Fluoride Concentration in Urine after Supplementation with Quelites in a Population of Adolescents
Abstract
:1. Introduction
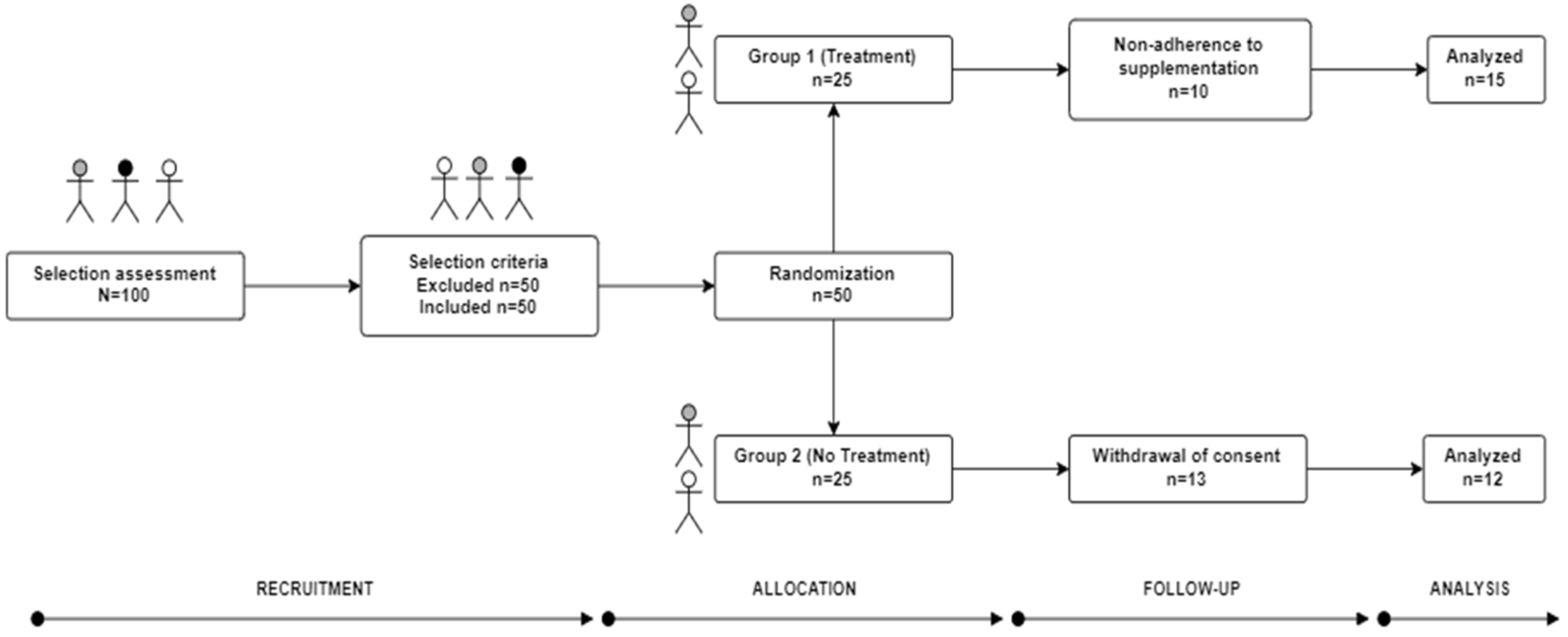
2. Materials and Methods
2.1. Study Design
2.2. Ethical Principles for Clinical Research (Volunteers)
2.3. Supplementation
2.4. Anthropometric Assessment
2.5. Dietary Evaluation
2.6. Chemicals and Reagents
2.7. Preparation of Urine Samples for Analysis of F−
2.8. Determination of Fluoride in Water and Urine
2.9. Statistical Analysis
