Solid-State Fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on Tannin Content, Phenolic Profile, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material, Microorganisms, and Chemicals
2.2. Sample Preparation
2.3. Proximal Physicochemical Characterization
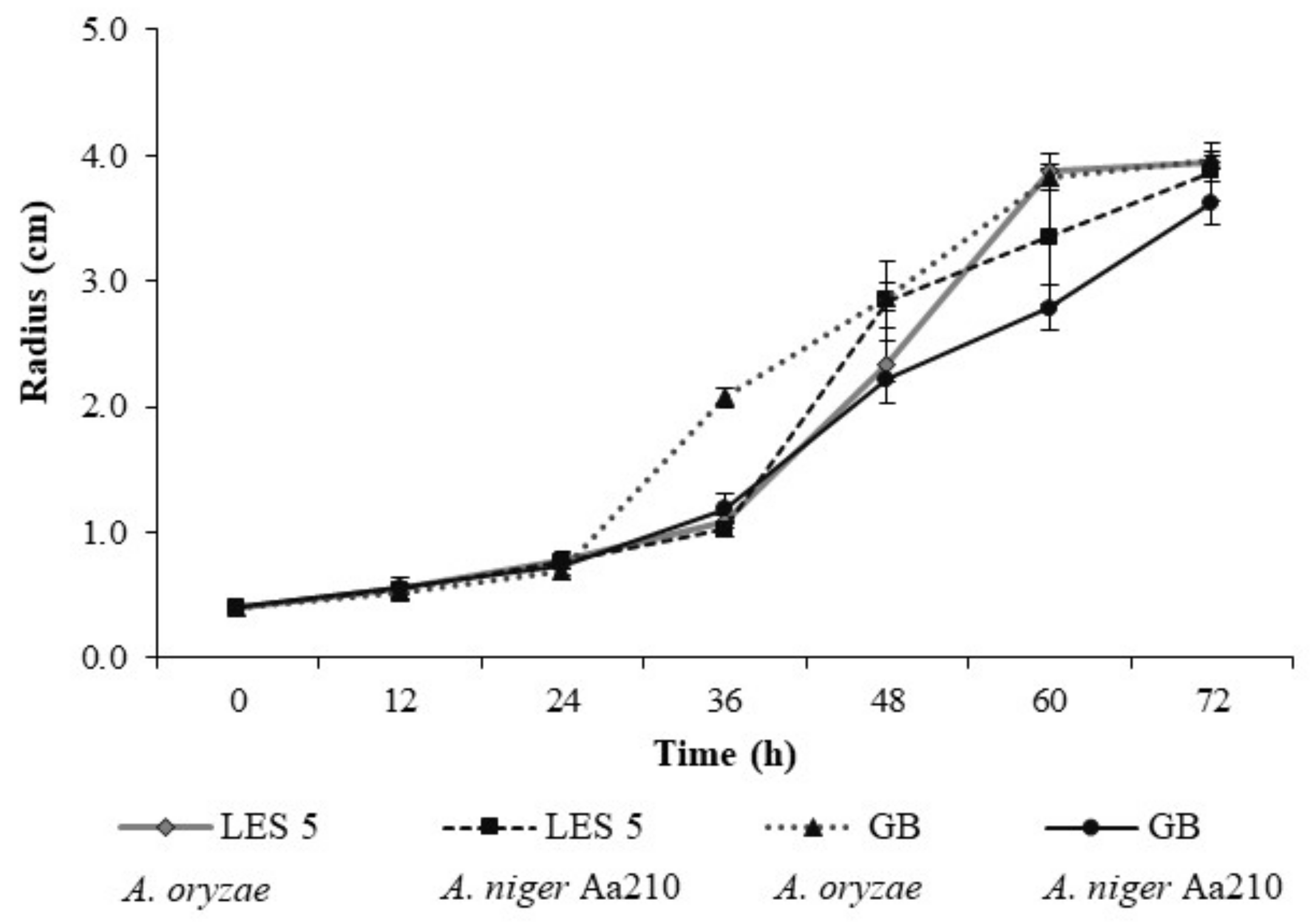
2.4. Solid-State Fermentation (SSF)
2.5. Determination of Phenolic Content
2.6. Phenolic Profile by High-Performance Liquid Chromatography Coupled to Mass Spectrometry (HPLC-MS)
2.7. Antioxidant Activity
2.7.1. ABTS
2.7.2. DPPH
2.7.3. FRAP
2.8. Statistical Analysis
3. Results
3.1. Proximal Physicochemical Characterization
3.2. Solid-State Fermentation (SSF)—Assisted Extraction
3.2.1. Phenolic Content
3.2.2. Phenolic Profile
3.3. Antioxidant Activity
4. Discussion
4.1. Proximal Physicochemical Characterization
4.2. Solid-State Fermentation (SSF)—Assisted Extraction
4.2.1. Phenolic Content
4.2.2. Phenolic Profile
4.3. Antioxidant Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Przybylska-Balcerek, A.; Frankowski, J.; Stuper-Szablewska, K. Bioactive Compounds in Sorghum. Eur. Food Res. Technol. 2019, 245, 1075–1080. [Google Scholar] [CrossRef]
- Hossain, M.S.; Islam, M.N.; Rahman, M.M.; Mostafa, M.G.; Khan, M.A.R. Sorghum: A Prospective Crop for Climatic Vulnerability, Food and Nutritional Security. J. Agric. Food Res. 2022, 8, 100300. [Google Scholar] [CrossRef]
- Luthria, D.L.; Liu, K. Localization of Phenolic Acids and Antioxidant Activity in Sorghum Kernels. J. Funct. Foods 2013, 5, 1751–1760. [Google Scholar] [CrossRef]
- Wu, G.; Bennett, S.J.; Bornman, J.F.; Clarke, M.W.; Fang, Z.; Johnson, S.K. Phenolic Profile and Content of Sorghum Grains under Different Irrigation Managements. Food Res. Int. 2017, 97, 347–355. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Bolívar, G.; Ramírez, C.T. Effect of Solid State Fermentation with Rhizopus Oryzae on Biochemical and Structural Characteristics of Sorghum (Sorghum Bicolor (L.) Moench). Int. J. Food Ferment. Technol. 2018, 8, 27–36. [Google Scholar] [CrossRef]
