Effect of Cationic Modified Microcrystalline Cellulose on the Emulsifying Properties and Water/Oil Interface Behavior of Soybean Protein Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
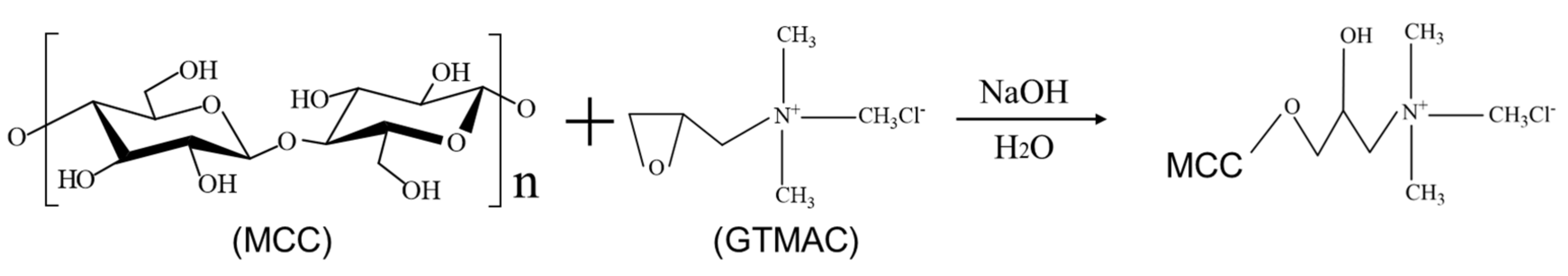
2.2. Cationic Modification of Microcrystalline Cellulose
2.3. Characterization of CMCC
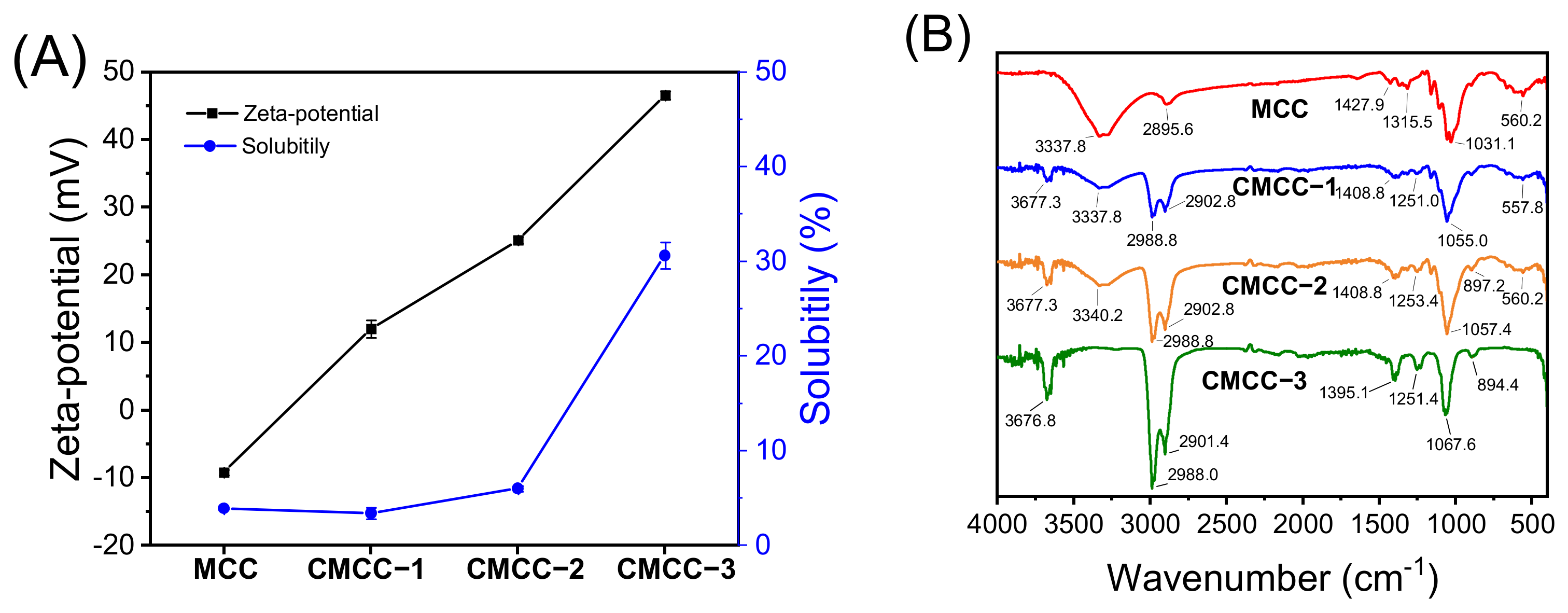
2.3.1. Measurement of Surface Charge and Solubility
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
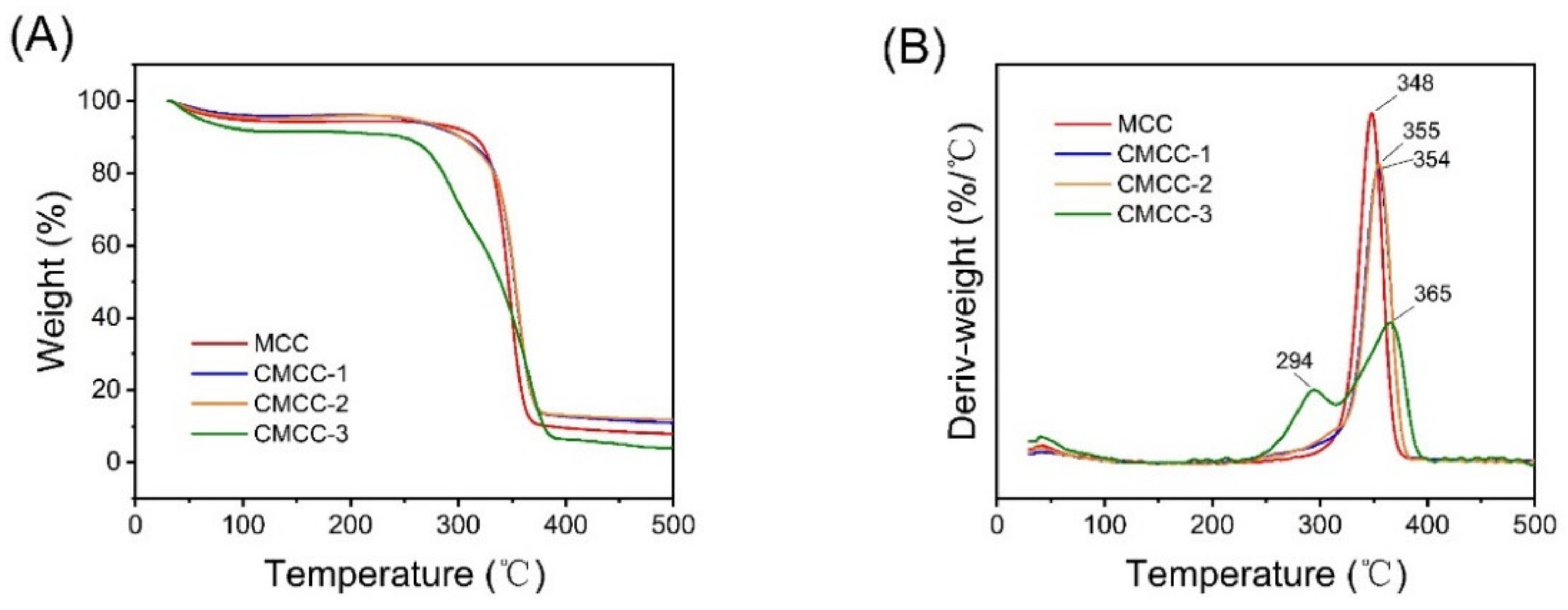
2.3.3. Thermogravimetric (TG) Analysis
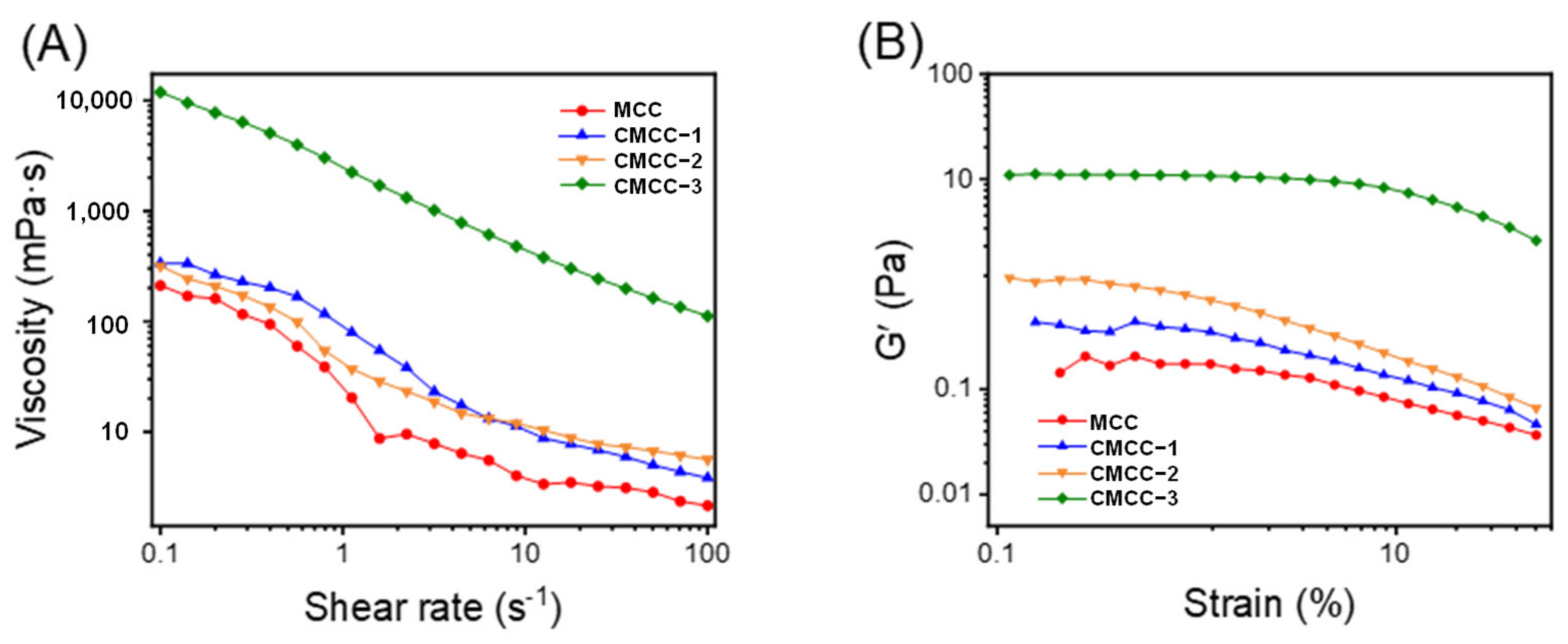
2.3.4. Rheological Properties Analysis
2.4. Preparation of Composite Emulsions Stabilized by Complexes of SPI and CMCC
2.5. Characterization of Emulsions
2.5.1. Measurement of Droplet Size, Zeta-Potential, and Morphology
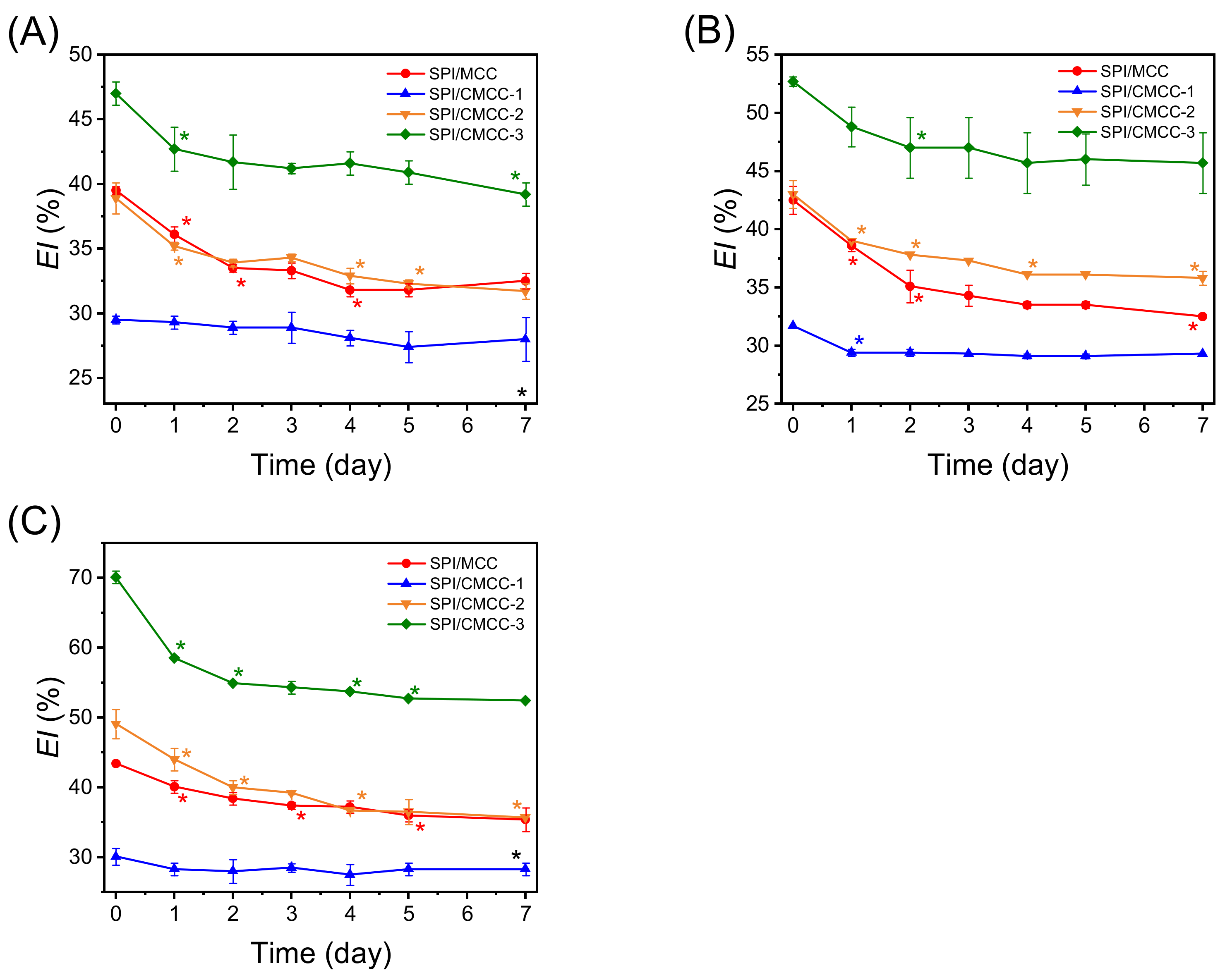
2.5.2. Measurement of Emulsification Index
2.6. Interfacial Behavior and Interaction Mechanism
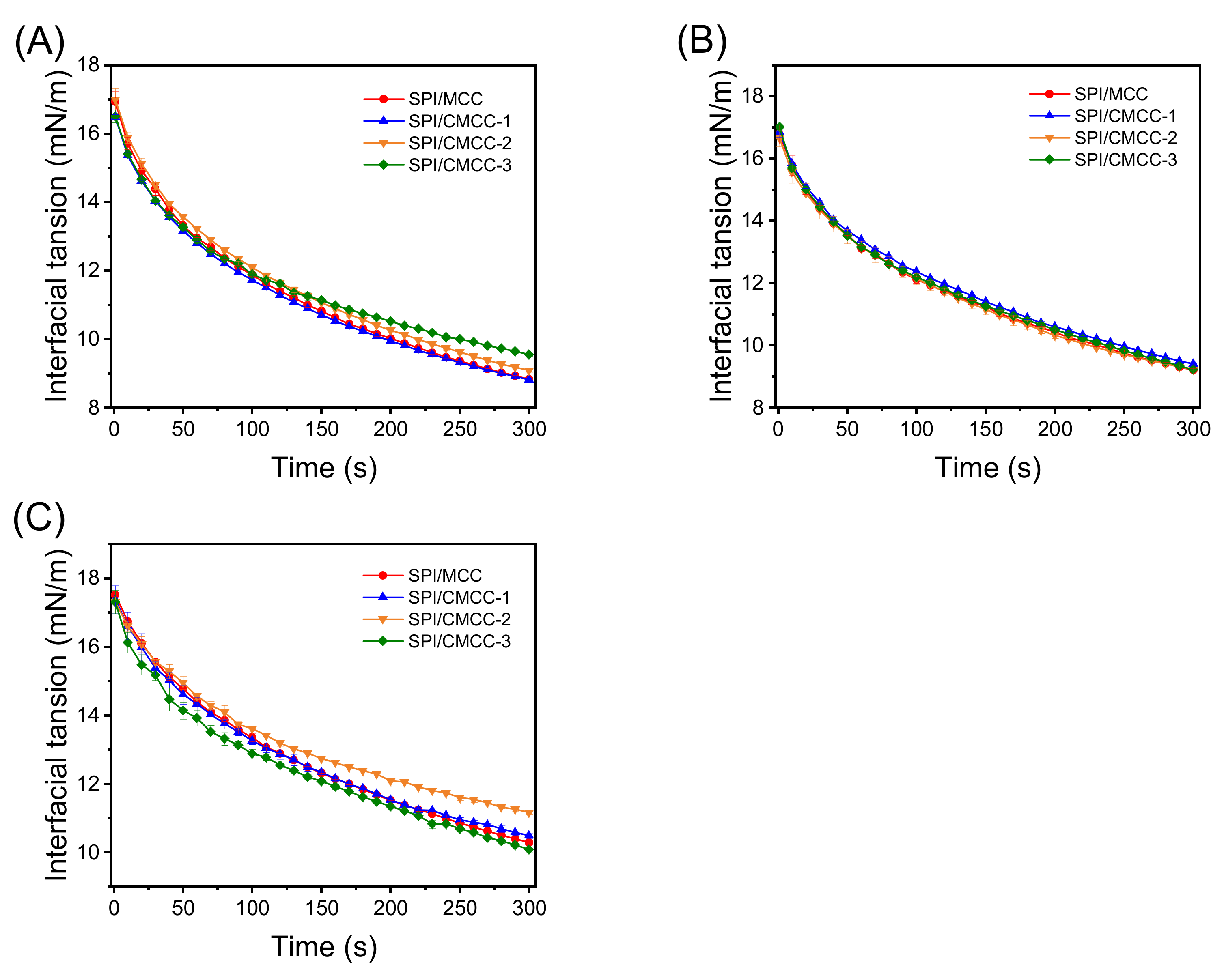
2.6.1. Measurement of Interfacial Tension
2.6.2. Measurement of Rheological Properties
