Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing of Marinated Pork
2.2. Materials and Chemical Reagents
2.3. Simultaneous Extraction and Purification of HAs and PAHs in Unmarinated/Marinated Pork and Juice by QuEChERS
2.4. Separation and Identification of HAs in Unmarinated/Marinated Pork and Juice by UPLC-MS/MS
2.5. Separation and Identification of PAHs in Unmarinated/Marinated Pork and Juice by GC-MS/MS
2.6. Method Validation of HAs and PAHs
2.7. Determination of HA Precursors
2.8. Determination of Antioxidant Activity
2.9. Determination of Bioactive Compounds in Cinnamon Powder by UPLC-MS/MS
2.10. Determination of Bioactive Compounds in Green Tea Powder by HPLC-Diode Array Detection (DAD)
2.11. Determination of PAH Precursors
2.12. Principal Component Analysis (PCA)
2.13. Statistical Analysis
3. Results and Discussion
3.1. HA Contents in Unmarinated/Marinated Pork and Juice as Affected by Flavorings and Heating Time
3.1.1. Amino Acid Change in Unmarinated/Marinated Pork and Juice
3.1.2. Reducing Sugar Content Change in Unmarinated/Marinated Pork and Juice
3.1.3. Creatine and Creatinine Change in Unmarinated/Marinated Pork and Juice
3.1.4. Antioxidant Capacity of Unmarinated/Marinated Pork and Juice
3.2. Analysis of PAHs in Unmarinated/Marinated Pork and Juice
Analysis of PAH Precursors in Unmarinated/Marinated Pork and Juice
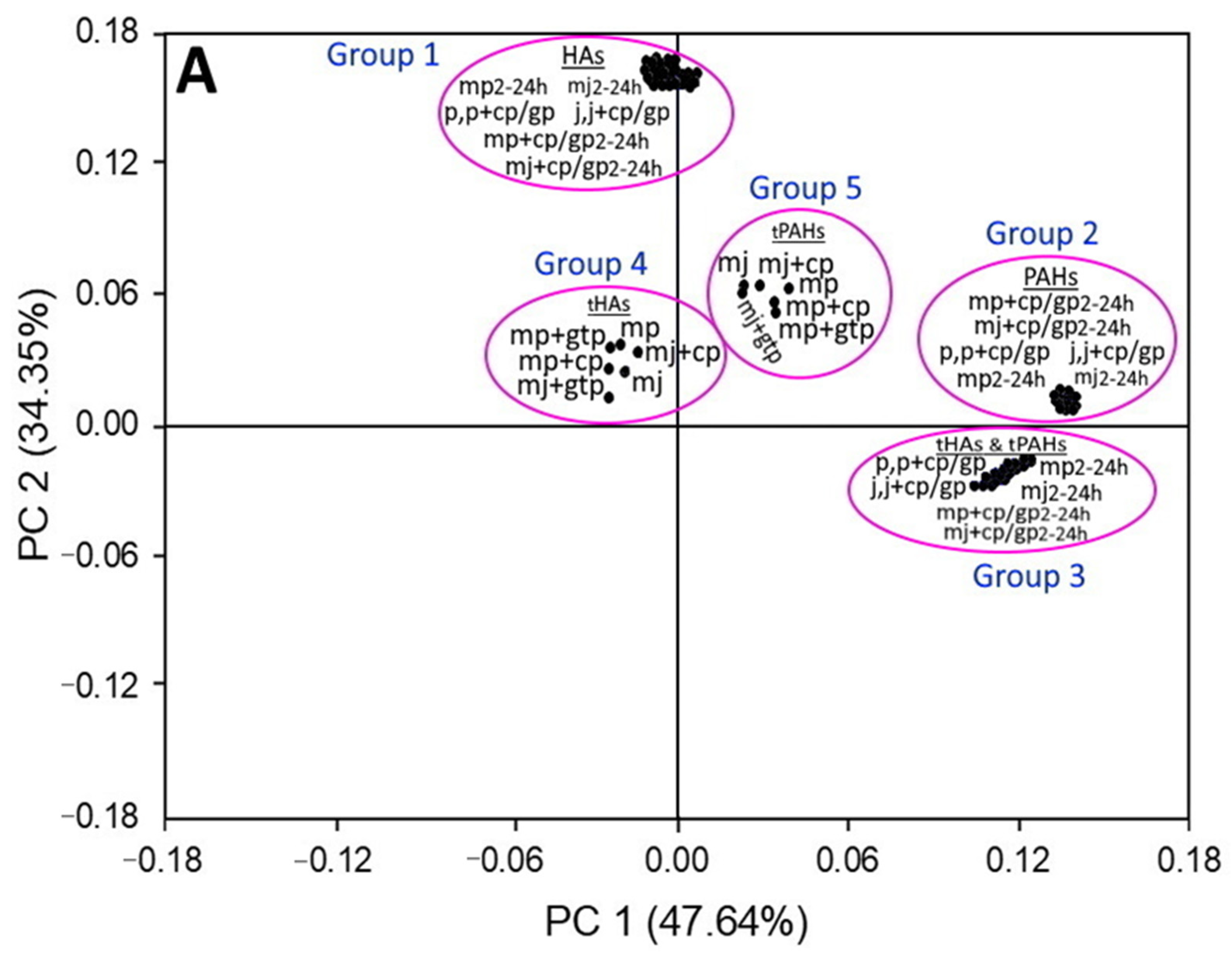
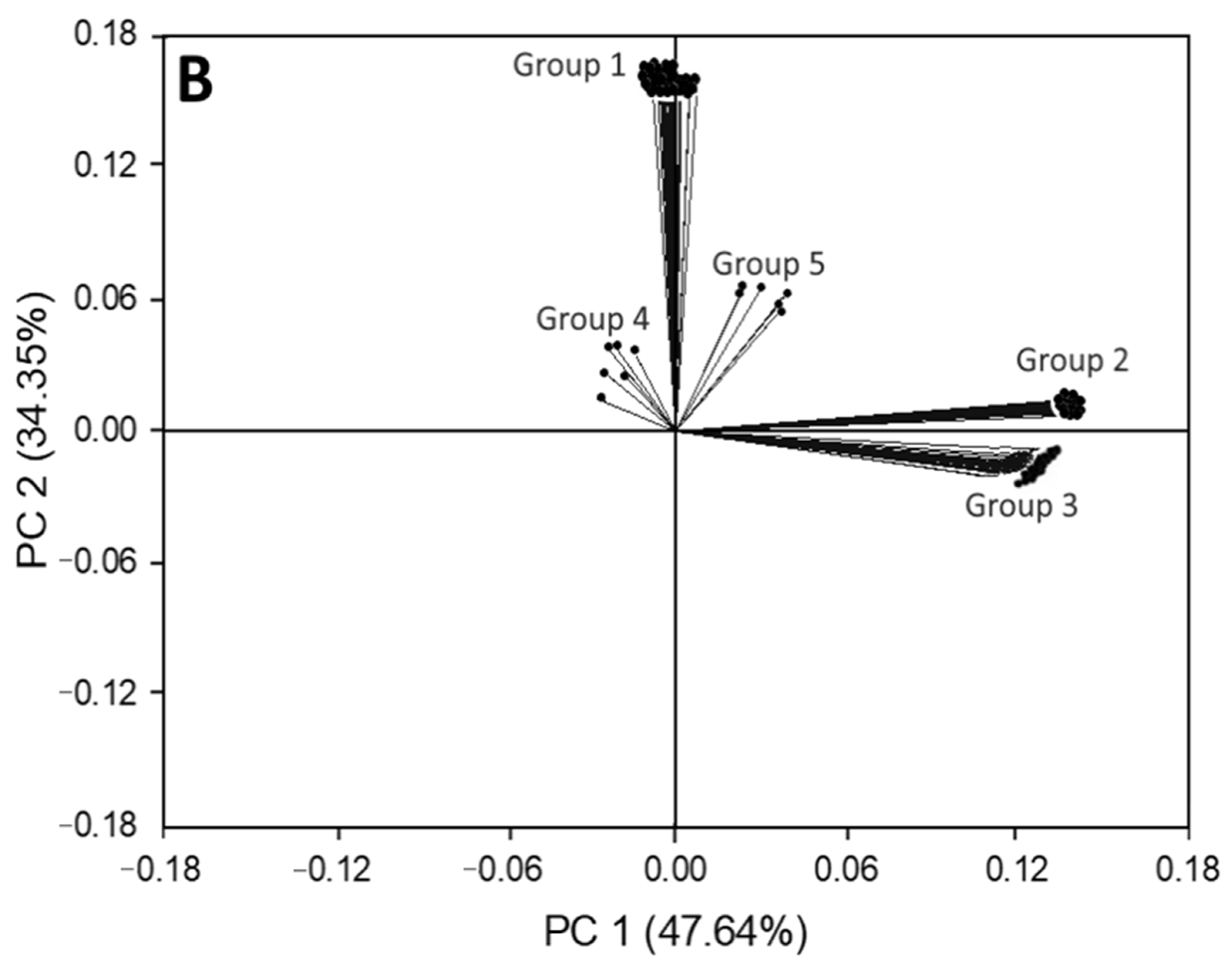
3.3. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Names |
| HAs | |
| DMIP | 2-amino-1,6-dimethylimidazo [4,5-b]-pyridine |
| IFP | 2-amino-1,6-dimethyl-furo [3,2-e]imidazo [4,5-b]-pyridine |
| iso-IQ | 2-amino-1-methyl-imidazo [4,5-f]-quinoline |
| IQ | 2-amino-3-methyl-imidazo [4,5-f]-quinoline |
| MeIQ | 2-amino-3,4-dimethyl-imidazo [4,5-f]- quinolin |
| IQ [4,5-b] | 2-amino-1-methyl-imidazo [4,5-b]-quinoline |
| IQx | 2-amino-3-methyl-imidazo [4,5-f]-quinoxaline |
| 8-MeIQx | 2-amino-3,8-dimethyl-imidazo [4,5-f]- quinoxaline |
| 7,8-DiMeIQx | 2-amino-3,7,8-dimethyl-imidazo [4,5-f]-quinoxaline |
| 4,8-DiMeIQx | 2-amino-3,4,8-dimethyl-imidazo [4,5-f]-quinoxaline |
| 4,7,8-TriMeIQx | 2-amino-3,4,7,8-dimethyl-imidazo [4,5-f]- quinoxaline (internal standard) |
| Phe-P-1 | 2-amino-5-phenylpyridine |
| AαC | 2-amino-9 H-pyrido [2,3-b]indole |
| Trp-P-2 | 3-amino-1-methyl-5 H-pyrido [4,3-b]indole |
| Trp-P-1 | 3-amino-1,4-methyl-5 H-pyrido [4,3-b]indole |
| GIu-P-2 | 2-aminodipyrido-[1,2-a:3′,2′-d]imidazole |
| Glu-P-1 | 2-amino-6-methyldipyrido-[1,2-a:3′,2′-d]imidazole |
| PhIP | 2-amino-1-methyl-6-phenylimidazo [4,5-b]-pyridine |
| Harman | 1-methyl-9 H-pyrido [3,4-b]indole |
| Norharman | 9 H-pyrido [3,4-b]indole |
| MeAαC | 2-Amino-3-methyl-9 H-pyrido [2,3-b]indole |
| PAHs | |
| Nap | naphthalene |
| AcPy | acenaphylene |
| AcP | acenaphthene |
| Flu | fluorene |
| Phe | phenanthrene |
| Ant | anthracene |
| FL | fluoranthene |
| Pyr | pyrene |
| BaA | benzo[a]anthracene |
| CHR | chrysene |
| BbFL | benzo[b]fluoranthene |
| BaP | benzo[a]pyrene |
| IP | indeno [1,2,3-c,d]pyrene |
| DBahA | dibenzo[a,h]anthracene |
| BghiP | benzo[g,h,i]perylene |
| BjF | benzo[j]fluoranthene |
| BcF | benzo[c]fluorene |
| CcdP | cyclopenta[c,d]pyrene |
| MCH | 5-methylchrysene |
| DBalP | dibenzo[a,l]pyrene |
| DBaeP | dibenzo[a,e]pyrene |
| DBaiP | dibenzo[a,i]pyrene |
| DBahP | dibenzo[a,h]pyrene |
| Triphenylene | Triphenylene (internal standard) |
References
- Hsu, K.Y.; Chen, B.H. Analysis and reduction of heterocyclic amines and cholesterol oxidation products in chicken by controlling flavorings and roasting condition. Food Res. Int. 2020, 131, 109004. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.T.; Lee, Y.T.; Inbaraj, B.S.; Sridhar, K.; Chen, B.H. Analysis and formation of polycyclic aromatic hydrocarbons and cholesterol oxidation products in thin slices of dried pork during processing. Food Chem. 2021, 353, 129474. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Chen, S.; Chen, C.J.; Huang, C.W.; Chen, B.H. Evaluation of analysis of polycyclic aromatic hydrocarbons by the QuEChERS method and gas chromatography–mass spectrometry and their formation in poultry meat as affected by marinating and frying. J. Agric. Food Chem. 2012, 60, 1380–1389. [Google Scholar] [CrossRef]
- Alaejos, M.S.; Afonso, A.M. Factors that affect the content of heterocyclic aromatic amines in foods. Comp. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Nagao, M.; Fujita, Y.; Wakabayashi, K.; Sugimura, T. Ultimate forms of mutagenic and carcinogenic heterocyclic amines produced by pyrolysis. Biochem. Biophys. Res. Commun. 1983, 114, 626–631. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Boczek, P.; Viell, B. Formation of the comutagenic beta-carboline norharman in a simple tryptophan-containing model system at low temperature (40 °C–80 °C). Adv. Exp. Med. Biol. 1999, 467, 693–696. [Google Scholar]
- Chen, B.H.; Chen, Y.C. Formation of polycyclic aromatic hydrocarbons in the smoke from heated model lipids and food lipids. J. Agric. Food Chem. 2001, 49, 5238–5243. [Google Scholar] [CrossRef]
- IARC, International Agency for Research on Cancer. Agents Classified by the IARC Monographs; IARC: Lyon, France, 2012; pp. 1–104. [Google Scholar]
- IARC. Some Naturally Occurring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation; IARC: Lyon, France, 1985. [Google Scholar]
- IARC, International Agency for Research on Cancer. Some food additives, feed additives and naturally occurring substances. IARC Monogr. Eval. Carcinog. Risks Hum. 1987, 31, 314. [Google Scholar]