- Chung, I.; Kim, E.; Yeo, M.; Kim, S.; Cheol, M.; Moon, H. Antidiabetic Effects of Three Korean Sorghum Phenolic Extracts in Normal and Streptozotocin-Induced Diabetic Rats. Food Res. Int. 2011, 44, 127–132. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S.; Kaur, M. Effect of Duration of Solid State Fermentation by Aspergillus awamorinakazawa on Antioxidant Properties of Wheat Cultivars. LWT-Food Sci. Technol. 2016, 71, 323–328. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and Anti-Inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-State Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Sawangwan, T.; Saman, P. Prebiotic Synthesis from Rice Using Aspergillus oryzae with Solid State Fermentation. Agric. Nat. Resour. 2016, 50, 227–231. [Google Scholar] [CrossRef][Green Version]
- ISTA Reglas Internacionales Para El Análisis de Las Semillas. Available online: https://vri.umayor.cl/images/ISTA_Rules_2016_Spanish.pdf (accessed on 1 August 2022).
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Sepúlveda, L.; Aguilar, C.N.; Prado-Barragán, A. Potential Use of Different Agroindustrial By-Products as Supports for Fungal Ellagitannase Production under Solid-State Fermentation. Food Bioprod. Process. 2014, 92, 376–382. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists (1980) Official Methods of Analysis, 16th ed.; AOAC: Arlington, TX, USA, 1980. [Google Scholar]
- Ankom Technology. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction. Available online: https://www.ankom.com/sites/default/files/document-files/Crude_Fat_Abstract.pdf (accessed on 1 August 2022).
