Hypoglycemic Effects of Novel Panax notoginseng Polysaccharide in Mice with Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction and Purification of P. notoginseng Polysaccharides
2.3. Structural Characteristics Analysis
2.4. Antioxidant Activity Analysis
2.5. MTT Assay
2.6. Animals and Treatments
2.7. Oral Glucose Tolerance Test (OGTT)
2.8. Western Blot
2.9. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight, Monosaccharide Composition and Uronic Acid Levels
3.2. Methylation Analysis
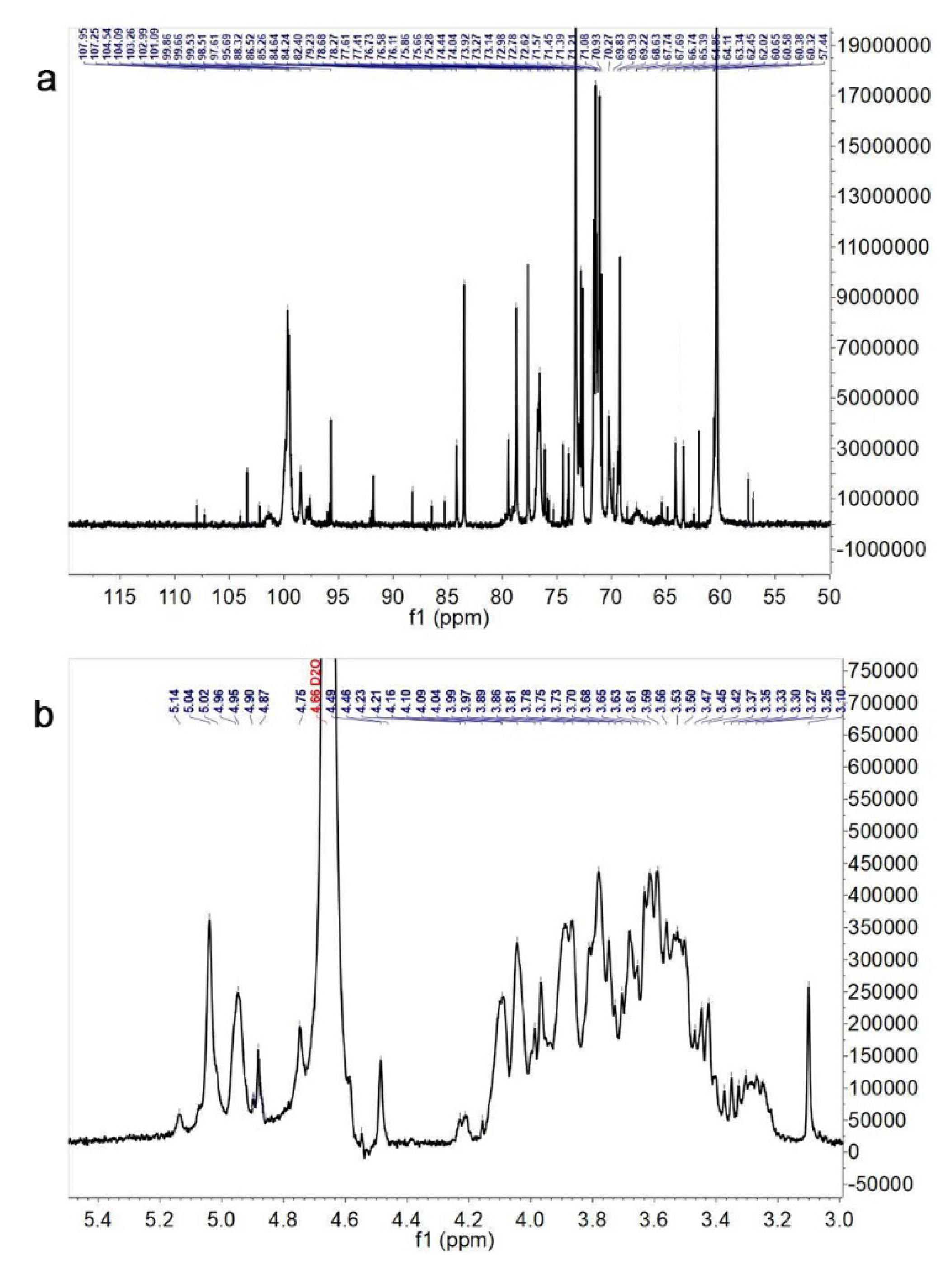
3.3. NMR Spectroscopy Assay
3.4. Antioxidant Activity Assay
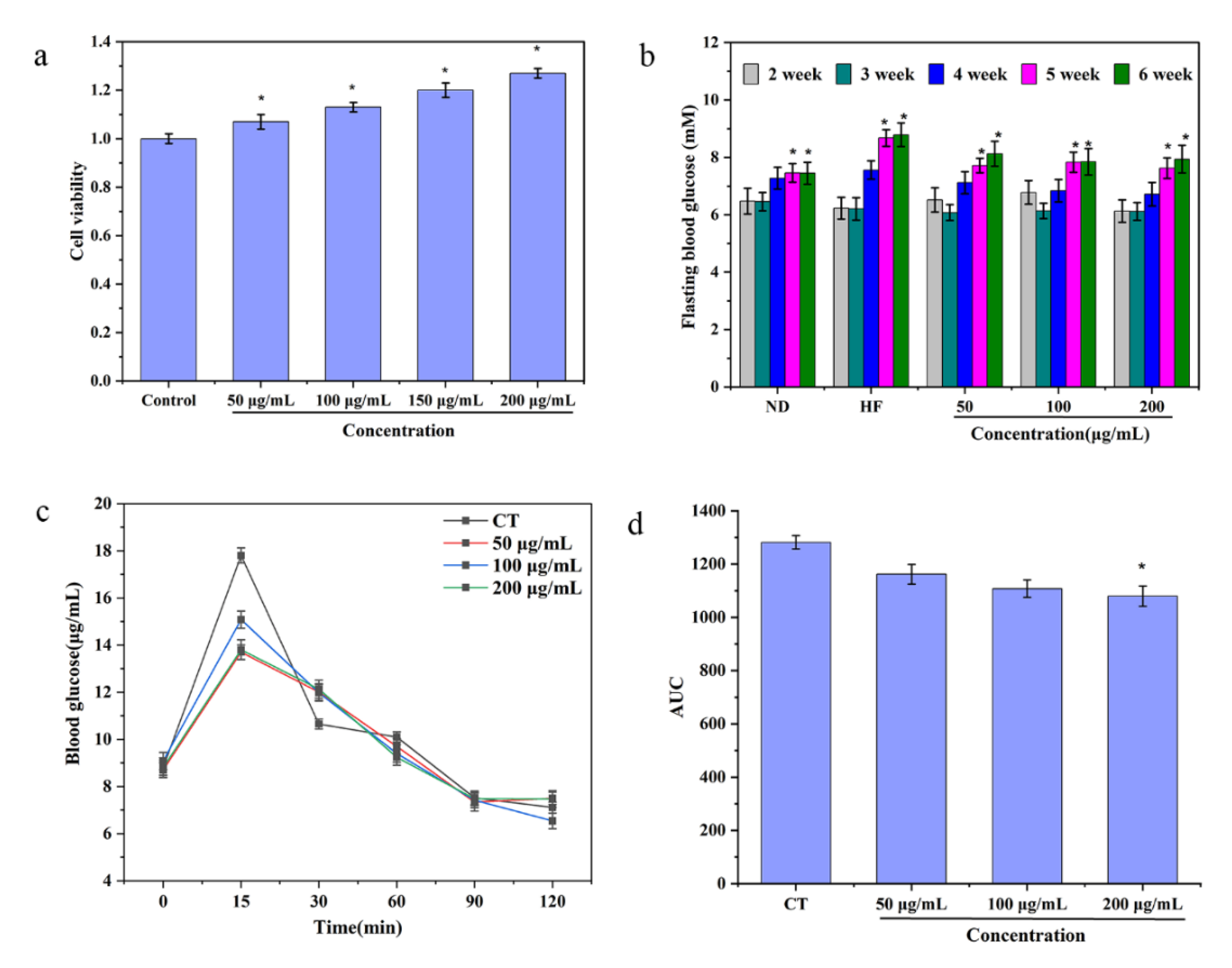
3.5. Cytotoxicity Assay
3.6. Variation in Mice Hyperglycemia
3.7. The Underlying Hypoglycemic Mechanism of Pan
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samsom, M.; Trivedi, T.; Orekoya, O.; Vyas, S. Understanding the importance of gene and environment in the etiology and prevention of type 2 diabetes mellitus in high-risk populations. Oral. Health Case Rep. 2016, 2, 112. [Google Scholar]
- Ghoorah, K.; Campbell, P.; Kent, A.; Maznyczka, A.; Kunadian, V. Obesity and cardiovascular outcomes: A review. Eur. Heart J. Acute Cardiovas. Care 2016, 5, 77–85. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Correction: Corrigendum: Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2017, 8, 16130. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Zhang, H.-H.; Yu, C.-H.; Fang, J.; Ying, H.-Z. Ethanol extract of Atractylodis macrocephalae Rhizoma ameliorates insulin resistance and gut microbiota in type 2 diabetic db/db mice. J. Funct. Foods 2017, 39, 139–151. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Mou, Y.-C.; Su, C.-C.; Chiang, B.-H. Antihepatocarcinoma activity of lactic acid bacteria fermented Panax notoginseng. J. Agric. Food Chem. 2010, 58, 8528–8534. [Google Scholar] [CrossRef]
- Chan, M.K.; Yu, Y.; Wulamu, S.; Wang, Y.; Wang, Q.; Zhou, Y.; Sun, L. Structural analysis of water-soluble polysaccharides isolated from Panax notoginseng. Int. J. Biol. Macromol. 2020, 155, 376–385. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Qu, Y.; Guo, X.; Yang, X.; Cui, X.; Wang, C. Structural characterization of a novel polysaccharide from Panax notoginseng residue and its immunomodulatory activity on bone marrow dendritic cells. Int. J. Biol. Macromol. 2020, 161, 797–809. [Google Scholar] [CrossRef]
