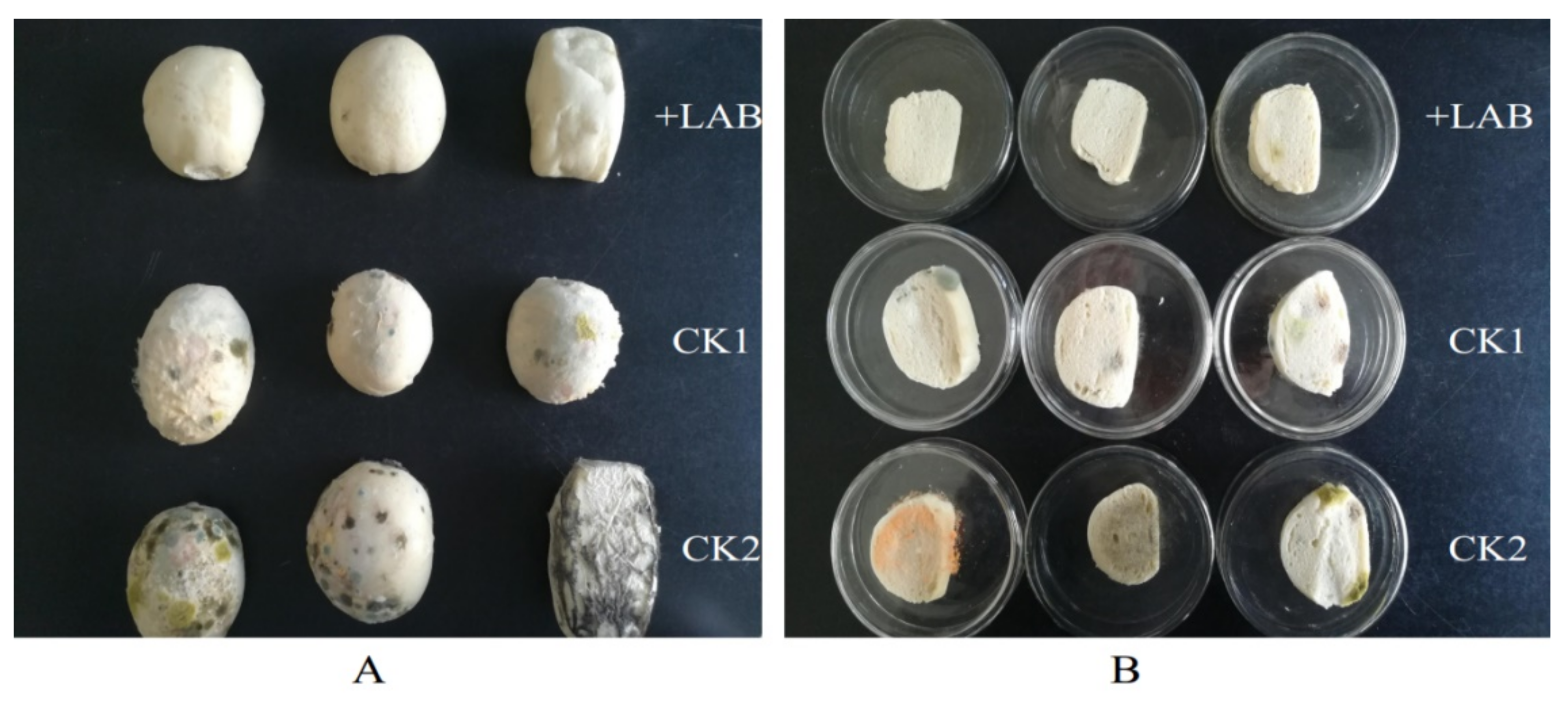
2.8. Testing of ZZUA493 for Extending the Shelf-Life of CSB
2.9. Statistical Analysis
3. Results and Discussion
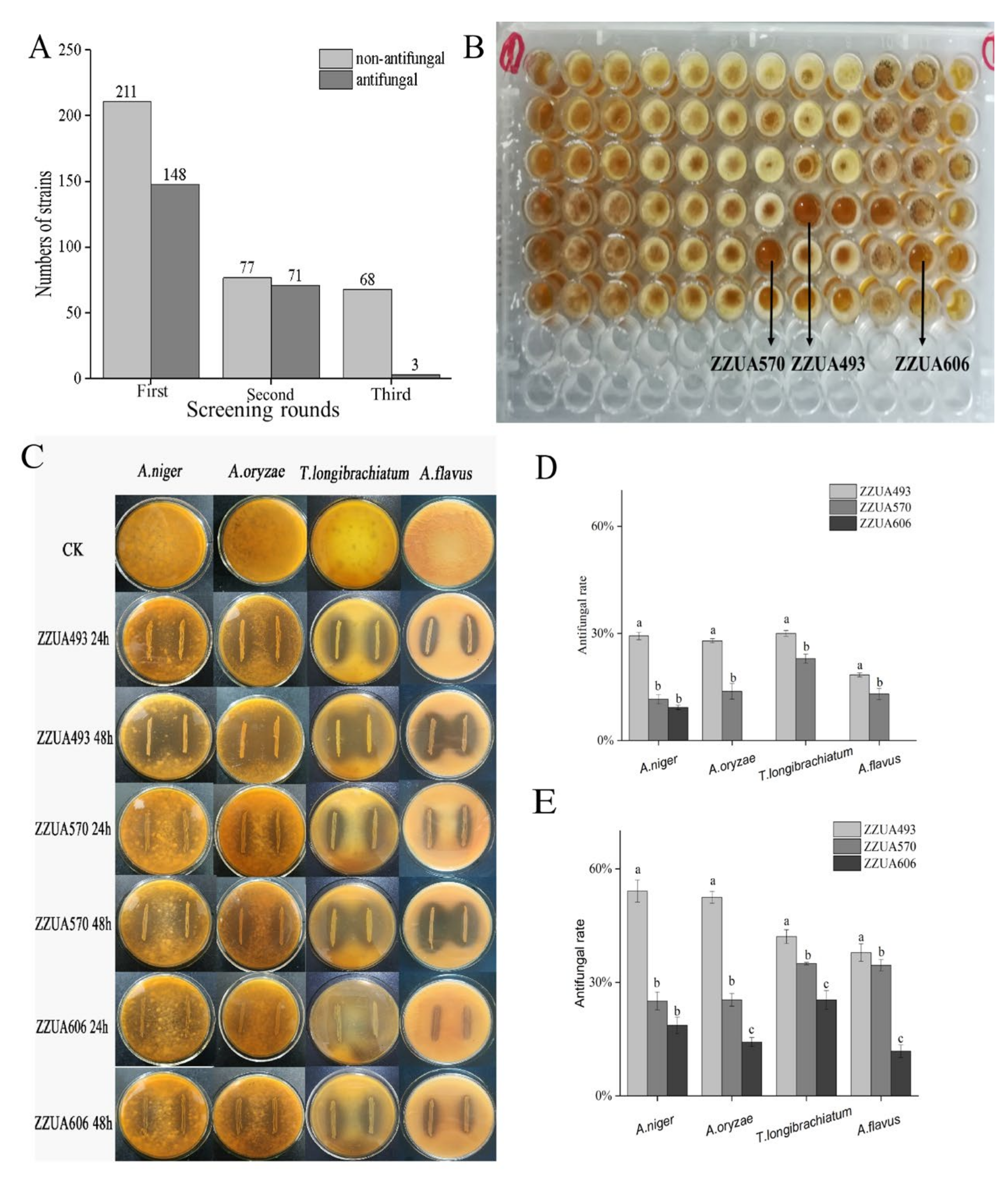
3.1. Screening of LAB Strains for Antifungal Activity
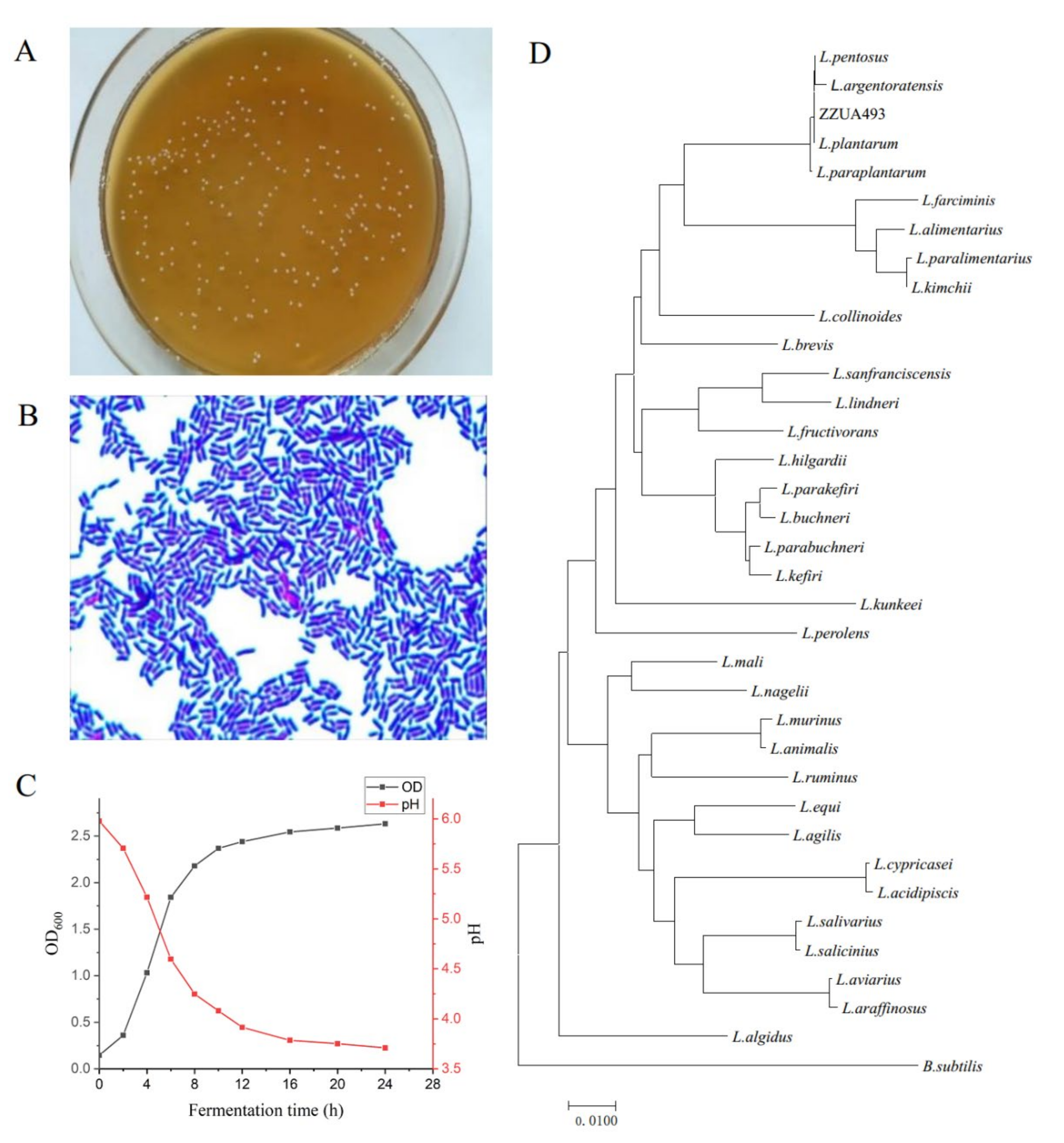
3.2. Morphological, Physiological and Biochemical Properties and Molecular Identification of ZZUA493
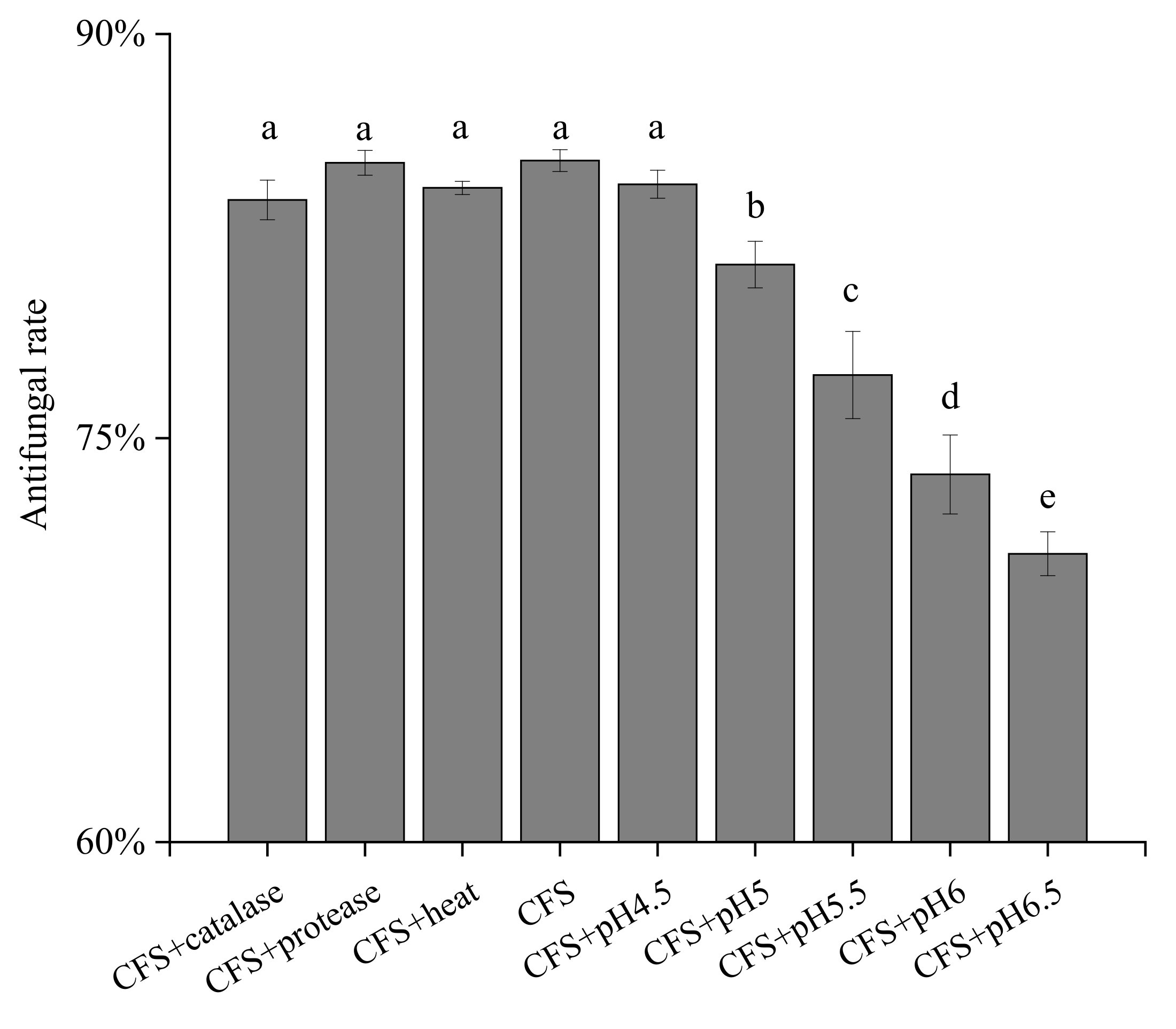
3.3. Studies on Antifungal Activity and Properties of Antifungal Substances of ZZUA493
3.4. Effect of ZZUA493 on the Shelf-Life of CSB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Legan, J.D. Mould spoilage of bread: The problem and some solutions. Int. Biodeter. Biodegr. 1993, 32, 33–53. [Google Scholar] [CrossRef]
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem. In Mycotoxigenic Fungi. Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1542. [Google Scholar] [CrossRef]
- Maftei, N.A.; Ramos-Villarroel, A.Y.; Nicolau, A.I.; Martín-Belloso, O.; Soliva-Fortuny, R. Influence of processing parameters on the pulsed-light inactivation of Penicillium expansum in apple juice. Food Control 2014, 41, 27–31. [Google Scholar] [CrossRef]
- De Souza, V.R.; Popovic, V.; Warriner, K.; Koutchma, T. A comparative study on the inactivation of Penicillium expansum spores on apple using light emitting diodes at 277 nm and a low-pressure mercury lamp at 253.7 nm. Food Control 2020, 110, 107039. [Google Scholar] [CrossRef]