- Ankom Technology. Crude Fiber Analysis in Feeds-Filter Bag Technique (for A200, A200I, A2000 and A2000I). Available online: https://www.ankom.com/sites/default/files/document-files/Crude_Fiber_Abstract_0.pdf (accessed on 1 August 2022).
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate Quantification of Total Phenolic Content from Plant Extracts Obtained by Conventional and Ultrasound Methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic Acid Production Using Polyphenols from Orange Peel Waste by Submerged Fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The Complete Biodegradation Pathway of Ellagitannins by Aspergillus niger in Solid-State Fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
- Hou, F.; Su, D.; Xu, J.; Gong, Y.; Zhang, R.; Wei, Z.; Chi, J.; Zhang, M. Enhanced Extraction of Phenolics and Antioxidant Capacity from Sorghum (Sorghum bicolor L. Moench) Shell Using Ultrasonic-Assisted Ethanol–Water Binary Solvent. J. Food Process. Preserv. 2016, 40, 1171–1179. [Google Scholar] [CrossRef]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of Grapefruit By-Products as Solid Support for Solid-State Fermentation to Produce Antioxidant Bioactive Extracts. Waste Biomass Valorization 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.; de Pascual-Teresa, S.; León, A.E. The influence of different air-drying conditions on bioactive compounds and antioxidant activity of berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2009, 228, 441–448. [Google Scholar] [CrossRef]
- Abhay, S.M.; Hii, C.L.; Law, C.L.; Suzannah, S.; Djaeni, M. Effect of hot-air drying temperature on the polyphenol content and the sensory properties of cocoa beans. Int. Food Res. J. 2016, 23, 1479–1484. Available online: http://www.ifrj.upm.edu.my/23%20(04)%202016/(19).pdf (accessed on 15 August 2022).
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry. Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J.A. Colonization of Aspergillus japonicus on Synthetic Materials and Application to the Production of Fructooligosaccharides. Carbohydr. Res. 2009, 344, 795–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid State Fermentation of Fig (Ficus carica L.) by-Products Using Fungi to Obtain Phenolic Compounds with Antioxidant Activity and Qualitative Evaluation of Phenolics Obtained. Process. Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Contreras-Jiménez, B.; Hernández-Becerra, E.; Rodriguez-Garcia, M.E. Physicochemical Changes of Starch during Malting Process of Sorghum Grain. J. Cereal Sci. 2020, 95, 103069. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Avelino, J.; Salatino, A. Contents of Tannins of Cultivars of Sorghum Cultivated in Brazil, as Determined by Four Quantification Methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic Compositions and Antioxidant Activities Differ Significantly among Sorghum Grains with Different Applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef]