- IARC, World Health Organization International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1993, 56, 165–242. [Google Scholar]
- Cheng, K.W.; Chen, F.; Wang, M. Heterocyclic amines: Chemistry and health. Mol. Nutr. Food Res. 2006, 50, 1150–1170. [Google Scholar] [CrossRef]
- IARC. IARC working group on the evaluation of carcinogenic risks to humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–853. [Google Scholar]
- Hsu, K.Y.; Inbaraj, B.S.; Chen, B.H. Evaluation of analysis of cholesterol oxidation products and heterocyclic amines in duck and their formation as affected by roasting methods. J. Food Drug Anal. 2020, 28, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kao, T.H.; Chen, C.J.; Huang, C.W.; Chen, B.H. Reduction of carcinogenic polycyclic aromatic hydrocarbons in meat by sugar-smoking and dietary exposure assessment in Taiwan. J. Agric. Food Chem. 2013, 61, 7645–7653. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Xu, Z.; Cao, D.; Chen, J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Cont. 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Phenolic compounds in beer inhibit formation of polycyclic aromatic hydrocarbons from charcoal-grilled chicken wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Yebra-Pimentel, I.; Martinez-Carballo, E.; Simal-Gandara, J.; Ferreira, I.M. Effect of beer marinades on formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. J. Agric. Food Chem. 2014, 62, 2638–2643. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Wang, C.; Zhu, Z.; Huang, M. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Cont. 2021, 119, 107490. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. The influence of natural antioxidants on polycyclic aromatic hydrocarbon formation in charcoal-grilled chicken wings. Food Cont. 2019, 98, 34–41. [Google Scholar] [CrossRef]
- Li, M.; Lin, S.; Wang, R.; Gao, D.; Bao, Z.; Chen, D.; Tang, Y.; Sun, N.; Zhang, S. Inhibitory effect and mechanism of various fruit extracts on the formation of heterocyclic aromatic amines and flavor changes in roast large yellow croaker (Pseudosciaena crocea). Food Cont. 2022, 131, 108410. [Google Scholar] [CrossRef]
- Lai, Y.W.; Lee, Y.T.; Cao, H.; Zhang, H.L.; Chen, B.H. Extraction of heterocyclic amines and polycyclic aromatic hydrocarbons from pork jerky and the effect of flavoring on formation and inhibition. Food Chem. 2022, 402, 134291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chien, J.; Inbaraj, B.S.; Chen, B.H. Formation and inhibition of cholesterol oxidation products during marinating of pig feet. J Agric. Food Chem. 2012, 60, 173–179. [Google Scholar] [CrossRef] [PubMed]
- TFDA, Taiwan Food and Drug Administration. Method of Test for Free Amino Acids, Glucosamine and Taurine in Foods; TFDA: Taipei, Taiwan, 2017. [Google Scholar]
- Gibis, M.; Loeffler, M. Effect of creatine and glucose on formation of heterocyclic amines in grilled chicken breasts. Foods 2019, 8, 616. [Google Scholar] [CrossRef]
- Serpen, A.; Gokmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, C.C.; Lin, Y.H.; Chen, B.H. Preparation of catechin nanoemulsion from oolong tea leaf waste and its inhibition of prostate cancer cells DU-145 and tumors in mice. Molecules 2021, 26, 3260. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef]
- SAS, Institute Inc. SAS® 9.4 Output Delivery System: User’s Guide, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Pfau, W.; Skog, K. Exposure to β-carbolines norharman and harman. J. Chromatogr. B 2004, 802, 115–126. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Z.; Guo, H.; Na, N.; Chen, L.; Zhang, D. Potential sources of β-carbolines harman and norharman in braised sauce meat. Sci. Agric. Sin. 2013, 46, 3003–3009. [Google Scholar]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Comp. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Nagao, M.; Yahagi, T.; Sugimura, T. Differences in effects of norharman with various classes of chemical mutagens and amounts of S-9. Biochem. Biophys. Res. Commun. 1978, 83, 373–378. [Google Scholar] [CrossRef]
- Bertram, H.C.; Aaslyng, M.D. Pelvic suspension and fast post-mortem chilling: Effects on technological and sensory quality of pork–A combined NMR and sensory study. Meat Sci. 2007, 76, 524–535. [Google Scholar] [CrossRef]
- Gibis, M.; Thellmann, K.; Thongkaew, C.; Weiss, J. Interaction of polyphenols and multilayered liposomal-encapsulated grape seed extract with native and heat-treated proteins. Food Hydrocoll. 2014, 41, 119–131. [Google Scholar] [CrossRef]
- Dundar, A.; Sarıçoban, C.; Yılmaz, M.T. Response surface optimization of effects of some processing variables on carcinogenic/mutagenic heterocyclic aromatic amine (HAA) content in cooked patties. Meat Sci. 2012, 91, 325–333. [Google Scholar] [CrossRef]
- Unal, K.; Karakaya, M.; Oz, F. The effects of different spices and fat types on the formation of heterocyclic aromatic amines in barbecued sucuk. J. Sci. Food Agric. 2018, 98, 719–725. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, antimicrobial mechanisms, and antibiotic activities of cinnamaldehyde against pathogenic bacteria in animal feeds and human foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Khatib, A.; Manap, M.Y.A.; Abdull Razis, A.F.; Hajeb, P. Inhibitory effect of mixture herbs/spices on formation of heterocyclic amines and mutagenic activity of grilled beef. Food Addit. Contam. A 2018, 35, 1911–1927. [Google Scholar] [CrossRef]