- Han, S.-Y.; Li, H.-X.; Ma, X.; Zhang, K.; Ma, Z.-Z.; Jiang, Y.; Tu, P.-F. Evaluation of the anti-myocardial ischemia effect of individual and combined extracts of Panax notoginseng and Carthamus tinctorius in rats. J. Ethnopharmacol. 2013, 145, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-J.; Yang, H.-R.; Wu, X.-L.; Peng, F.; Huang, Z.; Pu, L.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int. J. Biol. Macromol. 2019, 137, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Huang, M.; Zhao, M.; Sun, W.; Sun, X.; Zheng, F. Structural characterization and immuno-stimulating activities of a novel polysaccharide from Huangshui, a byproduct of Chinese Baijiu. Food Res. Int. 2020, 136, 109493. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-J.; Yang, H.-R.; Wang, H.-F.; Zong, M.-H.; Lou, W.-Y. Immune enhancement activity of a novel polysaccharide produced by Dendrobium officinale endophytic fungus Fusarium solani DO7. J. Funct. Foods 2019, 53, 266–275. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y.; Li, H.; Zhao, C.; Yang, Y.; Hou, Y.; et al. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; You, L.J.; Fu, X. Chemical property and impacts of different polysaccharide fractions from Fructus Mori. on lipolysis with digestion model in vitro. Carbohydr. Polym. 2017, 178, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cheng, H.; Xu, Z.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Antioxidant and anti-aging activities and structural elucidation of polysaccharides from Panax notoginseng root. Process Biochem. 2019, 78, 189–199. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- You, L.J. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. 2009, 2, 235–240. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Li, D.; Hu, Z.; He, Q.; Guo, Y.; Chong, Y.; Xu, J.; Qin, L. Lactoferrin alleviates acute alcoholic liver injury by improving redox-stress response capacity in female C57BL/6J mice. J. Agric. Food Chem. 2021, 69, 14856–14867. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Yamamoto, A.; Fujiwara, Y.; Yamane, T.; Satsu, H.; Ohkubo, I. Dipeptidyl peptidase-IV inhibitory activity of katsuobushi-derived peptides in Caco-2 cell assay and oral glucose tolerance test in ICR mice. J. Agric. Food Chem. 2020, 68, 6355–6367. [Google Scholar] [CrossRef]