- Mokrane, H.; Amoura, H.; Belhaneche-Bensemra, N.; Courtin, C.M.; Delcour, J.A.; Nadjemi, B. Assessment of Algerian Sorghum Protein Quality [Sorghum bicolor (L.) Moench] Using Amino Acid Analysis and in Vitro Pepsin Digestibility. Food Chem. 2010, 121, 719–723. [Google Scholar] [CrossRef]
- De Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, Bioactive Compounds, and Potential Impact on Human Health. Crit. Rev. Food Sci. Nutr. 2016, 57, 372–390. [Google Scholar] [CrossRef]
- Motlhaodi, T.; Bryngelsson, T.; Chite, S.; Fatih, M.; Ortiz, R.; Geleta, M. Nutritional Variation in Sorghum [Sorghum bicolor (L.) Moench] Accessions from Southern Africa Revealed by Protein and Mineral Composition. J. Cereal Sci. 2018, 83, 123–129. [Google Scholar] [CrossRef]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.D.S. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Maxwell, O.I.; Chinwuba, U.B.; Onyebuchukwu, M.G. Protein Enrichment of Potato Peels Using Saccharomyces cerevisiae via Solid-State Fermentation Process. Adv. Chem. Eng. Sci. 2019, 09, 99–108. [Google Scholar] [CrossRef]
- Pastrana, L. Fundamentos De La Fermentación En Estado Sólido Y Aplicación a La Industria Alimentaria. CYTA-J. Food 2009, 1, 4–12. [Google Scholar] [CrossRef]
- Putri, S.N.A.; Utari, D.P.; Martati, E.; Putri, W.D.R. Study of Sorghum (Sorghum bicolor (L.) Moench) Grains Fermentation with Lactobacillus plantarum ATCC 14977 on Tannin Content. IOP Conf. Series Earth Environ. Sci. 2021, 924, 012037. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Rajagopal, V.; Kumaravel. Bioprocessing of paddy straw for the production and purification of gallic acid using Penicillium chrysogenum. Electron. J. Environ. Agric. Food Chem. 2011, 9, 1460–1470. [Google Scholar]
- Badui Dergal, S. Quimica de Los Alimentos, 4th ed.; Pearson Educación: Mexico City, Mexico, 2006; ISBN 9702606705. [Google Scholar]
- Ritthibut, N.; Oh, S.J.; Lim, S.T. Enhancement of Bioactivity of Rice Bran by Solid-State Fermentation with Aspergillus Strains. LWT-Food Sci. Technol. 2021, 135, 110273. [Google Scholar] [CrossRef]
- Tanasković, S.J.; Šekuljica, N.; Jovanović, J.; Gazikalović, I.; Grbavčić, S.; Đorđević, N.; Sekulić, M.V.; Hao, J.; Luković, N.; Knežević-Jugović, Z. Upgrading of Valuable Food Component Contents and Anti-Nutritional Factors Depletion by Solid-State Fermentation: A Way to Valorize Wheat Bran for Nutrition. J. Cereal Sci. 2021, 99, 103159. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Surekha; Duhan, J.S. Bioaugmentation of Phenolics and Antioxidant Activity of Oryza sativa by Solid State Fermentation Using Aspergillus spp. Int. Food Res. J. 2017, 24, 1160–1166. [Google Scholar]
- Pandey, A.; Negi, S.; Soccol, C.R. Current Developments in Biotechnology and Bioengineering Production, Isolation and Purification. Fedor, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780444636621. [Google Scholar]
- Sharanagat, V.S.; Suhag, R.; Anand, P.; Deswal, G.; Kumar, R.; Chaudhary, A.; Singh, L.; Singh Kushwah, O.; Mani, S.; Kumar, Y.; et al. Physico-Functional, Thermo-Pasting and Antioxidant Properties of Microwave Roasted Sorghum [Sorghum bicolor (L.) Moench]. J. Cereal Sci. 2019, 85, 111–119. [Google Scholar] [CrossRef]