- Zhang, X.M.; Fu, M.R. Inhibitory effect of chlorine dioxide (ClO2) fumigation on growth and patulin production and its mechanism in Penicillum expansum. LWT-Food Sci. Technol. 2018, 96, 335–343. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Siahmoshteh, F.; Hamidi-Esfahani, Z.; Spadaro, D.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 2018, 89, 300–307. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.W.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Assaf, L.A.; Pedrozo, L.P.; Nally, M.C.; Pesce, V.M.; Toro, M.E.; Castellanos de Figueroa, L.I.; Vazquez, F. Use of yeasts from different environments for the control of Penicillium expansum on table grapes at storage temperature. Int. J. Food Microbiol. 2020, 320, 108520. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zielinska, K.J.; Wrobel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microb. Biot. 2019, 35, 76. [Google Scholar] [CrossRef] [Green Version]
- Ammor, S.; Tauveron, G.; Dufour, E.; Chevallier, I. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility: 2—Behaviour of pathogenic and spoilage bacteria in dual species biofilms including a bacteriocin-like-producing lactic acid bacteria. Food Control 2006, 17, 462–468. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: Identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biot. 1998, 50, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.T.; Chen, Y.S.; Wu, H.C.; Yanagida, F. Bio-protective potential of lactic acid bacteria isolated from fermented wax gourd. Folia Microbiol. 2012, 57, 99–105. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z.; Sadon, S.K. Antifungal Activity of Lactobacillus fermentum Te007, Pediococcus pentosaceus Te010, Lactobacillus pentosus G004, and L. paracasi D5 on Selected Foods. J. Food Sci. 2011, 76, M493–M499. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, W.F.; Wang, L.F.; Ju, X.R. Insight into the effect of gluten-starch ratio on the properties of Chinese steamed bread (Mantou). Int. J. Biol. Macromol. 2020, 163, 1821–1827. [Google Scholar] [CrossRef]