- Teng, H.; Chen, Y.; Lin, X.; Lv, Q.; Chai, T.T.; Wong, F.C.; Chen, L.; Xiao, J. Inhibitory effect of the extract from Sonchus olearleu on the formation of carcinogenic heterocyclic aromatic amines during the pork cooking. Food Chem. Toxicol. 2019, 129, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Quelhas, I.; Petisca, C.; Viegas, O.; Melo, A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of green tea marinades on the formation of heterocyclic aromatic amines and sensory quality of pan-fried beef. Food Chem. 2010, 122, 98–104. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Shirotori, T.; Takabatake, E. Determination of polycyclic aromatic hydrocarbons in foods 2) 3, 4-benzopyrene in Japanese daily foods. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1973, 14, 173–178. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Owens, C.V.; Todd Skeen, J. Does glucose enhance the formation of nitrogen containing polycyclic aromatic compounds and polycyclic aromatic hydrocarbons in the pyrolysis of proline? Fuel 2004, 83, 1417–1432. [Google Scholar] [CrossRef]
- Nie, W.; Cai, K.Z.; Li, Y.Z.; Zhang, S.; Wang, Y.; Guo, J.; Chen, C.G.; Xu, B.C. Small molecular weight aldose (d-Glucose) and basic amino acids (l-Lysine, l-Arginine) increase the occurrence of PAHs in grilled pork sausages. Molecules 2018, 23, 3377. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic aromatic hydrocarbons (PAH) formation from the pyrolysis of different municipal solid waste fractions. Waste Manag. 2015, 36, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Ray, C.A. Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef]
- Sinaga, K.; Legowo, A.; Supriatna, E.; Pramono, Y. Reduction of benzo(a)pyrene in charcoal grilled duck meat by marinating with andaliman (Zanthoxylum acanthopodium, DC) fruit juice. J. Indon. Trop. Anim. Agric. 2016, 41, 204–208. [Google Scholar] [CrossRef][Green Version]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef]
- Kim, Y.; Goodner, K.L.; Park, J.D.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Janoszka, B. HPLC-fluorescence analysis of polycyclic aromatic hydrocarbons (PAHs) in pork meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2011, 126, 1344–1353. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.M.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Qi, J.; Yu, Y.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Effect of tea marinades on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled chicken wings. Food Cont. 2018, 93, 325–333. [Google Scholar] [CrossRef]
- Cao, J.; Yang, L.; Ye, B.; Chai, Y.; Liu, L. Effect of apple polyphenol and three antioxidants on the formation of polycyclic aromatic hydrocarbon in barbecued pork. Polycycl. Aromat. Compd. 2022; in press. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Li, C.; Xu, X.; Zhou, G. Effects of phenolic acid marinades on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled chicken wings. J. Food Prot. 2019, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Bhat, R.; Sharma, A. Status of free radicals in indian monsooned coffee beans γ-irradiated for disinfestation. J. Agric Food Chem. 2003, 51, 4960–4964. [Google Scholar] [CrossRef]



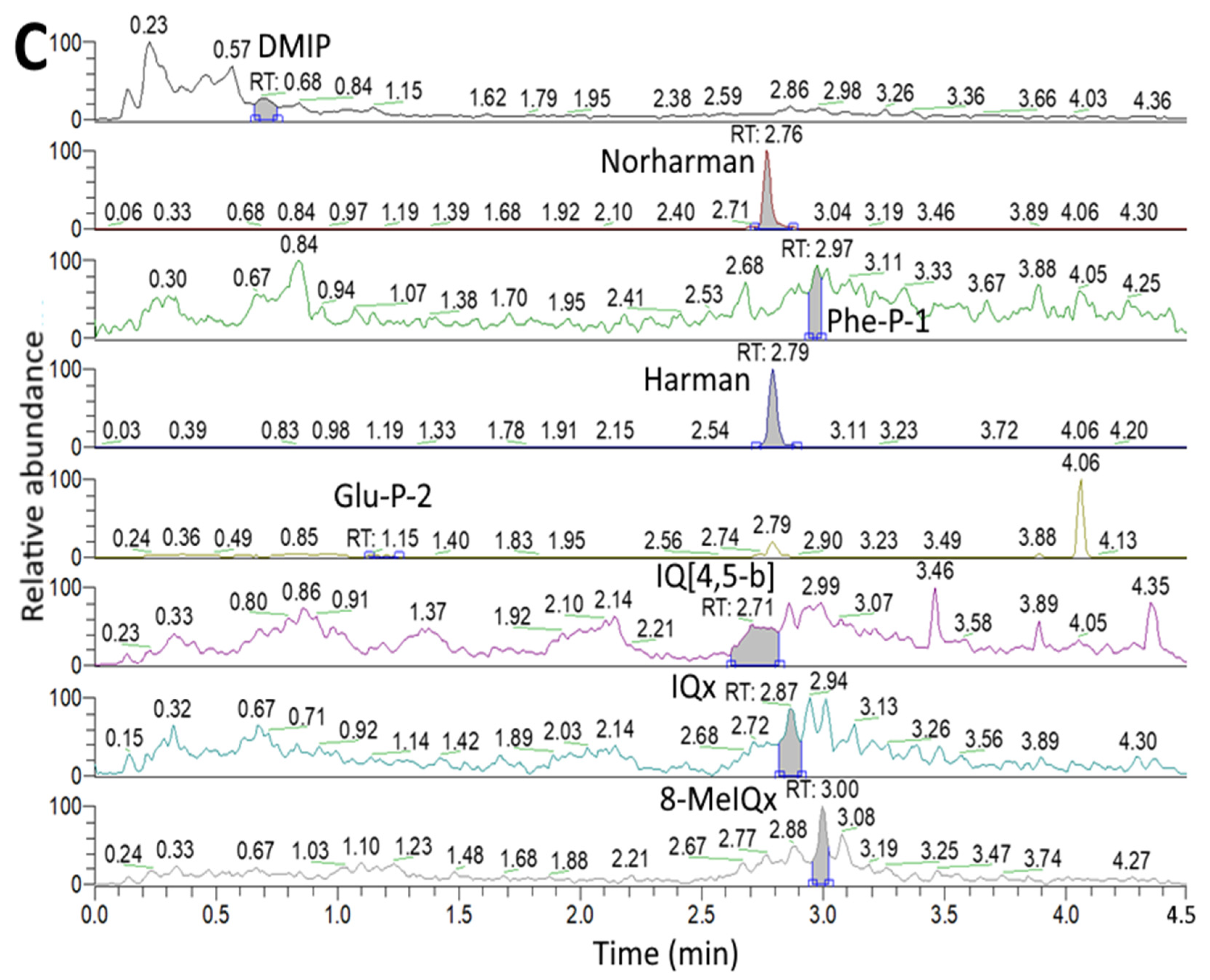
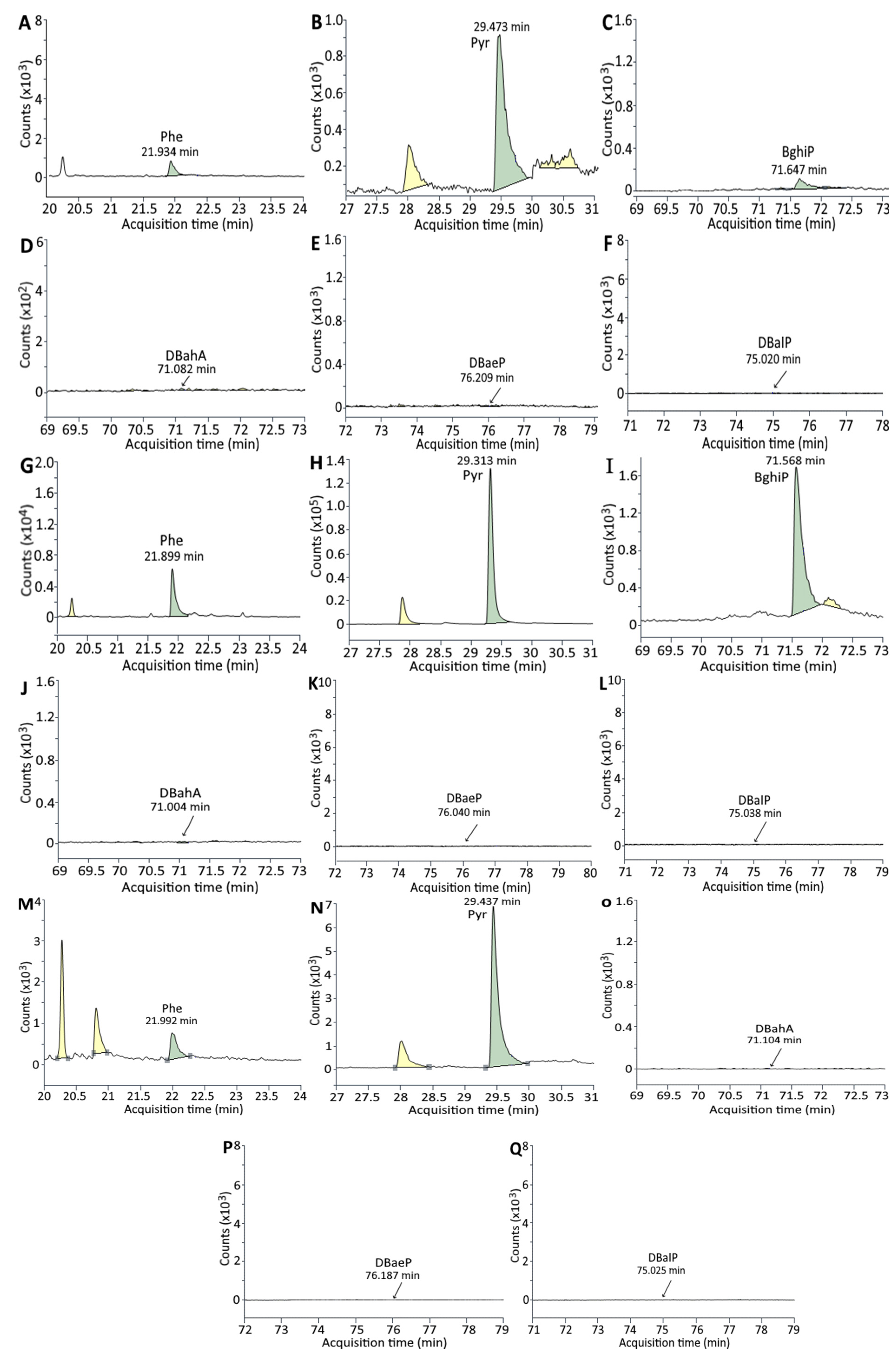

| HAs | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| Unmarinated pork/marinated pork 1 | ||||||
| DMIP | nd 2 | nd | nd | 0.21 ± 0.02 c | 0.367 ± 0.04 b | 0.86 ± 0.05 a |
| Norharman | 7.92 ± 0.30 f | 79.66 ± 2.27 e | 120.75 ± 5.37 d | 212.19 ± 9.04 c | 243.57 ± 9.41 b | 335.49 ± 15.04 a |
| Phe-P-1 | nd | 23.98 ± 1.93 e | 30.57 ± 1.68 d | 64.66 ± 4.07 c | 118.57 ± 6.81 b | 189.12 ± 4.67 a |
| Harman | 53.51 ± 4.48 f | 403.18 ± 6.46 e | 686.35 ± 12.27 d | 1243.92 ± 11.53 c | 1624.68 ± 18.25 b | 2456.96 ± 41.45 a |
| Glu-P-2 | 0.15 ± 0.01 c | 0.58 ± 0.09 b | 0.63 ± 0.10 b | 0.70 ± 0.08 b | 0.99 ± 0.10 a | 1.01 ± 0.16 a |
| IQ [4,5-b] | nd | nd | nd | nd | 0.15 ± 0.02 b | 1.53 ± 0.10 a |
| IQx | nd | nd | 0.21 ± 0.01 b | 0.24 ± 0.04 b | 0.28 ± 0.02 b | 0.47 ± 0.04 a |
| 8-MeIQx | nd | nd | nd | nd | trace 3 | 0.10 ± 0.02 a |
| PhIP | nd | nd | 0.24 ± 0.03 d | 0.32 ± 0.05 c | 0.68 ± 0.05 b | 0.95 ± 0.12 a |
| Total | 61.58 ± 4.69 f | 507.39 ± 10.69 e | 838.75 ± 15.97 d | 1522.23 ± 14.34 c | 1989.28 ± 33.09 b | 2986.47 ± 60.52 a |
| Unmarinated pork/marinated pork + 0.5% cinnamon powder 1 | ||||||
| DMIP | nd | nd | 0.20 ± 0.08 c | 0.37 ± 0.05 b | 0.42 ± 0.12 b | 0.98 ± 0.04 a |
| Norharman | 8.44 ± 0.61 f | 64.32 ± 3.47 e | 110.40 ± 8.48 d | 137.97 ± 6.25 c | 180.39 ± 3.75 b | 279.45 ± 8.89 a |
| Phe-P-1 | nd | 4.27 ± 0.03 e | 9.87 ± 0.81 d | 17.78 ± 0.75 c | 31.31 ± 1.75 b | 69.67 ± 1.93 a |
| Harman | 50.18 ± 3.50 f | 285.69 ± 7.25 e | 607.12 ± 11.64 d | 891.23 ± 21.56 c | 1376.23 ± 26.68 b | 2287.77 ± 42.26 a |
| Glu-P-2 | 0.20 ± 0.03 e | 0.50 ± 0.05 d | 0.65 ± 0.10 d | 0.87 ± 0.12 c | 1.47 ± 0.10 b | 1.73 ± 0.08 a |
| IQ [4,5-b] | nd | nd | nd | nd | nd | 1.62 ± 0.18 a |
| IQx | nd | 0.19 ± 0.01 c | 0.25 ± 0.03 c | 0.32 ± 0.05 b | 0.36 ± 0.05 b | 0.54 ± 0.02 a |
| 8-MeIQx | nd | nd | trace | trace | 0.06 ± 0.01 b | 0.15 ± 0.04 a |
| Total | 58.82 ± 4.10 f | 354.98 ± 10.47 e | 728.48 ± 20.86 d | 1048.53 ± 28.13 c | 1590.24 ± 30.21 b | 2641.90 ± 44.61 a |
| Unmarinated pork/marinated pork + 0.5% green tea powder 1 | ||||||
| DMIP | nd | nd | nd | 0.25 ± 0.12 b | 0.34 ± 0.04 b | 0.50 ± 0.08 a |
| Norharman | 8.54 ± 0.53 e | 69.32 ± 3.60 d | 107.53 ± 5.06 c | 177.52 ± 6.34 b | 238.32 ± 19.22 a | 263.84 ± 28.33 a |
| Phe-P-1 | nd | 16.35 ± 0.93 e | 29.40 ± 1.27 d | 61.43 ± 2.66 c | 88.39 ± 6.68 b | 174.72 ± 9.83 a |
| Harman | 52.03 ± 1.52 f | 346.31 ± 29.96 e | 629.19 ± 17.17 d | 1051.16 ± 29.38 c | 1606.89 ± 21.77 b | 2086.39 ± 24.34 a |