3. Results
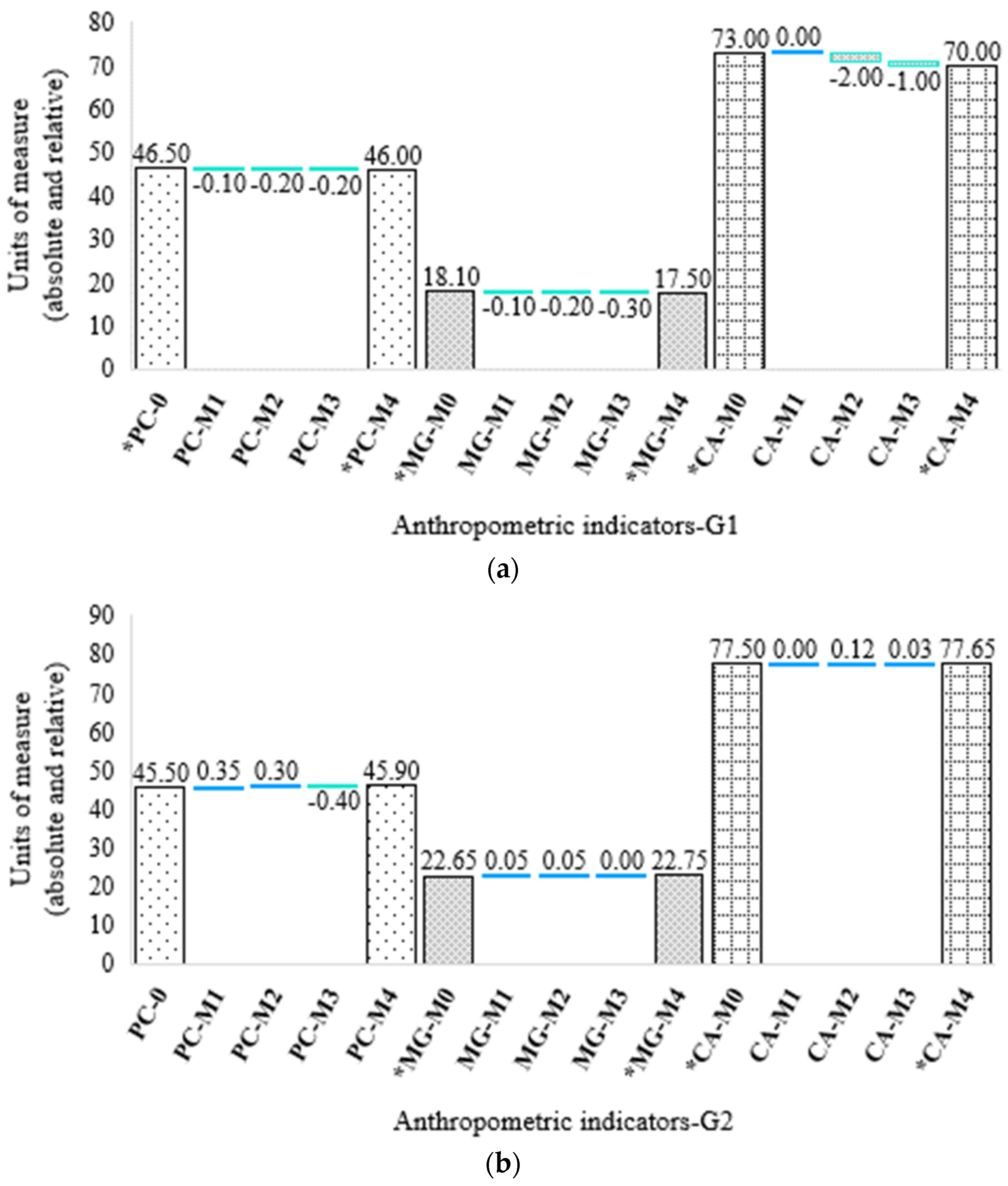
3.1. Anthropometric Assessment
3.2. Dietary Evaluation
3.3. F− Concentration in Drinking Water and Urine
3.4. Number Needed to Treat (NNT)
4. Discussion
4.1. Drinking Water (F−)
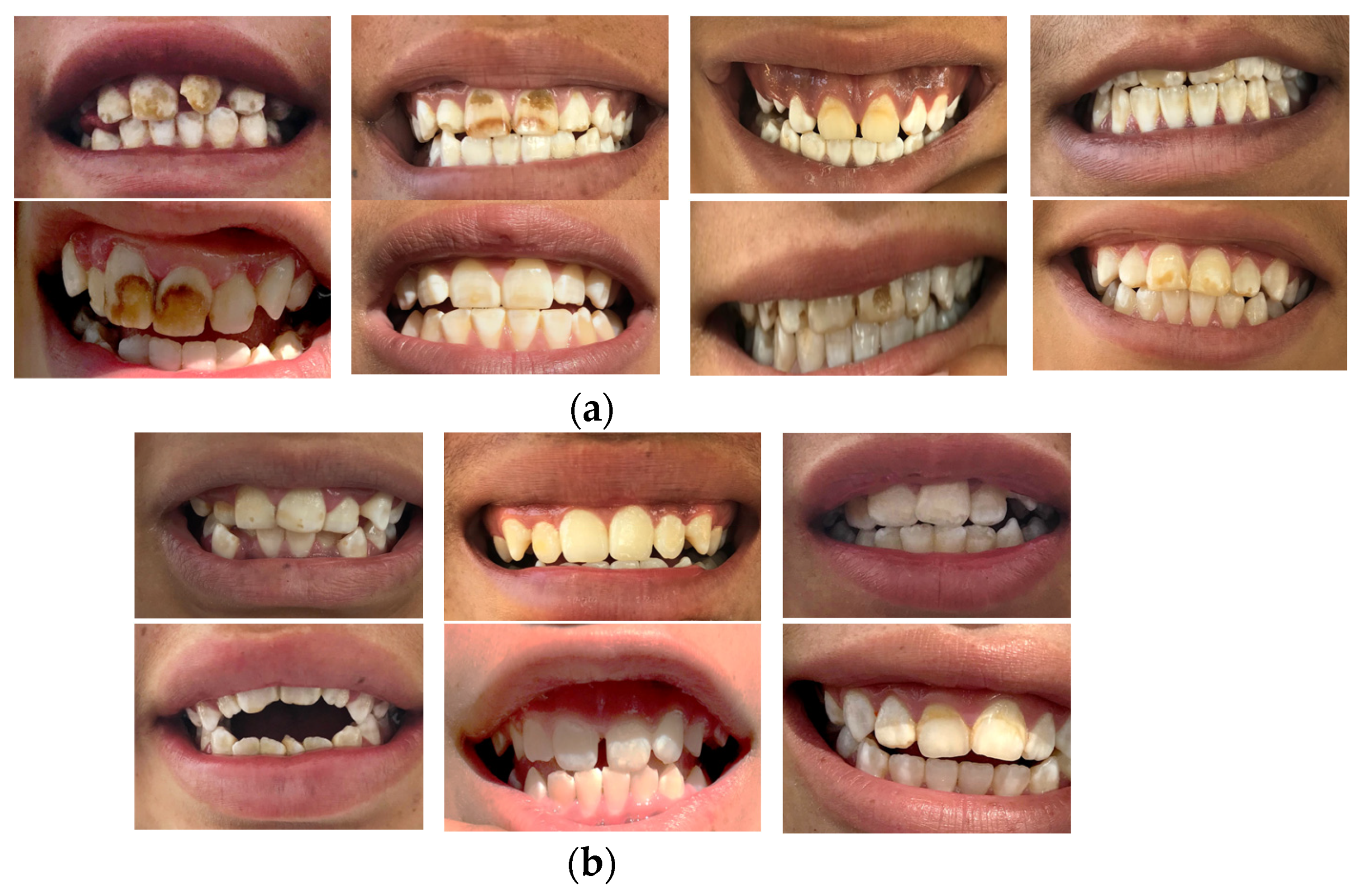
4.2. Fluoride in Urine (F−)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meza-Lozano, B.; Ortíz-Pérez, M.D.; Ponce-Palomares, M.; Castillo-Gutiérrez, S.G.; Flores-Ramírez, R.; Cubillas-Tejeda, A.C. Implementación y evaluación de un programa de comunicación de riesgos por exposición a flúor en la comunidad de el fuerte, Santa María del Río, San Luís Potosí, México. Rev. Int. Contam. Ambie. 2016, 32, 87–100. [Google Scholar]
- Gupta, S.; Deshpande, R.; Agarwal, M. Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeol. J. 2005, 13, 596–605. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine, ATSDR. 2003. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp11 (accessed on 5 February 2022).