- Turpin, J.; Frumence, E.; El Safadi, D.; Meilhac, O.; Krejbich-Trotot, P.; Viranaïcken, W. Improvement of immunodetection of the transcription factor C/EBP homologous protein by western blot. Anal. Biochem. 2020, 601, 113775. [Google Scholar] [CrossRef]
- Zeng, Y.-J.; Yang, H.-R.; Ou, X.-Y.; Su, H.-H.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Fungal polysaccharide similar with host Dendrobium officinale polysaccharide: Preparation, structure characteristics and biological activities. Int. J. Biol. Macromol. 2019, 141, 460–470. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef]
- Hu, H.-B.; Liang, H.-P.; Li, H.-M.; Yuan, R.-N.; Sun, J.; Zhang, L.-L.; Han, M.-H.; Wu, Y. Isolation, purification, characterization and antioxidant activity of polysaccharides from the stem barks of Acanthopanax leucorrhizus. Carbohydr. Polym. 2018, 196, 359–367. [Google Scholar] [CrossRef]
- Yang, H.; Zong, X.; Xu, Y.; Zeng, Y.; Zhao, H. Improvement of multiple-stress tolerance and ethanol production in yeast during very-high-gravity fermentation by supplementation of wheat-gluten hydrolysates and their ultrafiltration fractions. J. Agric. Food. Chem. 2018, 66, 10233–10241. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Maschio, D.A.; Carvalho, C.P.F.; Collares-Buzato, C.B. Influence of gender and time diet exposure on endocrine pancreas remodeling in response to high fat diet-induced metabolic disturbances in mice. Ann. Anat. 2015, 200, 88–97. [Google Scholar] [CrossRef]
- Bennett, C.M.; Guo, M.; Dharmage, S.C. HbA(1c) as a screening tool for detection of Type 2 diabetes: A systematic review. Diabetic Med. 2007, 24, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Bloomgarden, Z.T.; Zimmet, P.Z. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: Response to the International Expert Committee. Diabetes Care 2009, 32, e159. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Beechey, S.P. Molecular biology of intestinal glucose transport. Nutr. Res. Rev. 1995, 8, 27–41. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, S.; Xu, H.; Li, M.; Tong, Z.; Xu, M.; Xu, X. Hypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanism. Mol. Nutr. Food Res. 2016, 60, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.N.; Purslow, P.P.; Tosh, S.M.; Bakovic, M. Oat β-glucan depresses SGLT1- and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutr. Res. 2016, 36, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Wang, L.M.; Lonnroth, P.; Wesslau, C.; Pierce, J.H.; Smith, U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA 1997, 94, 4171–4175. [Google Scholar] [CrossRef]