- Sim, S.Y.; Aziah, A.A.N.; Cheng, L.H. Characteristics of wheat dough and Chinese steamed bread added with sodium alginates or konjac glucomannan. Food Hydrocolloid 2011, 25, 951–957. [Google Scholar] [CrossRef]
- Li, D.X.; Ni, K.K.; Pang, H.L.; Wang, Y.P.; Cai, Y.M.; Jin, Q.S. Identification and Antimicrobial Activity Detection of Lactic Acid Bacteria Isolated from Corn Stover Silage. Asian Austral. J. Anim. 2015, 28, 620–631. [Google Scholar] [CrossRef] [Green Version]
- Gerez, C.L.; Torres, M.J.; de Valdez, G.F.; Rollan, G. Control of spoilage fungi by lactic acid bacteria. Biol. Control 2013, 64, 231–237. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis Strain Si3 Produces a Broad-Spectrum Proteinaceous Antifungal Compound. Appl. Environ. Microb. 2001, 67, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yan, Y.; Wang, J.; Zhang, H.; Qi, W. Production and Characterization of Antifungal Compounds Produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doulgeraki, A.I.; Paraskevopoulos, N.; Nychas, G.J.E.; Panagou, E.Z. An in vitro study of Lactobacillus plantarum strains for the presence of plantaricin genes and their potential control of the table olive microbiota. Antonie Leeuwenhoek Int. J. Gen. 2013, 103, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.X.; Zhang, L.W.; Tuo, Y.F.; Han, X.; Du, M. A novel method for rapid detection of class IIa bacteriocin-producing lactic acid bacteria. Food Control 2010, 21, 426–430. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Gilles, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gendy, S.M.; Abdel-Galil, H.; Shahin, Y.; Hegazi, F.Z. Characteristics of Salt-Tolerant Lactic Acid Bacteria, in Particular Lactobacilli, Leuconostocs and Pediococci, Isolated from Salted Raw Milk. J. Food Prot. 1983, 46, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.; Venancio, A.; Abrunhosa, L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit Contam Part A Chem Anal. Control Expo. Risk Assess. 2018, 35, 1803–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, B.; Vimont, A.; Desfosses-Foucault, E.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control 2017, 78, 350–356. [Google Scholar] [CrossRef]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and Characterization of an Antifungal Compound (3-Phenyllactic Acid) Produced by Lactobacillus plantarum Strain. Food Bioproc. Technol. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Quattrini, M.; Bernardi, C.; Stuknyte, M.; Masotti, F.; Passera, A.; Ricci, G.; Vallone, L.; De Noni, I.; Brasca, M.; Fortina, M.G. Functional characterization of Lactobacillus plantarum ITEM 17215: A potential biocontrol agent of fungi with plant growth promoting traits, able to enhance the nutritional value of cereal products. Food Res. Int. 2018, 106, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; O’Connor, P.M.; Colquhoun, I.J.; Vior, N.M.; Rodriguez, J.M.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Production of multiple bacteriocins, including the novel bacteriocin gassericin M, by Lactobacillus gasseri LM19, a strain isolated from human milk. Appl. Microbiol. Biot. 2020, 104, 3869–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saugar, J.M.; Rodríguez-Hernández, M.J.; de la Torre, B.G.; Pachón-Ibañez, M.E.; Fernández-Reyes, M.; Andreu, D.; Pachón, J.; Rivas, L. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: Molecular basis for the differential mechanisms of action. Antimicrob. Agents Chemother. 2006, 50, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G.; Shin, S.Y.; Kim, D.-H.; Seo, M.Y.; Kang, J.H.; Lee, Y.; Kim, K.L.; Hahm, K.-S. Antifungal mechanism of a cysteine-rich antimicrobial peptide, Ib-AMP1, from Impatiens balsamina against Candida albicans. Biotechnol. Lett. 1999, 21, 1047–1050. [Google Scholar] [CrossRef]
- Cookson, A.L.; Noel, S.J.; Kelly, W.J.; Attwood, G.T. The use of PCR for the identification and characterisation of bacteriocin genes from bacterial strains isolated from rumen or caecal contents of cattle and sheep. FEMS Microbiol. Ecol. 2004, 48, 199–207. [Google Scholar] [CrossRef]
- Macwana, S.J.; Muriana, P.M. A ‘bacteriocin PCR array’ for identification of bacteriocin-related structural genes in lactic acid bacteria. J. Microbiol. Meth. 2012, 88, 197–204. [Google Scholar] [CrossRef]
- Devi, S.M.; Halami, P.M. Genetic Variation of pln Loci Among Probiotic Lactobacillus plantarum Group Strains with Antioxidant and Cholesterol-Lowering Ability. Probiotics Antimicro 2019, 11, 11–22. [Google Scholar] [CrossRef]
- Guyot, J.-P. Cereal-based fermented foods in developing countries: Ancient foods for modern research. Int. J. Food Sci. Technol. 2012, 47, 1109–1114. [Google Scholar] [CrossRef]