- Khoddami, A.; Truong, H.H.; Liu, S.Y.; Roberts, T.H.; Selle, P.H. Concentrations of Specific Phenolic Compounds in Six Red Sorghums Influence Nutrient Utilisation in Broiler Chickens. Anim. Feed Sci. Technol. 2015, 210, 190–199. [Google Scholar] [CrossRef]
- Ohara, A.; Gonçalves, J.; Alencar, J.; Menezes, P.; Barbosa, M.; Furlan, F.; Bagagli, M.; Sato, H.; Janser, R.; de Castro, S. A Multicomponent System Based on a Blend of Agroindustrial Wastes for the Simultaneous Production of Industrially Applicable Enzymes by Solid-State Fermentation. Food Sci. Technol. 2018, 2061, 131–137. [Google Scholar] [CrossRef]
- Gharaati, S. Extraction Techniques of Phenolic Compounds from Plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Alfieri, M.; Balconi, C.; Cabassi, G.; Habyarimana, E.; Redaelli, R. Antioxidant Activity in a Set of Sorghum Landraces and Breeding Lines. Maydica 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Sorour, M.; Mehanni, A.; Taha, E.; Rashwan, A. Changes of Total Phenolics, Tannins, Phytate and Antioxidant Activity of Two Sorghum Cultivars as Affected by Processing. J. Food Dairy Sci. 2017, 8, 267–274. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Kayitesi, E. Fermentation by Lactobacillus fermentum Strains (Singly and in Combination) Enhances the Properties of Ting from Two Whole Grain Sorghum Types. J. Cereal Sci. 2018, 82, 49–56. [Google Scholar] [CrossRef]
- Selle, P.H.; Cadogan, D.J.; Li, X.; Bryden, W.L. Implications of Sorghum in Broiler Chicken Nutrition. Anim. Feed Sci. Technol. 2010, 156, 57–74. [Google Scholar] [CrossRef]
- De Oliveira, S.G.; Berchielli, T.; Pedreira, M.D.S.; Primavesi, O.; Frighetto, R.; Lima, M. Effect of Tannin Levels in Sorghum Silage and Concentrate Supplementation on Apparent Digestibility and Methane Emission in Beef Cattle. Anim. Feed Sci. Technol. 2007, 135, 236–248. [Google Scholar] [CrossRef]
- De Oliveira, K.G.; Queiroz, V.A.V.; Carlos, L.D.A.; Cardoso, L.D.M.; Pinheiro-Sant’Ana, H.M.; Anunciação, P.C.; de Menezes, C.B.; da Silva, E.C.; Barros, F. Effect of the Storage Time and Temperature on Phenolic Compounds of Sorghum Grain and Flour. Food Chem. 2017, 216, 390–398. [Google Scholar] [CrossRef]
- Wen, Y.L.; Yan, L.P.; Chen, C.S. Effects of Fermentation Treatment on Antioxidant and Antimicrobial Activities of Four Common Chinese Herbal Medicinal Residues by Aspergillus oryzae. J. Food Drug Anal. 2013, 21, 219–226. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Satpal; Sangwan, S. Characterization of Phenolic Compounds and Antioxidant Activity in Sorghum [Sorghum bicolor (L.) Moench] Grains. Cereal Res. Commun. 2021, 49, 343–353. [Google Scholar] [CrossRef]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J.; Gutierrez-Uribe, J.A. Phenolic Compounds, Antioxidant Capacity and Gelling Properties of Glucoarabinoxylans from Three Types of Sorghum Brans. J. Cereal Sci. 2015, 65, 277–284. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Busconi, M.; Marocco, A.; Trevisan, M.; Lucini, L. Pigmented Sorghum Polyphenols as Potential Inhibitors of Starch Digestibility: An in Vitro Study Combining Starch Digestion and Untargeted Metabolomics. Food Chem. 2020, 312, 126077. [Google Scholar] [CrossRef]