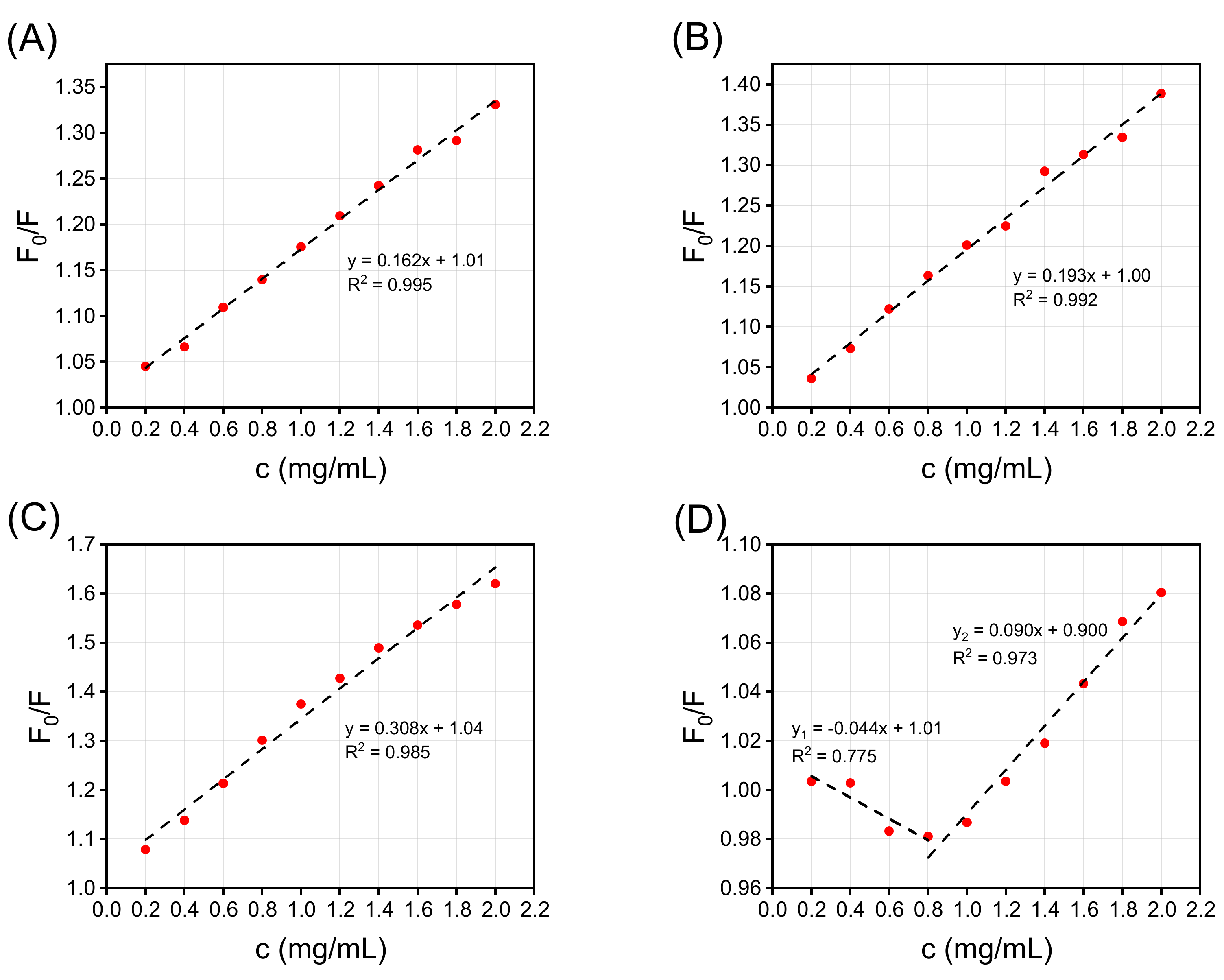
2.6.3. Fluorescence Quenching
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CMCC
3.2. Emulsifying Properties of SPI-CMCC Complexes
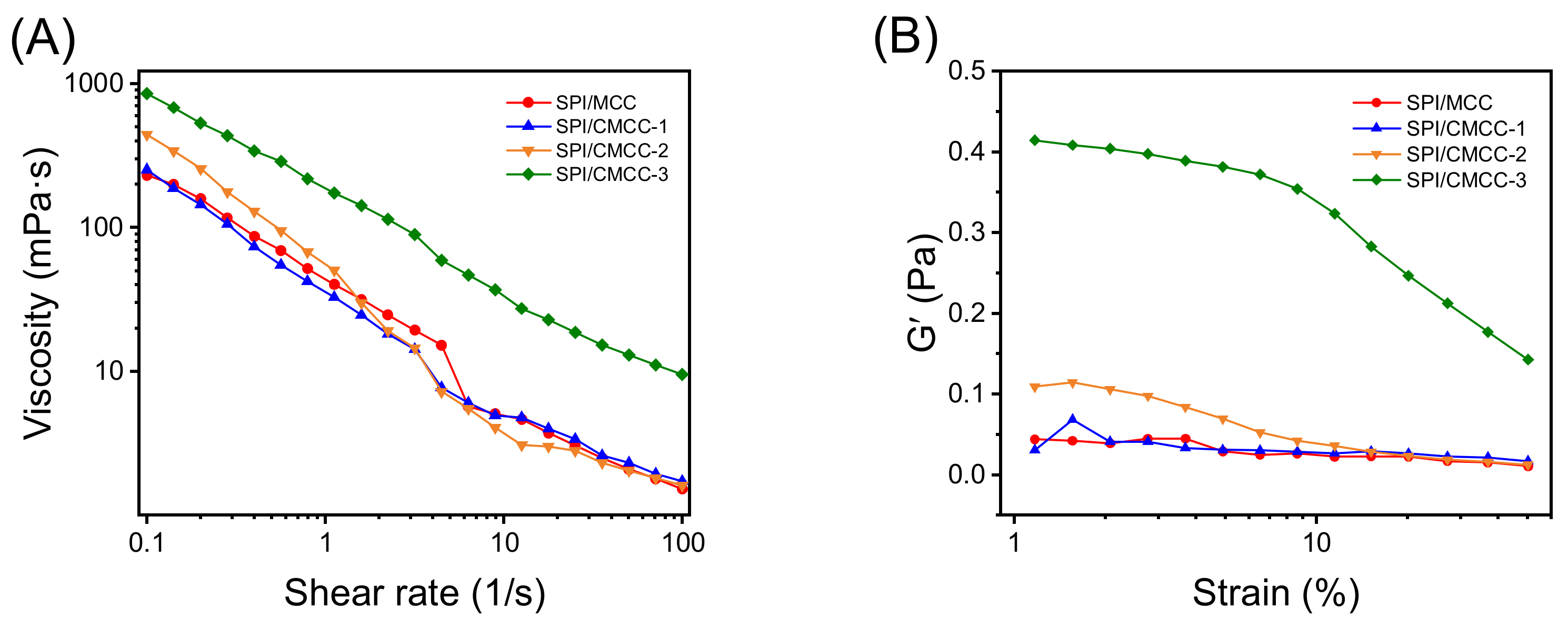
3.3. Interface Characteristics of SPI/CMCC Complexes and Their Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez-Avila, C.; Trujillo, A. Ultra-high pressure homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Tang, C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Shao, G.; Qu, D.; Zhao, H.; Yang, L.; Zhu, L.; He, Y.; Liu, H.; Zhu, D. Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocoll. 2020, 101, 105490. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Concentrated O/W pickering emulsions stabilized by soy protein/cellulose nanofibrils: Influence of pH on the emulsification performance. Food Hydrocoll. 2020, 108, 106025. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.R.; Li, Y.; Li, B. Influence of anionic alginate and cationic chitosan on physicochemical stability and carotenoids bioaccessibility of soy protein isolate-stabilized emulsions. Food Res. Int. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Kundu, D.; Banerjee, T. Development of microcrystalline cellulose based hydrogels for the in vitro delivery of Cephalexin. Heliyon 2020, 6, e03027. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, X.; Chen, L.; Li, X.; Xie, F. Starch/microcrystalline cellulose hybrid gels as gastric-floating drug delivery systems. Carbohydr. Polym. 2019, 215, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules 2009, 10, 334–341. [Google Scholar] [CrossRef]
- Hao, J.; Xu, S.; Xu, N.; Li, D.; Linhardt, R.J.; Zhang, Z. Impact of degree of oxidation on the physicochemical properties of microcrystalline cellulose. Carbohydr. Polym. 2017, 155, 483–490. [Google Scholar] [CrossRef]
- Huang, B.; Tang, Y.; Pei, Q.; Zhang, K.; Liu, D.; Zhang, X. Hemicellulose-based films reinforced with unmodified and cationically modified nanocrystalline cellulose. J. Polym. Environ. 2018, 26, 1625–1634. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Gong, J.; Kuang, Y.; Mo, L.; Song, T. Cellulose nanocrystals (CNCs) with different crystalline allomorph for oil in water Pickering emulsions. Carbohydr. Polym. 2018, 183, 303–310. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.M. Exploring the structure-function relationship of Great Northern and navy bean (Phaseolus vulgaris L.) protein hydrolysates: A study on the effect of enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 162, 1516–1525. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.-H.; Ge, Y.-S.; Liu, X.-R.; Jiang, F.-L.; Liu, Y. Biophysical studies on the interactions of a classic mitochondrial uncoupler with bovine serum albumin by spectroscopic, isothermal titration calorimetric and molecular modeling methods. J. Fluoresc. 2011, 21, 475–485. [Google Scholar] [CrossRef]
- Kuo, Y.N.; Hong, J. Investigation of solubility of microcrystalline cellulose in aqueous NaOH. Polym. Adv. Technol. 2005, 16, 425–428. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent advances in modified cellulose for tissue culture applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Berry, R.C.; Tam, K.C. Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 2013, 20, 1747–1764. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Ramalhete, S.M.; Skuze, W.J.; Soni, R.; Khimyak, Y.Z.; Edler, K.J.; Scott, J.L. Unravelling cationic cellulose nanofibril hydrogel structure: NMR spectroscopy and small angle neutron scattering analyses. Soft Matter 2018, 14, 255–263. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Quaternized cellulose hydrogels as sorbent materials and pickering emulsion stabilizing agents. Materials 2016, 9, 645. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.; Li, W.; Wei, W.; Jiang, M.; Liu, D.; Zhou, Z. An eco-friendly approach to preparing cellulose nanocrystals by precisely controlling the dissolution of natural cellulose in TBAH/H2O solvent. Cellulose 2020, 27, 9311–9324. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Sun, R. An efficient method for the synthesis of hemicellulosic derivatives with bifunctional groups in butanol/water medium and their rheological properties. Carbohydr. Polym. 2011, 83, 1922–1928. [Google Scholar] [CrossRef]
- Kim, D.H.; Song, Y.S. Rheological behavior of cellulose nanowhisker suspension under magnetic field. Carbohydr. Polym. 2015, 126, 240–247. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Niu, F.; Han, B.; Fan, J.; Kou, M.; Zhang, B.; Feng, Z.-J.; Pan, W.; Zhou, W. Characterization of structure and stability of emulsions stabilized with cellulose macro/nano particles. Carbohydr. Polym. 2018, 199, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef]
- Perez, A.A.; Carrara, C.R.; Sánchez, C.C.; Patino, J.M.R.; Santiago, L.G. Interactions between milk whey protein and polysaccharide in solution. Food Chem. 2009, 116, 104–113. [Google Scholar] [CrossRef]
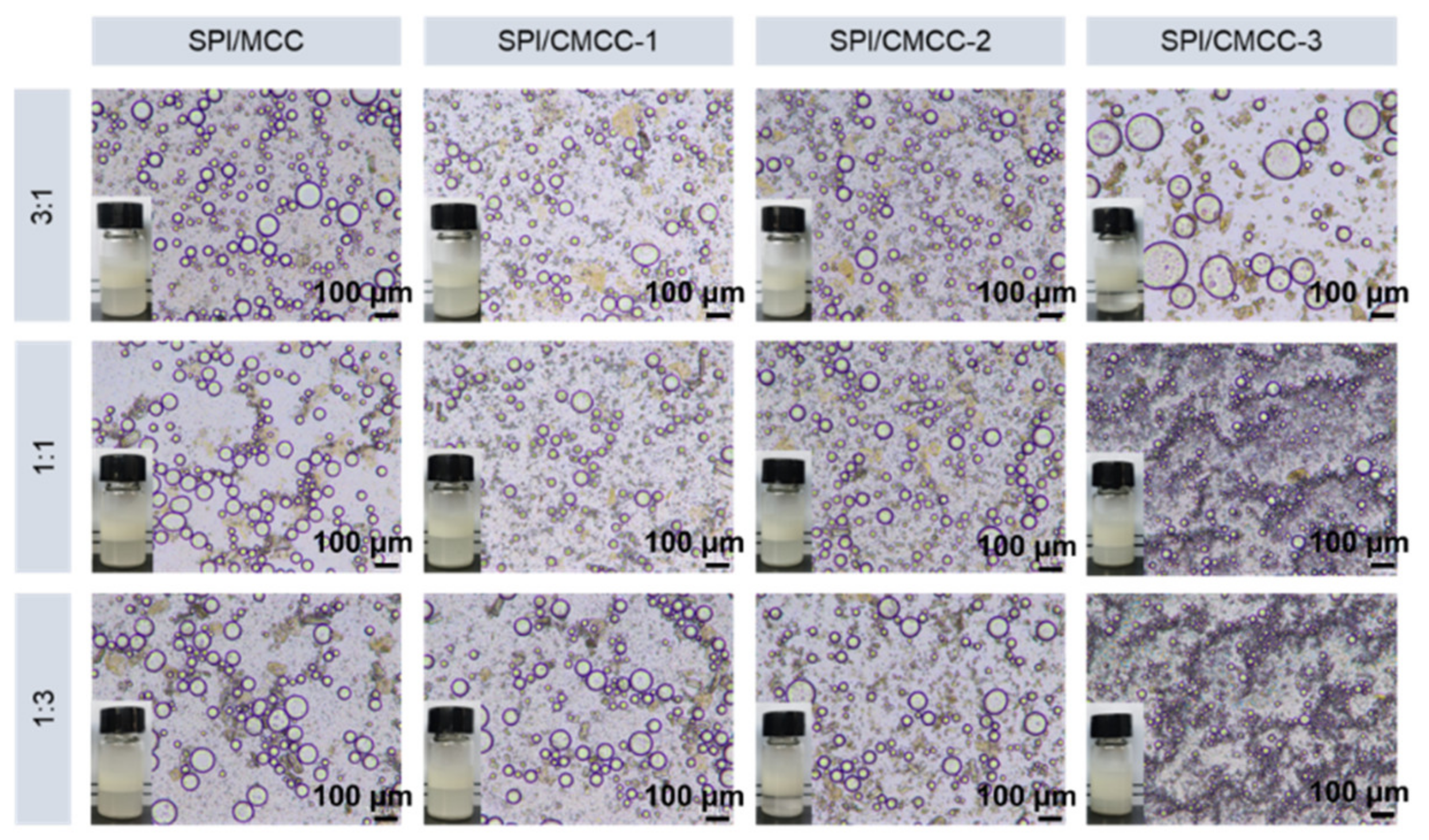
| Ratio of SPI: MCC/CMCC | SPI/MCC | SPI/CMCC-1 | SPI/CMCC-2 | SPI/CMCC-3 | |
|---|---|---|---|---|---|
| 3:1 | d43 (µm) | 60.70 ± 2.28 Bb | 50.81 ± 2.28 Bc | 45.76 ± 2.59 Bd | 97.06 ± 1.79 Aa |
| Zeta-potential (mV) | −41.1 ± 0.7 Ab | −41.8 ± 0.9 Ab | −40.9 ± 0.9 Ab | −2.4 ± 0.3 Ca | |