| Glu-P-2 | 0.18 ± 0.06 c | 0.52 ± 0.15 b | 0.90 ± 0.07 a | 0.92 ± 0.09 a | 0.97 ± 0.15 a | 0.99 ± 0.18 a |
| IQ [4,5-b] | nd | nd | nd | nd | nd | 0.87 ± 0.10 a |
| IQx | nd | nd | nd | 0.25 ± 0.011 b | 0.28 ± 0.05 b | 0.45 ± 0.05 a |
| 8-MeIQx | nd | nd | nd | trace | trace | 0.16 ± 0.03 a |
| PhIP | nd | nd | nd | 0.18 ± 0.02 c | 0.30 ± 0.09 b | 0.85 ± 0.05 a |
| Total | 60.76 ± 1.69 f | 432.50 ± 32.36 e | 767.02 ± 22.01 d | 1291.71 ± 34.05 c | 1935.50± 40.63 b | 2528.75 ± 57.76 a |
| Unmarinated juice/marinated juice 1 | ||||||
| DMIP | nd 2 | nd | nd | nd | 0.27 ± 0.02 b | 1.33 ± 0.04 a |
| Norharman | 16.13 ± 0.68 f | 45.50 ± 0.626 e | 59.25 ± 2.26 d | 94.23 ± 3.06 c | 134.02 ± 5.93 b | 204.82 ± 8.24 a |
| Phe-P-1 | 2.41 ± 0.17 d | 11.11 ± 0.49 c | 11.65 ± 0.85 c | 13.14 ± 0.55 c | 54.08 ± 2.70 b | 102.72 ± 4.67 |
| Harman | 120.42 ± 1.44 f | 304.32 ± 10.70 e | 373.96 ± 7.65 d | 598.07 ± 4.40 c | 1016.41 ± 9.09 b | 1481.22 ± 41.84 a |
| Glu-P-2 | 0.30 ± 0.05 c | 0.35 ± 0.01 c | 0.57 ± 0.05 b | 0.59 ± 0.01 b | 0.59 ± 0.04 b | 0.96 ± 0.23 a |
| IQ [4,5-b] | nd | nd | nd | nd | nd | trace 3 |
| IQx | nd | 0.18 ± 0.09 c | 0.26 ± 0.06 b | 0.26 ± 0.07 b | 0.36 ± 0.05 a | 0.37 ± 0.04 a |
| 8-MeIQx | nd | nd | nd | nd | 0.06 ± 0.01 b | 0.18 ± 0.02 a |
| PhIP | nd | nd | nd | 0.17 ± 0.01 c | 0.22 ± 0.03 b | 0.48 ± 0.04 a |
| Total | 139.27 ± 2.16 f | 361.46 ± 11.02 e | 445.68 ± 10.17 d | 706.46 ± 4.68 c | 1206.01 ± 13.55 b | 1792.08 ± 54.65 a |
| HAs (ng/g) | Unmarinated juice/marinated juice + 0.5% cinnamon powder 1 | |||||
| DMIP | nd | nd | nd | nd | nd | 0.51 ± 0.03 a |
| Norharman | 16.91 ± 0.38 e | 45.54 ± 2.20 d | 48.39 ± 2.70 | 77.62 ± 5.76 c | 116.80 ± 3.50 b | 141.38 ± 5.40 a |
| Phe-P-1 | 1.72 ± 0.14 e | 9.95 ± 0.19 d | 18.95 ± 0.93 c | 19.53 ± 1.22 c | 23.64 ± 1.53 b | 73.03 ± 3.93 a |
| Harman | 116.23 ± 3.35 f | 273.02 ± 6.80 e | 309.93 ± 5.27 d | 538.04 ± 7.62 c | 900.94 ± 14.20 b | 1065.64 ± 22.30 a |
| Glu-P-2 | 0.38 ± 0.06 c | 0.36 ± 0.01 c | 0.53 ± 0.08 b | 0.55 ± 0.05 b | 0.61 ± 0.03 b | 0.71 ± 0.04 a |
| IQ [4,5-b] | nd | nd | nd | nd | nd | 0.22 ± 0.04 a |
| IQx | nd | 0.21 ± 0.01 c | 0.22 ± 0.06 c | 0.36 ± 0.04 b | 0.34 ± 0.03 b | 0.42 ± 0.06 a |
| 8-MeIQx | nd | nd | nd | trace | trace | 0.10 ± 0.03 a |
| PhIP | nd | nd | nd | nd | 0.13 ± 0.02 b | 0.32 ± 0.02 a |
| Total | 135.23 ± 3.47 f | 329.08 ± 9.15 e | 378.02 ± 7.84 d | 636.10 ± 11.33 c | 1042.46 ± 19.24 b | 1282.32 ± 30.81 a |
| HAs (ng/g) | Unmarinated juice/marinated juice + 0.5% green tea powder 1 | |||||
| DMIP | nd | nd | nd | nd | nd | 0.98 ± 0.05 a |
| Norharman | 18.23 ± 0.88 e | 23.21 ± 2.05 e | 32.39 ± 2.26 d | 50.15 ± 2.89 c | 92.06 ± 3.47 b | 130.70 ± 5.45 a |
| Phe-P-1 | 0.32 ± 0.07 d | 0.50 ± 0.04 d | 0.91 ± 0.17 cd | 1.63 ± 0.26 c | 7.47 ± 0.41 b | 15.83 ± 1.41 a |
| Harman | 118.63 ± 2.33 f | 137.13 ± 7.52 e | 202.93 ± 4.54 d | 385.25 ± 25.68 c | 778.41 ± 35.98 b | 1249.16 ± 31.11 a |
| Glu-P-2 | 0.35 ± 0.05 d | 0.47 ± 0.04 cd | 0.56 ± 0.06 c | 0.70 ± 0.01 b | 0.70 ± 0.09 b | 0.96 ± 0.13 a |
| IQ [4,5-b] | nd | nd | nd | nd | nd | 0.45 ± 0.05 a |
| IQx | nd | nd | 0.21 ± 0.03 b | 0.27 ± 0.02 b | 0.27 ± 0.06 b | 0.37 ± 0.07 a |
| 8-MeIQx | nd | nd | nd | trace | trace | 0.10 ± 0.02 a |
| Total | 137.52 ± 3.24 e | 161.30 ± 9.24 e | 237.001 ± 6.431 d | 438.00 ± 28.570 c | 878.91 ± 39.72 b | 1398.56 ± 38.23 a |
| AA (mg/g) | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| marinated pork 1 | ||||||
| Asp | 0.41 ± 0.04 | 0.40 ± 0.59 | 0.36 ± 0.01 | 0.40 ± 0.01 | 0.28 ± 0.02 | 0.41 ± 0.02 |
| Glu | 0.94 ± 0.05 | 0.77 ± 0.00 | 0.76 ± 0.04 | 0.82 ± 0.03 | 0.69 ± 0.01 | 0.77 ± 0.01 |
| Ser | 0.23 ± 0.01 | 0.21 ± 0.00 | 0.20 ± 0.00 | 0.21 ± 0.00 | 0.19 ± 0.00 | 0.21 ± 0.00 |
| His | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.00 | 0.08 ± 0.01 | 0.11 ± 0.00 |
| Gly | 0.50 ± 0.02 | 0.46 ± 0.03 | 0.48 ± 0.04 | 0.38 ± 0.01 | 0.46 ± 0.05 | 0.43 ± 0.01 |
| Thr | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.08 ± 0.01 | 0.10 ± 0.00 | 0.14 ± 0.01 | 0.16 ± 0.02 |
| Arg | 0.49 ± 0.01 | 0.42 ± 0.01 | 0.42 ± 0.02 | 0.40 ± 0.01 | 0.42 ± 0.01 | 0.42 ± 0.00 |
| Ala | 0.47 ± 0.01 | 0.43 ± 0.00 | 0.43 ± 0.03 | 0.41 ± 0.01 | 0.39 ± 0.00 | 0.42 ± 0.00 |
| Tyr | 0.22 ± 0.00 2 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.30 ± 0.02 | 0.16 ± 0.00 |
| Cys | 0.15 ± 0.01 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.00 |
| Val | 0.23 ± 0.04 | 0.24 ± 0.00 | 0.20 ± 0.06 | 0.28 ± 0.04 | 0.17 ± 0.01 | 0.19 ± 0.02 |
| Met | 0.23 ± 0.14 | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.23 ± 0.07 | 0.14 ± 0.00 | 0.16 ± 0.01 |
| Phe | 0.21 ± 0.01 | 0.23 ± 0.00 | 0.23 ± 0.01 | 0.20 ± 0.03 | 0.17 ± 0.01 | 0.20 ± 0.00 |
| Ile | 0.24 ± 0.06 | 0.28 ± 0.00 | 0.27 ± 0.01 | 0.23 ± 0.06 | 0.30 ± 0.01 | 0.27 ± 0.01 |
| Leu | 0.54 ± 0.20 | 0.66 ± 0.01 | 0.69 ± 0.03 | 0.67 ± 0.02 | 0.50 ± 0.01 | 0.55 ± 0.01 |
| Lys | 0.53 ± 0.05 | 0.55 ± 0.01 | 0.59 ± 0.04 | 0.61 ± 0.02 | 0.55 ± 0.04 | 0.53 ± 0.02 |
| Pro | 0.26 ± 0.00 | 0.40 ± 0.02 | 0.41 ± 0.04 | 0.45 ± 0.01 | 0.43 ± 0.03 | 0.42 ± 0.01 |
| Total | 5.81 ± 0.14 | 5.83 ± 0.63 | 5.45 ± 0.10 | 5.61 ± 0.03 | 5.24± 0.02 | 5.45 ± 0.04 |
| marinated pork + 0.5% cinnamon powder 1 | ||||||
| Asp | 0.42 ± 0.01 | 0.40 ± 0.02 | 0.36 ± 0.01 | 0.38 ± 0.01 | 0.41 ± 0.01 | 0.36 ± 0.01 |
| Glu | 0.78 ± 0.02 | 0.72 ± 0.02 | 0.68 ± 0.00 | 0.74 ± 0.00 | 0.78 ± 0.00 | 0.80 ± 0.01 |
| Ser | 0.21 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.00 | 0.20 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.00 |
| His | 0.03 ± 0.01 | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Gly | 0.58 ± 0.02 | 0.55 ± 0.04 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.10 ± 0.00 |