- Shelembe, J.S.; Cromarty, D.; Bester, M.; Minnaar, A.; Duodu, K.G. Effect of Acidic Condition on Phenolic Composition and Antioxidant Potential of Aqueous Extracts from Sorghum (Sorghum bicolor) Bran. J. Food Biochem. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid Composition of Red Sorghum Genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum Polyphenols and Other Bioactive Components as Functional and Health Promoting Food Ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Althwab, S.; Carr, T.P.; Weller, C.L.; Dweikat, I.M.; Schlegel, V. Advances in Grain Sorghum and Its Co-Products as a Human Health Promoting Dietary System. Food Res. Int. 2015, 77, 349–359. [Google Scholar] [CrossRef]
- Peñarrieta, M.; Tejeda, L.; Mollinedo, P.; Vila, J.L.; Bravo, J.A. Phenolic Compounds in Food. Rev. Boliv. Química 2014, 31, 68–81. [Google Scholar]
- Salazar-López, N.J.; González-Aguilar, G.; Rouzaud-Sández, O.; Robles-Sánchez, M. Technologies Applied to Sorghum (Sorghum bicolor L. Moench): Changes in Phenolic Compounds and Antioxidant Capacity. Food Sci. Technol. 2018, 38, 369–382. [Google Scholar] [CrossRef]
- Pontieri, P.; Del Giudice, F.; Dimitrov, M.D.; Pesheva, M.G.; Venkov, P.V.; Di Maro, A.; Pacifico, S.; Gadgil, P.; Herald, T.J.; Tuinstra, M.R.; et al. Measurement of Biological Antioxidant Activity of Seven Food-Grade Sorghum Hybrids Grown in a Mediterranean Environment. Aust. J. Crop. Sci. 2016, 10, 904–910. [Google Scholar] [CrossRef]
- Ciulu, M.; de la Luz Cádiz-Gurrea, M.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef]
- López-Contreras, J.J.; Zavala-García, F.; Urías-Orona, V.; Martínez-Ávila, G.C.G.; Rojas, R.; Niño-Medina, G. Chromatic, Phenolic and Antioxidant Properties of Sorghum bicolor Genotypes. Not. Bot. Horti Agrobot Cluj Napoca 2015, 43, 366–370. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum: Its Unique Nutritional and Health-Promoting Attributes; Elsevier: London, UK, 2017; ISBN 9780081008911. [Google Scholar]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of Phenolic Compounds and Antioxidant Activity in Sorghum Grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic Compounds in Cereal Grains and Their Health Benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Zheng, W.; Gao, B.; Zhu, H.; Xu, R.; Deng, H.; Wang, B.; Wu, Y.; Sun, X.; et al. Triacylglycerols Compositions, Soluble and Bound Phenolics of Red Sorghums, and Their Radical Scavenging and Anti-Inflammatory Activities. Food Chem. 2021, 340, 128123. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Kim, J.M.; Lee, Y.G.; Kim, C. Antioxidant Activity and Contents of Total Phenolic Compounds and Anthocyanins According to Grain Colour in Several Varieties of Sorghum bicolor (L.) Moench. Cereal Res. Commun. 2019, 47, 228–238. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced Raw 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