- Robert, H.; Gabriel, V.; Fontagné-Faucher, C. Biodiversity of lactic acid bacteria in French wheat sourdough as determined by molecular characterization using species-specific PCR. Int. J. Food Microbiol. 2009, 135, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Van Kranenburg, R.; Kleerebezem, M.; van Hylckama Vlieg, J.; Ursing, B.M.; Boekhorst, J.; Smit, B.A.; Ayad, E.H.E.; Smit, G.; Siezen, R.J. Flavour formation from amino acids by lactic acid bacteria: Predictions from genome sequence analysis. Int. Dairy J. 2002, 12, 111–121. [Google Scholar] [CrossRef]

| Gram Stain | Shape | Fermentation Type | Growth at Temperature (°C) | Growth in NaCl (w/v %) | Gas from Glucose | Catalase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 10 | 20 | 30 | 40 | 50 | 3.0 | 6.5 | |||||
| + | Rod | Homo | w | + | + | + | + | w | + | + | - | - |
| Growth at pH | ||||||||||||
| 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 7.0 | 8.0 | 8.5 | 9.0 | 9.5 | 10 |
| + | + | + | + | + | ++ | ++ | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Hao, X.; Yang, F.; Wang, Y.; Fan, X.; Wang, Y. Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods 2022, 11, 195. https://doi.org/10.3390/foods11020195
Zhao S, Hao X, Yang F, Wang Y, Fan X, Wang Y. Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods. 2022; 11(2):195. https://doi.org/10.3390/foods11020195
Chicago/Turabian StyleZhao, Shanshan, Xiangmei Hao, Fengyuan Yang, Yuan Wang, Xiaomiao Fan, and Yanping Wang. 2022. "Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns" Foods 11, no. 2: 195. https://doi.org/10.3390/foods11020195
APA StyleZhao, S., Hao, X., Yang, F., Wang, Y., Fan, X., & Wang, Y. (2022). Antifungal Activity of Lactobacillus plantarum ZZUA493 and Its Application to Extend the Shelf Life of Chinese Steamed Buns. Foods, 11(2), 195. https://doi.org/10.3390/foods11020195