| Thr | 0.14 ± 0.01 | 0.15 ± 0.00 | 0.42 ± 0.01 | 0.43 ± 0.00 | 0.44 ± 0.00 | 0.49 ± 0.00 |
| Arg | 0.53 ± 0.00 | 0.48 ± 0.00 | 0.96 ± 0.00 | 0.98 ± 0.01 | 1.02 ± 0.01 | 1.18 ± 0.00 |
| Ala | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.40 ± 0.00 | 0.41 ± 0.00 | 0.43 ± 0.01 | 0.49 ± 0.00 |
| Tyr | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.13 ± 0.00 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.14 ± 0.00 |
| Cys | 0.14 ± 0.01 | 0.14 ± 0.00 | 0.04 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.00 |
| Val | 0.18 ± 0.00 | 0.16 ± 0.01 | 0.16 ± 0.00 | 0.18 ± 0.00 | 0.20 ± 0.00 | 0.17 ± 0.01 |
| Met | 0.16 ± 0.01 | 0.15 ± 0.00 | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.13 ± 0.00 |
| Phe | 0.23 ± 0.07 | 0.19 ± 0.01 | 0.17 ± 0.00 | 0.19 ± 0.00 | 0.21 ± 0.00 | 0.21 ± 0.01 |
| Ile | 0.36 ± 0.16 | 0.25 ± 0.01 | 0.28 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 |
| Leu | 0.43 ± 0.09 | 0.56 ± 0.00 | 0.49 ± 0.01 | 0.53 ± 0.01 | 0.56 ± 0.02 | 0.65 ± 0.01 |
| Lys | 0.45 ± 0.06 | 0.58 ± 0.04 | 0.60 ± 0.01 | 0.56 ± 0.01 | 0.55 ± 0.01 | 0.44 ± 0.01 |
| Pro | 0.29 ± 0.02 | 0.56 ± 0.00 | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.38 ± 0.00 |
| Total | 5.49 ± 0.11 | 5.79 ± 0.10 | 5.56 ± 0.02 | 5.85 ± 0.06 | 6.07 ± 0.04 | 6.10 ± 0.07 |
| marinated pork + 0.5% green tea powder 1 | ||||||
| Asp | 0.53 ± 0.05 | 0.43 ± 0.09 | 0.41 ± 0.08 | 0.65 ± 0.37 | 0.36 ± 0.06 | 0.34 ± 0.08 |
| Glu | 0.94 ± 0.08 | 0.81 ± 0.21 | 0.99 ± 0.23 | 1.04 ± 0.27 | 1.04 ± 0.22 | 1.06 ± 0.26 |
| Ser | 0.23 ± 0.02 | 0.24 ± 0.05 | 0.23 ± 0.05 | 0.23 ± 0.05 | 0.24 ± 0.05 | 0.23 ± 0.06 |
| His | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Gly | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.18 ± 0.06 | 0.17 ± 0.06 | 0.19 ± 0.07 | 0.17 ± 0.07 |
| Thr | 0.44 ± 0.01 | 0.46 ± 0.08 | 0.53 ± 0.08 | 0.48 ± 0.08 | 0.57 ± 0.08 | 0.56 ± 0.08 |
| Arg | 1.13 ± 0.06 | 1.10 ± 0.20 | 1.19 ± 0.23 | 1.24 ± 0.25 | 1.33 ± 0.23 | 1.32 ± 0.23 |
| Ala | 0.48 ± 0.03 | 0.46 ± 0.09 | 0.50 ± 0.10 | 0.52 ± 0.11 | 0.56 ± 0.10 | 0.56 ± 0.10 |
| Tyr | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.03 | 0.01 ± 0.00 | 0.09 ± 0.02 | 0.20 ± 0.04 |
| Cys | 0.01 ± 0.00 | 0.07 ± 0.04 | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Val | 0.23 ± 0.02 | 0.19 ± 0.03 | 0.12 ± 0.04 | 0.24 ± 0.05 | 0.24 ± 0.05 | 0.22 ± 0.06 |
| Met | 0.14 ± 0.02 | 0.17 ± 0.03 | 0.21 ± 0.07 | 0.17 ± 0.05 | 0.22 ± 0.06 | 0.22 ± 0.07 |
| Phe | 0.16 ± 0.01 | 0.18 ± 0.03 | 0.24 ± 0.05 | 0.20 ± 0.03 | 0.24 ± 0.03 | 0.24 ± 0.04 |
| Ile | 0.36 ± 0.04 | 0.34 ± 0.09 | 0.23 ± 0.01 | 0.38 ± 0.11 | 0.43 ± 0.13 | 0.36 ± 0.09 |
| Leu | 0.52 ± 0.03 | 0.57 ± 0.11 | 0.45 ± 0.08 | 0.60 ± 0.19 | 0.70 ± 0.13 | 0.72 ± 0.13 |
| Lys | 0.50 ± 0.15 | 0.71 ± 0.20 | 0.71 ± 0.18 | 0.57 ± 0.14 | 0.64 ± 0.18 | 0.57 ± 0.13 |
| Pro | 0.31 ± 0.01 | 0.42 ± 0.04 | 0.38 ± 0.02 | 0.27 ± 0.02 | 0.35 ± 0.02 | 0.35 ± 0.01 |
| Total | 6.16 ± 0.51 | 6.36 ± 1.14 | 6.54 ± 1.00 | 6.63 ± 1.41 | 7.25 ± 1.32 | 7.23 ± 1.42 |
| marinated juice 1 | ||||||
| Asp | 0.41 ± 0.04 | 0.71 ± 0.59 | 0.36 ± 0.01 | 0.40 ± 0.01 | 0.28 ± 0.02 | 0.41 ± 0.02 |
| Glu | 0.94 ± 0.05 | 0.77 ± 0.00 | 0.76 ± 0.04 | 0.82 ± 0.03 | 0.69 ± 0.01 | 0.77 ± 0.01 |
| Ser | 0.23 ± 0.01 | 0.21 ± 0.00 | 0.20 ± 0.00 | 0.21 ± 0.00 | 0.19 ± 0.00 | 0.21 ± 0.00 |
| His | 0.45 ± 0.27 | 0.79 ± 0.50 | 0.83 ± 0.54 | 0.73 ± 0.50 | 0.71 ± 0.49 | 0.69 ± 0.45 |
| Gly | 0.50 ± 0.02 | 0.46 ± 0.03 | 0.48 ± 0.04 | 0.38 ± 0.01 | 0.46 ± 0.05 | 0.43 ± 0.01 |
| Thr | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.08 ± 0.01 | 0.10 ± 0.00 | 0.14 ± 0.01 | 0.16 ± 0.02 |
| Arg | 0.49 ± 0.01 | 0.42 ± 0.01 | 0.42 ± 0.02 | 0.40 ± 0.01 | 0.42 ± 0.01 | 0.42 ± 0.00 |
| Ala | 0.47 ± 0.01 | 0.43 ± 0.00 | 0.43 ± 0.03 | 0.41 ± 0.01 | 0.39 ± 0.00 | 0.42 ± 0.00 |
| Tyr | 0.22 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.30 ± 0.02 | 0.16 ± 0.00 |
| Cys | 0.15 ± 0.01 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.00 |
| Val | 0.23 ± 0.04 | 0.24 ± 0.00 | 0.20 ± 0.06 | 0.28 ± 0.04 | 0.17 ± 0.01 | 0.19 ± 0.02 |
| Met | 0.23 ± 0.14 | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.23 ± 0.07 | 0.14 ± 0.00 | 0.16 ± 0.01 |
| Phe | 0.21 ± 0.01 | 0.23 ± 0.00 | 0.23 ± 0.01 | 0.20 ± 0.03 | 0.17 ± 0.01 | 0.20 ± 0.00 |
| Ile | 0.24 ± 0.06 | 0.28 ± 0.00 | 0.27 ± 0.01 | 0.23 ± 0.06 | 0.30 ± 0.01 | 0.27 ± 0.01 |
| Leu | 0.54 ± 0.20 | 0.66 ± 0.01 | 0.69 ± 0.03 | 0.67 ± 0.02 | 0.50 ± 0.01 | 0.55 ± 0.01 |
| Lys | 0.53 ± 0.05 | 0.55 ± 0.01 | 0.59 ± 0.04 | 0.61 ± 0.02 | 0.55 ± 0.04 | 0.53 ± 0.02 |
| Pro | 0.26 ± 0.00 | 0.40 ± 0.02 | 0.41 ± 0.04 | 0.45 ± 0.01 | 0.43 ± 0.03 | 0.42 ± 0.01 |
| Total | 12.44 ± 1.64 | 13.11 ± 2.06 | 14.26 ± 3.04 | 14.94 ± 3.61 | 14.17 ± 3.05 | 13.86 ± 2.95 |
| marinated juice + 0.5% cinnamon powder 1 | ||||||
| Asp | 0.42 ± 0.01 | 0.40 ± 0.02 | 0.36 ± 0.01 | 0.38 ± 0.01 | 0.41 ± 0.01 | 0.36 ± 0.01 |
| Glu | 0.78 ± 0.02 | 0.72 ± 0.02 | 0.68 ± 0.00 | 0.74 ± 0.00 | 0.78 ± 0.00 | 0.80 ± 0.01 |
| Ser | 0.21 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.00 | 0.20 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.00 |
| His | 0.55 ± 0.36 | 0.70 ± 0.45 | 0.71 ± 0.52 | 0.62 ± 0.46 | 0.58 ± 0.38 | 0.53 ± 0.34 |
| Gly | 0.58 ± 0.02 | 0.55 ± 0.04 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.10 ± 0.00 |
| Thr | 0.14 ± 0.01 | 0.15 ± 0.00 | 0.42 ± 0.01 | 0.43 ± 0.00 | 0.44 ± 0.00 | 0.49 ± 0.00 |
| Arg | 0.53 ± 0.00 | 0.48 ± 0.00 | 0.96 ± 0.00 | 0.98 ± 0.01 | 1.02 ± 0.02 | 1.18 ± 0.00 |
| Ala | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.40 ± 0.00 | 0.41 ± 0.00 | 0.43 ± 0.01 | 0.49 ± 0.00 |