| 1:1 | d43 (µm) | 58.37.75 Ba | 50.05 ± 1.71 Bb | 46.99 ± 2.58 Bc | 24.81 ± 0.55 Bd |
| Zeta-potential (mV) | −44.0 ± 0.6 Bd | −42.8 ± 1.1 Adc | −41.4 ± 1.1 Ab | 45.5 ± 0.6 Ba | |
| 1:3 | d43 (µm) | 69.86 ± 1.67 Aa | 68.40 ± 2.59 Aa | 55.39 ± 2.08 Ab | 20.25 ± 0.46 Cc |
| Zeta-potential (mV) | −45.3 ± 0.8 Cc | −44.4 ± 0.9 Bc | −27.9 ± 1.0 Bb | 51.5 ± 1.0 Aa |
| Ratio of SPI: MCC/CMCC | γ(mN/m) | SPI/MCC | SPI/CMCC-1 | SPI/CMCC-2 | SPI/CMCC-3 |
|---|---|---|---|---|---|
| 3:1 | γ0 | 16.94 ± 0.30 Aa | 16.46 ± 0.07 Ba | 17.00 ± 0.31 Aa | 16.51 ± 0.18 Ba |
| γ∞ | 7.96 ± 0.17 Cc | 8.05 ± 0.07 Cb | 8.26 ± 0.14 Cb | 8.83 ± 0.01 Ba | |
| 1:1 | γ0 | 16.78 ± 0.30 Aa | 16.85 ± 0.17 ABa | 16.65 ± 0.27 Aa | 17.01 ± 0.08 ABa |
| γ∞ | 8.61 ± 0.09 Bab | 8.78 ± 0.04 Bab | 8.49 ± 0.12 Bb | 8.81 ± 0.27 Ca | |
| 1:3 | γ0 | 17.52 ± 0.04 Aa | 17.38 ± 0.41 Aa | 17.32 ± 0.32 Aa | 17.31 ± 0.34 Aa |
| γ∞ | 9.73 ± 0.05 Ab | 9.91 ± 0.14 Ab | 10.56 ± 0.03 Aa | 9.71 ± 0.17 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Feng, S.; Li, Z.; Jiang, M.; Xiao, Z.; Chen, L.; Zhang, Y. Effect of Cationic Modified