- Xiong, Y.; Damasceno Teixeira, T.V.; Zhang, P.; Warner, R.D.; Shen, S.; Fang, Z. Cellular Antioxidant Activities of Phenolic Extracts from Five Sorghum Grain Genotypes. Food Biosci. 2021, 41, 101068. [Google Scholar] [CrossRef]
| Parameter | LES 5 | GB | Mineral | LES 5 | GB |
|---|---|---|---|---|---|
| Moisture | 9.66 ± 0.34 a | 10.10 ± 0.48 a | K | 11.17 ± 0.04 b | 12.28 ± 0.01 a |
| Total carbohydrates | 71.35 ± 0.15 a | 71.49 ± 0.02 a | P | 1.99 ± 0.01 b | 2.29 ± 0.01 a |
| Protein | 10.89 ± 0.34 a | 10.50 ± 0.00 a | Ca | 2.19 ± 0.11 a | 1.95 ± 0.01 a |
| Lipids | 4.26 ±0.22 a | 4.00 ± 0.40 a | Cl | 1.27 ± 0.04 b | 1.46 ± 0.00 a |
| Ash | 1.85 ± 0.02 b | 2.01 ± 0.02 a | S | 1.00 ± 0.07 a | 1.19 ± 0.00 a |
| Crude fiber | 2.01 ± 0.13 a | 1.90 ± 0.07 a | Fe | 0.23 ± 0.01 b | 0.28 ± 0.00 a |
| Mn | 0.11 ± 0.00 a | 0.12 ± 0.01 a | |||
| Zn | 0.10 ± 0.01 b | 0.15 ± 0.00 a | |||
| Al | 0.06 ± 0.00 b | 0.13 ± 0.02 a |
| HT (mg GAE/100 g) | ||||
|---|---|---|---|---|
| Fermentation Time (h) | LES 5 SSF by A. oryzae | LES 5 SSF by A. niger Aa210 | GB SSF by A. oryzae | GB SSF by A. niger Aa210 |
| 0 | 3.33 ± 0.12 | 3.33 ± 0.12 | 3.20 ± 0.12 | 3.20 ± 0.12 |
| 12 | 3.73 ± 0.06 | 4.23 ± 0.06 * | 3.30 ± 0.06 | 4.27 ± 0.15 * |
| 24 | 3.77 ± 0.12 * | 3.97 ± 0.06 * | 3.90 ± 0.58 * | 3.90 ± 0.10 * |
| 36 | 3.27 ± 0.12 | 4.27 ± 0.06 * | 3.40 ± 0.23 | 4.50 ± 0.00 * |
| 48 | 3.53 ± 0.15 | 4.90 ± 0.27 * | 3.20 ± 0.06 | 4.80 ± 0.12 * |
| 60 | 3.40 ± 0.20 | 5.33 ± 0.06 * | 3.10 ± 0.10 | 4.90 ± 0.06 * |
| 72 | 3.57 ± 0.12 | 5.67 ± 0.12 * | 3.30 ± 0.00 | 5.90 ± 0.10 * |
| 84 | 3.70 ± 0.46 | 3.47 ± 0.12 | 3.40 ± 0.06 | 3.40 ± 0.25 |
| 96 | 3.53 ± 0.23 | 4.30 ± 0.00 * | 3.20 ± 0.44 | 4.70 ± 0.00 * |
| CT (mg CE/100 g) | ||||
|---|---|---|---|---|
| Fermentation Time (h) | SSF LES 5 by A. oryzae | SSF LES 5 by A. niger Aa210 | SSF GB by A. oryzae | SSF GB by A. niger Aa210 |
| 0 | 50.90 ± 5.15 | 50.90 ± 5.15 | 54.90 ± 3.12 | 54.90 ± 3.12 |
| 12 | 55.30 ± 3.05 | 61.63 ± 2.47 * | 50.36 ± 1.33 | 59.96 ± 3.43 |
| 24 | 49.50 ± 2.75 | 59.97 ± 6.24 | 46.86 ± 1.33 * | 59.76 ± 3.72 |
| 36 | 47.13 ± 1.96 | 55.30 ± 3.72 | 45.20 ± 2.95 * | 67.50 ± 5.38 * |
| 48 | 49.73 ± 4.57 | 72.27 ± 4.47 * | 50.50 ± 1.45 | 68.30 ± 2.18 * |
| 60 | 48.03 ± 0.40 | 70.70 ± 5.15 * | 55.30 ± 3.32 | 63.40 ± 3.37 |
| 72 | 48.00 ± 3.29 | 76.07 ± 3.50 * | 51.90 ± 3.27 | 67.70 ± 5.20 * |
| 84 | 45.20 ± 1.14 | 64.30 ± 4.59 * | 44.56 ± 2.65 * | 73.20 ± 5.49 * |
| 96 | 46.33 ± 3.16 | 69.03 ± 3.54 * | 44.90 ± 2.40 * | 62.50 ± 5.26 |
| No. | Dough | Compound | Family | LES 5 SSF | GB SSF | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ue | A. oryzae | A. niger Aa210 | Ue | A. oryzae | A. niger Aa210 | ||||
| 1 | 357.1 | Gardenin B | Methoxyflavones | 24, 48 | |||||
| 2 | 865.1 | Procyanidin trimer C1 | Proanthocyanidin trimers | 0 | 24, 48, 84 | 24, 48, 72 | 0 | 24 | |
| 3 | 864.1 | Procyanidin trimer C2 | Proanthocyanidin trimers | 0 | 24 | ||||
| 4 | 867.1 | Theaflavin 3,3’-O-digallate | Flavonoids | 0 | 24, 48, 72,84 | 24, 72 | 0 | 24, 48, 72, 84 | 24, 48, 72 |