| Tyr | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.10 ± 0.03 |
| Cys | 0.14 ± 0.01 | 0.14 ± 0.00 | 0.16 ± 0.13 | 0.18 ± 0.16 | 0.16 ± 0.01 | 0.12 ± 0.04 |
| Val | 0.18 ± 0.00 | 0.16 ± 0.01 | 0.16 ± 0.00 | 0.18 ± 0.00 | 0.20 ± 0.00 | 0.17 ± 0.01 |
| Met | 0.16 ± 0.01 | 0.15 ± 0.00 | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.16 ± 0.00 | 0.13 ± 0.00 |
| Phe | 0.23 ± 0.07 | 0.19 ± 0.01 | 0.17 ± 0.00 | 0.19 ± 0.00 | 0.21 ± 0.00 | 0.21 ± 0.01 |
| Ile | 0.36 ± 0.16 | 0.25 ± 0.01 | 0.28 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 |
| Leu | 0.43 ± 0.09 | 0.56 ± 0.00 | 0.49 ± 0.01 | 0.53 ± 0.01 | 0.56 ± 0.02 | 0.65 ± 0.01 |
| Lys | 0.45 ± 0.06 | 0.58 ± 0.04 | 0.60 ± 0.01 | 0.56 ± 0.01 | 0.55 ± 0.01 | 0.44 ± 0.01 |
| Pro | 0.29 ± 0.02 | 0.56 ± 0.00 | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.38 ± 0.00 |
| Total | 13.47 ± 2.56 | 13.61 ± 1.25 | 14.41 ± 3.46 | 14.85 ± 4.18 | 15.19 ± 4.37 | 15.25 ± 3.98 |
| marinated juice + 0.5% green tea powder 1 | ||||||
| Asp | 0.53 ± 0.05 | 0.43 ± 0.09 | 0.41 ± 0.08 | 0.65 ± 0.37 | 0.36 ± 0.06 | 0.34 ± 0.08 |
| Glu | 0.94 ± 0.08 | 0.81 ± 0.21 | 0.99 ± 0.23 | 1.04 ± 0.27 | 1.04 ± 0.22 | 1.06 ± 0.26 |
| Ser | 0.23 ± 0.02 | 0.24 ± 0.05 | 0.23 ± 0.05 | 0.23 ± 0.05 | 0.24 ± 0.05 | 0.23 ± 0.06 |
| His | 0.37 ± 0.20 | 0.49 ± 0.30 | 0.44 ± 0.31 | 0.30 ± 0.18 | 0.30 ± 0.19 | 0.28 ± 0.15 |
| Gly | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.18 ± 0.06 | 0.17 ± 0.06 | 0.19 ± 0.07 | 0.17 ± 0.07 |
| Thr | 0.44 ± 0.01 | 0.46 ± 0.08 | 0.53 ± 0.08 | 0.48 ± 0.08 | 0.57 ± 0.08 | 0.56 ± 0.08 |
| Arg | 1.13 ± 0.06 | 1.10 ± 0.20 | 1.19 ± 0.23 | 1.24 ± 0.25 | 1.33 ± 0.23 | 1.32 ± 0.23 |
| Ala | 0.48 ± 0.03 | 0.46 ± 0.09 | 0.50 ± 0.10 | 0.52 ± 0.11 | 0.56 ± 0.10 | 0.56 ± 0.10 |
| Tyr | 0.15 ± 0.01 | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.12 ± 0.05 | 0.09 ± 0.02 | 0.20 ± 0.04 |
| Cys | 0.14 ± 0.03 | 0.36 ± 0.23 | 0.24 ± 0.09 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Val | 0.23 ± 0.02 | 0.19 ± 0.03 | 0.12 ± 0.04 | 0.24 ± 0.05 | 0.24 ± 0.05 | 0.22 ± 0.06 |
| Met | 0.14 ± 0.02 | 0.17 ± 0.03 | 0.21 ± 0.07 | 0.17 ± 0.05 | 0.22 ± 0.06 | 0.22 ± 0.07 |
| Phe | 0.16 ± 0.01 | 0.18 ± 0.03 | 0.24 ± 0.05 | 0.20 ± 0.03 | 0.24 ± 0.03 | 0.24 ± 0.04 |
| Ile | 0.36 ± 0.04 | 0.34 ± 0.09 | 0.23 ± 0.01 | 0.38 ± 0.11 | 0.43 ± 0.13 | 0.36 ± 0.09 |
| Leu | 0.52 ± 0.03 | 0.57 ± 0.11 | 0.45 ± 0.08 | 0.60 ± 0.19 | 0.70 ± 0.13 | 0.72 ± 0.13 |
| Lys | 0.50 ± 0.15 | 0.71 ± 0.20 | 0.71 ± 0.18 | 0.57 ± 0.14 | 0.64 ± 0.18 | 0.57 ± 0.13 |
| Pro | 0.31 ± 0.01 | 0.42 ± 0.04 | 0.38 ± 0.02 | 0.27 ± 0.02 | 0.35 ± 0.02 | 0.35 ± 0.01 |
| Total | 11.88 ± 0.95 | 13.66 ± 2.60 | 14.93 ± 3.97 | 15.58 ± 2.23 | 16.38 ± 5.75 | 16.65 ± 5.50 |
| Time Length | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| Reducing sugar content (mg/g) 1 | ||||||
| marinated pork (MP) | 0.09 ± 0.25 e,A | 1.37 ± 0.46 d,B | 1.12 ± 0.08 d,B | 2.32 ± 0.28 c,B | 3.50 ± 0.02 b,C | 4.03 ± 0.05 a,C |
| MP + 0.5% cinnamon powder | 0.08 ± 0.48 c,A | 1.56 ± 1.70 b,A | 1.03 ± 0.44 b,B | 1.83 ± 0.37 b,C | 4.73 ± 0.10 a,B | 5.33 ± 0.66 a,B |
| MP + 0.5% green tea powder | 0.07 ± 0.40 d,A | 1.22 ± 0.76 c,B | 4.08 ± 1.65 b,A | 3.75 ± 0.47 b,A | 6.73 ± 1.81 a,A | 6.50 ± 0.21 a,A |
| Creatine content (mg/100 g) 1 | ||||||
| marinated pork (MP) | 57.85 ± 0.21 a,A | 25.70 ± 0.1 b,A | 20.43 ± 0.14 c,A | 15.94 ± 0.20 d,A | 11.34 ± 0.08 e,A | 6.72 ± 0.07 f,C |
| MP + 0.5% cinnamon powder | 56.99 ± 0.61 a,A | 22.98 ± 0.13 b,B | 14.12 ± 0.10 c,B | 11.82 ± 0.17 d,B | 8.96 ± 0.08 e,B | 7.27 ± 0.08 f,B |
| MP + 0.5% green tea powder | 57.14 ± 0.37 a,A | 21.16 ± 0.12 b,C | 13.46 ± 0.56 c,B | 10.66 ± 0.08 d,C | 8.72 ± 0.09 e,C | 7.62 ± 0.06 f,A |
| Creatinine content (mg/100 g) 1 | ||||||
| marinated pork (MP) | 5.97 ± 0.15 e,A | 34.57 ± 0.54 d,B | 60.33 ± 1.23 b,A | 76.43 ± 2.74 a,A | 60.26 ± 1.55 b,A | 44.47 ± 0.92 c,B |
| MP + 0.5% cinnamon powder | 5.84 ± 0.29 e,A | 37.07 ± 0.06 d,A | 46.38 ± 0.17 c,C | 56.60 ± 0.64 a,B | 50.22 ± 0.26 b,C | 45.43 ± 1.72 c,B |
| MP + 0.5% green tea powder | 5.11 ± 0.47 d,A | 37.17 ± 0.26 c,A | 49.03 ± 1.12 b,B | 51.65 ± 0.47 a,C | 51.57 ± 0.14 a,B | 49.31 ± 1.25 b,A |
| Reducing sugar content (mg/g) 1 | ||||||
| marinated juice (MJ) | 0.15 ± 0.06 d,A | 12.18 ± 0.13 a,A | 9.30 ± 0.22 b,B | 9.95 ± 0.29 b,B | 7.74 ± 0.17 c,C | 7.89 ± 0.11 c,C |
| MJ + 0.5% cinnamon powder | 0.10 ± 0.04 e,A | 11.01 ± 0.07 b,B | 10.91 ± 0.14 b,A | 11.61 ± 0.19 a,A | 9.43 ± 0.24 d,A | 9.74 ± 0.17 c,A |
| MJ + 0.5% green tea powder | 0.13 ± 0.02 e,A | 8.92 ± 0.06 a,C | 7.96 ± 0.08 d,C | 7.87 ± 0.09 d,C | 8.76 ± 0.10 b,B | 8.57 ± 0.09 c,B |
| Creatine content (mg/100 g) 1 | ||||||
| marinated juice (MJ) | 23.30 ± 0.18 a,A | 22.95 ± 0.07 b,A | 22.28 ± 0.06 c,A | 16.57 ± 0.24 d,A | 10.42 ± 0.15 e,B | 9.30 ± 0.14 f,,B |
| MJ + 0.5% cinnamon powder | 23.21 ± 0.03 a,A | 22.46 ± 0.02 b,B | 18.18 ± 0.08 c,B | 14.44 ± 0.49 d,B | 13.38 ± 0.13 e,A | 10.26 ± 0.02 f,A |
| MJ + 0.5% green tea powder | 23.01 ± 0.21 a,A | 20.43 ± 1.22 b,C | 15.84 ± 0.38 c,C | 13.14 ± 0.15 d,C | 10.21 ± 0.10 e,B | 10.79 ± 0.72 e,A |
| Creatinine content (mg/100 g) 1 | ||||||
| marinated juice (MJ) | nd 2 | 6.47 ± 0.10 e,B | 11.23 ± 0.23 b,A | 14.44 ± 0.52 a,A | 10.07 ± 0.26 c,A | 8.20 ± 0.17 d,B |
| MJ + 0.5% cinnamon powder | nd | 6.77 ± 0.01 d,A | 8.51 ± 0.03 c,C | 10.74 ± 0.12 a,B | 9.17 ± 0.05 b,C | 8.29 ± 0.31 c,B |
| MJ + 0.5% green tea powder | nd | 6.83 ± 0.05 d,A | 9.17 ± 0.21 c,B | 9.76 ± 0.09 a,C | 9.51 ± 0.03 ab,B | 9.37 ± 0.23 bc,A |
| Time Length | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| FRAP (TAC, mmol Trolox equivalent/kg) 1 | ||||||