Microcrystalline Cellulose on the Emulsifying Properties and Water/Oil Interface Behavior of Soybean Protein Isolate. Foods 2022, 11, 3100. https://doi.org/10.3390/foods11193100
Guo Y, Feng S, Li Z, Jiang M, Xiao Z, Chen L, Zhang Y. Effect of Cationic Modified Microcrystalline Cellulose on the Emulsifying Properties and Water/Oil Interface Behavior of Soybean Protein Isolate. Foods. 2022; 11(19):3100. https://doi.org/10.3390/foods11193100
Chicago/Turabian StyleGuo, Yunsi, Sirui Feng, Zhangpeng Li, Minghao Jiang, Zile Xiao, Lichun Chen, and Yue Zhang. 2022. "Effect of Cationic Modified Microcrystalline Cellulose on the Emulsifying Properties and Water/Oil Interface Behavior of Soybean Protein Isolate" Foods 11, no. 19: 3100. https://doi.org/10.3390/foods11193100
APA StyleGuo, Y., Feng, S., Li, Z., Jiang, M., Xiao, Z., Chen, L., & Zhang, Y. (2022). Effect of Cationic Modified Microcrystalline Cellulose on the Emulsifying Properties and Water/Oil Interface Behavior of Soybean Protein Isolate. Foods, 11(19), 3100. https://doi.org/10.3390/foods11193100