- Eckstein, S.S.; Weigert, C.; Lehmann, R. Divergent Roles of IRS (Insulin Receptor Substrate) 1 and 2 in Liver and Skeletal Muscle. Curr. Med. Chem. 2017, 24, 1827–1852. [Google Scholar] [CrossRef]
- Han, J.; Yi, J.; Liang, F.; Jiang, B.; Xiao, Y.; Gao, S.; Yang, N.; Hu, H.; Xie, W.F.; Chen, W. X-3, a mangiferin derivative, stimulates AMP-activated protein kinase and reduces hyperglycemia and obesity in db/db mice. Mol. Cell. Endocrinol. 2015, 405, 63–73. [Google Scholar] [CrossRef]
- Foretz, M.; Ancellin, N.; Andreelli, F.; Saintillan, Y.; Grondin, P.; Kahn, A.; Thorens, B.; Vaulont, S.; Viollet, B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 2005, 54, 1331–1339. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]




| Methylated Sugars | Linkage Types | Molar Ratio (%) |
|---|---|---|
| 2,4-Me2-Manp | 1,3,6-linked-Manp | 4.6 |
| 2,3,4-Me3-Rhap | 1-linked-Rhap | 12.03 |
| 3-Me-Rhap | 1,2,4-linked-Rhap | 8.25 |
| 2,3,4,6-Me4-Glcp | 1-linked-Glcp | 29.45 |
| 2,3,4-Me3-Glcp | 1,6-linked-Glcp | 111.89 |
| 2,4-Me2-Glcp | 1,3,6-linked-Glcp | 46.12 |
| 2,4,6-Me3-Glcp | 1,3-linked-Glcp | 2.56 |
| 2,3-Me2-Xylp | 1,4-linked-Xylp | 17.82 |
| 2,3,4,6-Me4-Galp | 1-linked-Galp | 39.36 |
| 2,3,6-Me3-Galp | 1,4-linked-Galp | 16.67 |
| 2,3-Me3-Galp | 1,4,6-linked-Galp | 32.55 |
| 2,3,5-Me3-Araf | 1-linked-Araf | 23 |
| 2,3-Me2-Araf | 1,5-linked-Araf | 33.97 |
| 2-Me-Araf | 1,3,5-linked-Araf | 11.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, H.; Yang, H.-R.; Zeng, Y.-J. Hypoglycemic Effects of Novel Panax notoginseng Polysaccharide in Mice with Diet-Induced Obesity. Foods 2022, 11, 3101. https://doi.org/10.3390/foods11193101
Li X, Liu H, Yang H-R, Zeng Y-J. Hypoglycemic Effects of Novel Panax notoginseng Polysaccharide in Mice with Diet-Induced Obesity. Foods. 2022; 11(19):3101. https://doi.org/10.3390/foods11193101
Chicago/Turabian StyleLi, Xue, Hao Liu, Hui-Rong Yang, and Ying-Jie Zeng. 2022. "Hypoglycemic Effects of Novel Panax notoginseng Polysaccharide in Mice with Diet-Induced Obesity" Foods 11, no. 19: 3101. https://doi.org/10.3390/foods11193101
APA StyleLi, X., Liu, H., Yang, H.-R., & Zeng, Y.-J. (2022). Hypoglycemic Effects of Novel Panax notoginseng Polysaccharide in Mice with Diet-Induced Obesity. Foods, 11(19), 3101. https://doi.org/10.3390/foods11193101