| marinated pork (MP) | 11.75 ± 0.17 f,B | 13.84 ± 0.21 e,C | 18.98 ± 0.23 d,C | 24.77 ± 0.14 c,C | 25.51 ± 0.20 b,C | 28.90 ± 0.70 a,C |
| MP + 0.5% cinnamon powder | 11.96 ± 0.19 f,B | 20.13 ± 0.20 e,B | 27.32 ± 0.64 d,B | 35.71 ± 0.69 c,B | 37.22 ± 0.49 b,B | 42.74 ± 0.34 a,B |
| MP + 0.5% green tea powder | 21.33 ± 0.29 f,A | 49.97 ± 0.52 c,A | 43.24 ± 0.77 e,A | 54.27 ± 0.17 a,A | 51.08 ± 0.05 b,A | 47.09 ± 0.58 d,A |
| DPPH (TAC, mmol Trolox equivalent/kg) 1 | ||||||
| marinated pork (MP) | 28.94 ± 0.11 c,C | 27.10 ± 0.22 e,C | 27.37 ± 0.13 d,B | 30.13 ± 0.07 b,C | 30.28 ± 0.08 b,B | 30.63 ± 0.09 a,B |
| MP + 0.5% cinnamon powder | 29.26 ± 0.19 d,B | 29.47 ± 0.07 c,B | 30.85 ± 0.02 ab,A | 30.78 ± 0.02 b,B | 30.83 ± 0.09 ab,A | 30.97 ± 0.09 a,A |
| MP + 0.5% green tea powder | 30.58 ± 0.07 c,A | 31.02 ± 0.04 a,A | 30.84 ± 0.06 ab,A | 31.01 ± 0.05 a,A | 30.75 ± 0.33 bc,A | 30.93 ± 0.02 ab,A |
| FRAP (TAC, mmol Trolox equivalent/kg) 1 | ||||||
| marinated juice (MJ) | 54.33 ± 0.96 a,C | 49.30 ± 0.47 c,C | 50.76 ± 0.19 b,C | 54.87 ± 0.64 a,C | 44.04 ± 0.91 e,C | 45.99 ± 1.09 d,C |
| MJ + 0.5% cinnamon powder | 56.27 ± 1.11 b,B | 52.87 ± 0.83 c,B | 53.50 ± 0.37 c,B | 57.62 ± 0.32 a,B | 58.31 ± 0.36 a,B | 51.36 ± 0.51 d,B |
| MJ + 0.5% green tea powder | 67.13 ± 0.14 b,A | 69.64 ± 0.15 a,A | 69.37 ± 0.46 a,A | 66.18 ± 0.22 c,A | 62.98 ± 0.81 d,A | 62.98 ± 0.81 d,A |
| DPPH (TAC, mmol Trolox equivalent/kg) 1 | ||||||
| marinated juice (MJ) | 30.32 ± 0.03 a,A | 29.94 ± 0.52 ab,A | 30.13 ± 0.03 a,A | 30.06 ± 0.05 a,A | 30.12 ± 0.11 a,A | 29.66 ± 0.07 b,A |
| MJ + 0.5% cinnamon powder | 30.41 ± 0.02 a,A | 30.23 ± 0.18 ab,A | 30.02 ± 0.25 b,A | 30.02 ± 0.05 b,A | 29.71 ± 0.06 c,B | 29.65 ± 0.02 c,A |
| MJ + 0.5% green tea powder | 30.18 ± 0.05 b,A | 30.34 ± 0.09 a,A | 30.11 ± 0.02 b,A | 29.95 ± 0.09 c,A | 29.68 ± 0.02 d,B | 29.50 ± 0.03 e,A |
| Maillard browning index 1 | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
| marinated juice (MJ) | 0.21 ± 0.01 d,B | 0.26 ± 0.00 c,A,2 | 0.30 ± 0.01 b,A | 0.31 ± 0.01 b,A | 0.31 ± 0.01 b,B | 0.38 ± 0.01 a,B |
| MJ + 0.5% cinnamon powder | 0.23 ± 0.01 d,A | 0.26 ± 0.02 d,A | 0.26 ± 0.03 d,A | 0.33 ± 0.01 c,A | 0.38 ± 0.01 b,B | 0.49 ± 0.02 a,AB |
| MJ + 0.5% green tea powder | 0.26 ± 0.01 b,A | 0.25 ± 0.02 b,A | 0.26 ± 0.05 b,A | 0.35 ± 0.07 b,A | 0.53 ± 0.07 a,A | 0.56 ± 0.11 a,A |
| Cinnamon Powder | ||||||||||
| Compound | Neochlorogenic Acid | Benzoic Acid | Caffeic Acid | Hyperoside + Isoquecetin | Coumarin | Quercetin | Cinnamic Acid | Cinnam-Aldehyde | Eugenol | p-Coumaric Acid |
| Content (mg/g) 1 | 0.017 ± 0.002 | 0.038 ± 0.006 | 0.004 ± 0.000 | 0.007 ± 0.001 | 0.001 ± 0.000 | 0.004 ± 0.000 | 0.323 ± 0.018 | 2.338 ± 0.012 | 0.008 ± 0.001 | 0.001 ± 0.000 |
| Green Tea Powder | ||||||||||
| Compound | Epicatechin (EC) | Epigallocatechin Gallate (EGCG) | Gallocatechin Gallate (GCG) | Epicatechin Gallate (ECG) | Total | |||||
| Content (mg/g) 1 | 60.41 ± 0.15 | 35.90 ± 0.10 | 1.30 ± 0.01 | 17.04 ± 0.10 | 114.64 ± 0.35 | |||||
| PAHs | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| marinated pork 1,2 | ||||||
| Pheneanthrene (Phe) | 0.60 ± 0.01 e | 0.63 ± 0.05 e | 0.78 ± 0.04 d | 1.24 ± 0.16 c | 1.47 ± 0.04 b | 1.64 ± 0.09 a |
| Pyrene (Pyr) | 31.00 ± 0.15 f | 38.81 ± 0.72 e | 41.79 ± 0.79 d | 43.87 ± 0.85 c | 46.94 ± 0.98 b | 50.51 ± 0.64 a |
| Benzo[ghi]perylene (BghiP) | nd 3 | trace 4 | 0.34 ± 0.01 d | 0.43 ± 0.02 c | 0.56 ± 0.04 b | 0.81 ± 0.01 a |
| Dibenz[a,h]anthracene (DBahA) | 1.54 ± 0.01 a | 1.53 ± 0.01 a | 1.53 ± 0.01 a | 1.53 ± 0.01 a | 1.53 ± 0.01 a | 1.54 ± 0.01 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.01 a | 0.71 ± 0.00 a | 0.71 ± 0.00 a | 0.71 ± 0.00 a | 0.71 ± 0.00 a | 0.71 ± 0.01 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.00 b | 0.72 ± 0.00 ab | 0.72 ± 0.00 ab | 0.72 ± 0.00 a | 0.72 ± 0.00 a | 0.72 ± 0.01 a |
| Total | 34.56 ± 0.17 f | 42.40 ± 0.77 e | 45.87 ± 0.76 d | 48.49 ± 0.999 c | 51.92 ± 0.94 b | 55.93 ± 0.73 a |
| marinated pork + 0.5% cinnamon powder 1,2 | ||||||
| Pheneanthrene (Phe) | trace | trace | trace | 0.44 ± 0.04 c | 4.83 ± 0.25 b | 7.29 ± 0.40 a |
| Pyrene (Pyr) | 30.61 ± 1.10 e | 36.08 ± 0.84 d | 37.15 ± 1.11 cd | 37.86 ± 1.01 bc | 39.66 ± 0.60 b | 45.02 ± 1.31 a |
| Benzo[ghi]perylene (BghiP) | nd | nd | trace | 0.45 ± 0.04 c | 1.23 ± 0.09 b | 3.46 ± 0.15 a |
| Dibenz[a,h]anthracene (DBahA) | 1.53 ± 0.01 ab | 1.53 ± 0.01 ab | 1.52 ± 0.00 ab | 1.52 ± 0.00 b | 1.52 ± 0.00 b | 1.55 ± 0.03 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.01 a | 0.71 ± 0.01 a | 0.71 ± 0.00 a | 0.71 ± 0.01 a | 0.71 ± 0.00 a | 0.72 ± 0.01 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.01 ab | 0.72 ± 0.00 b | 0.72 ± 0.00 b | 0.72 ± 0.00 b | 0.72 ± 0.00 b | 0.73 ± 0.01 a |
| Total | 33.57 ± 1.09 e | 39.04 ± 0.86 de | 40.10 ± 1.14 cd | 41.70 ± 1.06 c | 48.74 ± 0.50 b | 58.76 ± 1.87 a |
| marinated pork + 0.5% green tea powder 1,2 | ||||||
| Pheneanthrene (Phe) | 0.33 ± 0.01 d | 0.78 ± 0.06 c | 0.79 ± 0.06 c | 0.84 ± 0.04 c | 1.05 ± 0.13 b | 1.77 ± 0.09 a |
| Pyrene (Pyr) | 28.80 ± 1.50 d | 30.94 ± 1.12 d | 35.05 ± 0.80 c | 35.22 ± 1.79 bc | 37.35 ± 0.82 b | 49.77 ± 0.76 a |
| Benzo[ghi]perylene (BghiP) | nd | nd | nd | nd | nd | nd |
| Dibenz[a,h]anthracene (DBahA) | 1.53 ± 0.01 a | 1.57 ± 0.05 a | 1.54 ± 0.01 a | 1.54 ± 0.01 a | 1.55 ± 0.03 a | 1.55 ± 0.05 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.00 c | 0.71 ± 0.00 c | 0.71 ± 0.00 c | 0.72 ± 0.01 bc | 0.72 ± 0.00 b | 0.73 ± 0.00 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.00 c | 0.73 ± 0.01 b | 0.73 ± 0.00 b | 0.73 ± 0.08 b | 0.73 ± 0.01 b | 0.76 ± 0.01 a |