| 5 | 705.2 | (-)-Epicatechin-(2a-7)(4a-8)-epicatechin 3-O-galactoside | Proanthocyanidin dimers | 0 | 24, 48, 72, 84 | 24, 48, 72, 84 | 48, 72, 84 | 24, 48, 72, 84 | |
| 6 | 285.0 | Scutellarein | Flavones | 0 | 24, 48, 72, 84 | 24, 48, 72 | 24 | ||
| 7 | 271.0 | Arbutin | Other polyphenols | 24, 84 | 24, 84 | 0 | 24, 48, 72 | 24, 48, 72 | |
| 8 | 329.1 | 3,7-Dimethylquercetin | Methoxyflavonols | 24, 48 | |||||
| 9 | 289.0 | (+)-Catechin | Catechins | 0 | 48 | 24 | 0 | 48 | 24, 48, 72 |
| 10 | 330.8 | Galloyl glucose | Hydroxybenzoic acids | 72, 84 | 24, 48, 72 | 24 | |||
| 11 | 883.1 | Prodelphinidin trimer C-GC-C | Proanthocyanidin trimers | 0 | 0 | 24 | 24, 48, 72 | ||
| 12 | 287.0 | Eriodictyol | Flavanones | 72, 84 | 24 | 24, 72, 84 | |||
| 13 | 716.1 | Theaflavin 3’-O-gallate | Theaflavins | 72 | |||||
| 14 | 341.0 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | 48, 72, 84 | 0 | 84 | 48, 72, 84 | ||
| 15 | 377.0 | 3,4-DHPEA-EA | Tyrosols | 0 | 72, 84 | 0 | 84 | 48, 84 | |
| 16 | 272.9 | Phloretin | Dihydrochalcones | 24 | 24 | ||||
| 17 | 415.1 | Daidzin | Isoflavones | 0 | |||||
| 18 | 327.2 | p-Coumaroyl tyrosine | Hydroxycinnamic acids | 0 | |||||
| 19 | 289.0 | (-)-Epicatechin | Catechins | 72, 84 | 48, 72, 84 | ||||
| 20 | 387.1 | Medioresinol | Lignans | 84 | |||||
| 21 | 434.1 | Delphinidin 3-O-arabinoside | Anthocyanins | 48, 72, 84 | |||||
| Extract | Fermentation Time (h) | ABTS (mg TE/100 g) | DPPH (mg TE/100 g) | FRAP (mg TE/100 g) |
|---|---|---|---|---|
| LES 5 | 72 | 64.33 ± 1.15 a | 126.67 ± 1.15 b | 54.00 ± 3.17 ab |
| 84 | 61.67 ± 0.58 b | 127.67 ± 0.58 b | 50.20 ± 2.25 bc | |
| GB | 72 | 62.33 ± 0.58 b | 127.00 ± 1.00 b | 47.47 ± 1.75 c |
| 84 | 63.00 ± 1.00 ab | 133.67 ± 1.15 a | 59.23 ± 3.91 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Hernández, P.; Ruelas-Chacón, X.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Flores-Naveda, A.; Sepúlveda-Torre, L. Solid-State Fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on Tannin Content, Phenolic Profile, and Antioxidant Activity. Foods 2022, 11, 3121. https://doi.org/10.3390/foods11193121
Espitia-Hernández P, Ruelas-Chacón X, Chávez-González ML, Ascacio-Valdés JA, Flores-Naveda A, Sepúlveda-Torre L. Solid-State Fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on Tannin Content, Phenolic Profile, and Antioxidant Activity. Foods. 2022; 11(19):3121. https://doi.org/10.3390/foods11193121
Chicago/Turabian StyleEspitia-Hernández, Pilar, Xóchitl Ruelas-Chacón, Mónica L. Chávez-González, Juan A. Ascacio-Valdés, Antonio Flores-Naveda, and Leonardo Sepúlveda-Torre. 2022. "Solid-State Fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on Tannin Content, Phenolic Profile, and Antioxidant Activity" Foods 11, no. 19: 3121. https://doi.org/10.3390/foods11193121
APA StyleEspitia-Hernández, P., Ruelas-Chacón, X., Chávez-González, M. L., Ascacio-Valdés, J. A., Flores-Naveda, A., & Sepúlveda-Torre, L. (2022). Solid-State Fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on Tannin Content, Phenolic Profile, and Antioxidant Activity. Foods, 11(19), 3121. https://doi.org/10.3390/foods11193121