| Total | 32.09 ± 1.50 d | 34.72 ± 1.11 d | 38.82 ± 2.73 c | 39.05 ± 0.46 c | 41.40 ± 0.95 b | 54.59± 0.54 a |
| marinated juice1,2 | ||||||
| Pheneanthrene (Phe) | 0.34 ± 0.01 e | 0.41 ± 0.06 d | 0.45 ± 0.03 d | 0.63 ± 0.03 c | 0.71 ± 0.01 b | 0.80 ± 0.04 a |
| Pyrene (Pyr) | 23.56 ± 0.87 e | 23.70 ± 0.26 e | 26.00 ± 1.78 d | 33.07 ± 0.54 c | 37.33 ± 1.62 b | 40.36 ± 1.09 a |
| Dibenz[a,h]anthracene (DBahA) | 1.53 ± 0.00 b | 1.53 ± 0.00 b | 1.52 ± 0.00 b | 1.52 ± 0.01 b | 1.53 ± 0.01 b | 1.55 ± 0.00 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.00 c | 0.71 ± 0.00 c | 0.71 ± 0.00 bc | 0.71 ± 0.00 bc | 0.72 ± 0.00 b | 0.73 ± 0.01 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.00 d | 0.72 ± 0.00 d | 0.72 ± 0.00 cd | 0.73 ± 0.00 bc | 0.73 ± 0.01 b | 0.74 ± 0.00 a |
| Total | 26.85 ± 0.86 e | 27.06 ± 0.24 e | 29.41 ± 1.75 d | 36.67 ± 0.53 c | 41.01 ± 1.62 b | 44.17 ± 1.05 a |
| marinated juice + 0.5% cinnamon powder 1,2 | ||||||
| Pheneanthrene (Phe) | trace 3 | 0.38 ± 0.02 b | 0.42 ± 0.02 b | 0.70 ± 0.29 a | 0.77 ± 0.03 a | 0.85 ± 0.04 a |
| Pyrene (Pyr) | 24.19 ± 0.64 f | 34.96 ± 0.36 e | 42.76 ± 1.27 d | 46.41 ± 1.74 c | 49.13 ± 1.11 b | 54.52 ± 1.20 a |
| Dibenz[a,h]anthracene (DBahA) | 1.52 ± 0.00 b | 1.53 ± 0.00 b | 1.52 ± 0.00 b | 1.53 ± 0.01 b | 1.53 ± 0.01 b | 1.55 ± 0.01 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.00 b | 0.71 ± 0.00 b | 0.71 ± 0.00 b | 0.71 ± 0.01 b | 0.71 ± 0.00 b | 0.73 ± 0.01 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.00 c | 0.72 ± 0.00 c | 0.73 ± 0.00 b | 0.73 ± 0.00 b | 0.73 ± 0.01 ab | 0.74 ± 0.00 a |
| Total | 27.14 ± 0.63 f | 38.29 ± 0.34 e | 46.14 ± 1.26 d | 50.06 ± 1.58 c | 52.87 ± 1.12 b | 58.39 ± 1.17 a |
| marinated juice + 0.5% green tea powder 1,2 | ||||||
| Pheneanthrene (Phe) | 0.40 ± 0.03 f | 0.54 ± 0.01 e | 0.58 ± 0.01 d | 0.76 ± 0.03 c | 0.83 ± 0.02 b | 0.97 ± 0.03 a |
| Pyrene (Pyr) | 22.64 ± 1.05 f | 26.57 ± 2.12 e | 31.60 ± 0.84 d | 37.77 ± 0.54 c | 40.72 ± 0.89 b | 47.69 ± 1.81 a |
| Dibenz[a,h]anthracene (DBahA) | 1.53 ± 0.01 a | 1.53 ± 0.00 a | 1.53 ± 0.01 a | 1.53 ± 0.01 a | 1.54 ± 0.01 a | 1.54 ± 0.03 a |
| Dienzo[a,e]pyrene (DBaeP) | 0.71 ± 0.00 c | 0.71 ± 0.00 c | 0.71 ± 0.00 bc | 0.71 ± 0.00 ab | 0.71 ± 0.00 a | 0.72 ± 0.00 a |
| Dienzo[a,l]pyrene (DBalP) | 0.72 ± 0.00 d | 0.73 ± 0.00 d | 0.73 ± 0.00 cd | 0.73 ± 0.00 bc | 0.74 ± 0.00 b | 0.76 ± 0.00 a |
| Total | 26.00 ± 1.09 f | 30.07 ± 2.11 e | 35.14 ± 0.83 d | 41.50 ± 0.56 c | 44.54 ± 0.87 b | 51.68 ± 1.81 a |
| Time Length | 0 h | 2 h | 4 h | 8 h | 12 h | 24 h |
|---|---|---|---|---|---|---|
| 2-cyclohexene-1-one | ||||||
| marinated pork (MP) | 5.30 | 9.71 | 10.88 | 12.90 | 28.43 | 43.19 |
| MP + 0.5% cinnamon powder | 3.72 | 16.30 | 17.79 | 28.32 | 40.51 | 151.24 |
| MP + 0.5% green tea powder | nd1 | 11.25 | 21.46 | 45.09 | 54.02 | 181.89 |
| benzaldehyde | ||||||
| marinated pork (MP) | 140.93 | 155.59 | 191.86 | 226.28 | 289.67 | 240.47 |
| MP + 0.5% cinnamon powder | 183.88 | 922.47 | 522.05 | 338.95 | 294.31 | 253.05 |
| MP + 0.5% green tea powder | 200.81 | 121.63 | 177.61 | 155.51 | 176.41 | 180.34 |
| trans,trans-2,4-decadienal | ||||||
| marinated pork (MP) | 15.06 | 80.45 | 152.74 | 224.86 | 233.75 | 248.04 |
| MP + 0.5% cinnamon powder | 16.35 | 42.03 | 62.44 | 126.85 | 205.47 | 296.07 |
| MP + 0.5% green tea powder | 25.12 | 40.69 | 48.64 | 71.42 | 123.28 | 218.44 |
| Total (2-cyclohexene-1-one + benzaldehyde + trans,trans-2,4-decadienal) | ||||||
| marinated pork (MP) | 161.29 | 245.75 | 355.47 | 464.04 | 551.85 | 531.71 |
| MP + 0.5% cinnamon powder | 203.95 | 980.80 | 602.27 | 494.12 | 540.29 | 700.36 |
| MP + 0.5% green tea powder | 225.93 | 173.57 | 247.71 | 272.02 | 353.71 | 580.67 |
| 2-cyclohexene-1-one | ||||||
| marinated juice (MJ) | nd | 2.30 | 4.68 | 4.56 | 15.81 | 21.46 |
| MJ + 0.5% cinnamon powder | nd | 3.14 | 4.25 | 5.03 | 8.34 | 24.30 |
| MJ + 0.5% green tea powder | nd | 5.13 | 9.31 | 8.86 | 7.11 | 9.32 |
| benzaldehyde | ||||||
| marinated juice (MJ) | 24.25 | 39.23 | 37.19 | 37.79 | 36.90 | 13.77 |
| MJ + 0.5% cinnamon powder | 30.98 | 45.98 | 53.57 | 51.35 | 40.14 | 36.16 |
| MJ + 0.5% green tea powder | 18.38 | 24.36 | 24.95 | 32.15 | 34.96 | 33.80 |
| trans,trans-2,4-decadienal | ||||||
| marinated juice (MJ) | 5.92 | 6.10 | 7.22 | 44.38 | 102.00 | 93.32 |
| MJ + 0.5% cinnamon powder | nd | 6.43 | 5.16 | 9.84 | 51.90 | 81.73 |
| MJ + 0.5% green tea powder | 4.73 | 4.91 | 5.96 | 10.49 | 13.23 | 51.09 |
| Total (2-cyclohexene-1-one + benzaldehyde + trans,trans-2,4-decadienal) | ||||||
| marinated juice (MJ) | 30.17 | 47.63 | 49.08 | 86.73 | 154.72 | 128.55 |
| MJ + 0.5% cinnamon powder | 30.98 | 55.55 | 62.98 | 66.22 | 100.38 | 142.20 |
| MJ + 0.5% green tea powder | 23.11 | 34.40 | 40.23 | 51.50 | 55.30 | 94.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-W.; Lee, Y.-T.; Inbaraj, B.S.; Chen, B.-H. Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating. Foods 2022, 11, 3080. https://doi.org/10.3390/foods11193080
Lai Y-W, Lee Y-T, Inbaraj BS, Chen B-H. Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating. Foods. 2022; 11(19):3080. https://doi.org/10.3390/foods11193080
Chicago/Turabian StyleLai, Yu-Wen, Yu-Tsung Lee, Baskaran Stephen Inbaraj, and Bing-Huei Chen. 2022. "Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating" Foods 11, no. 19: 3080. https://doi.org/10.3390/foods11193080
APA StyleLai, Y.-W., Lee, Y.-T., Inbaraj, B. S., & Chen, B.-H. (2022). Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating. Foods, 11(19), 3080. https://doi.org/10.3390/foods11193080