- Santiago-Saenz, Y.O.; Monroy-Torres, R.; Rocha-Amador, D.O.; Hernández-Fuentes, A.D. Effect of a supplementation with two Quelites on urinary excretion of arsenic in adolescents exposed to water contaminated with the metalloid in a community in the state of Guanajuato, Mexico. Nutrients 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Fluoride in Drinking Water, WHO. 2006. Available online: https://www.who.int/water_sanitation_health/publications/fluoride_drinking_water_full (accessed on 5 February 2022).
- Valdez-Jiménez, L.; Soria-Fregozo, C.; Miranda-Beltrán, M.L.; Gutiérrez-Coronado, O.; Pérez- Vega, M.I. Effects of the fluoride on the central nervous system. Neurologia 2011, 26, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Jarquín-Yañez, L.; Mejía-Saavedra, J.J.; Molina-Frechero, N.; Gaona, E.; Rocha-Amador, D.O.; López-Guzmán, O.D.; Bologna-Molina, R. Association between urine fluoride and dental fluorosis as a toxicity factor in a rural community in the state of San Luis Potosi. Sci. World J. 2015, 2015, 647184. [Google Scholar] [CrossRef]
- Rocha-Amador, D.O.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderón, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. Saude Publica 2007, 23, S579–S587. [Google Scholar] [CrossRef]
- Monroy-Torres, R.; Espinoza-Pérez, J.A.; Ramírez-Gómez, X.; Carrizalez-Yañez, L.; Linares-Segovia, B.; Mejía-Saavedra, J.J. Efecto de una suplementación multivitamínica de cuatro semanas sobre el estado nutricio y excreción urinaria de arsénico en adolescentes. Nutr. Hosp. 2018, 35, 894–902. [Google Scholar] [CrossRef]
- Méndez, M.; Armienta, M.A. Arsenic phase distribution in Zimapán mine tailings, Mexico. Geofis. Int. 2003, 42, 131–140. [Google Scholar] [CrossRef]
- Del Razo, L.M.; Corona, J.C.; García-Vargas, G.; Albores, A.; Cebrián, M.E. Fluoride levels in well-water from a chronic arsenicism area of Northern Mexico. Environ. Pollut. 1993, 80, 91–94. [Google Scholar] [CrossRef]
- Orteaga-Guerrero, M.A. Presencia, distribución, hidrogeoquímica y origen de arsénico, fluoruro y otros elementos traza disueltos en agua subterránea, a escala de cuenca hidrológica tributaria de Lerma-Chapala, México. Rev. Mex. Cienc. Geol. 2009, 26, 143–161. [Google Scholar]
- Grijalva-Haro, M.I.; Barba-Leyva, M.E.; Laborín-Alvarez, A. Ingestión y excreción de fluoruros en niños de Hermosillo, Sonora, México. Salud. Públ. Méx. 2001, 43, 127–134. [Google Scholar] [CrossRef]
- Arreguín-Cortés, F.I.; Chávez-Guillén, R.; Soto-Navarro, P.R. Una Revisión de la Presencia de Arsénico en el Agua Subterránea en México; Comisión Nacional del Agua (CONAGUA), Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): Ciudad de México, Mexico, 2013. [Google Scholar]
- García-Camba de la Muela, J.M.; García-Hoyos, F.; Varela Morales, M.; González Sanz, A. Absorción sistémica de flúor en niños de secundaria al cepillado con dentífrico fluorado. Rev. Esp. Salud. Pública 2009, 83, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Fluoride in Drinking–Water. Background Document for Development of WHO Guidelines for Drinking–Water Quality. WHO. 2004. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/fluoride (accessed on 5 February 2022).
- Trejo, V.R. Posibles Efectos en la Salud Humana por Ingestión Excesiva de Fluoruros; XXVIII Congreso interamericano de ingeniería sanitaria y ambiental: Cancún, Mexico, 2002. [Google Scholar]
- Denny, A.; Buttriss, J. Plant foods and health: Focus on plant bioactives; European Food Information Resource (EuroFIR): Norwich, England, 2007. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. Detoxification capacity and protective effects of medicinal plants (part 2): Plant based review. IOSR J. Pharm. 2016, 6, 63–84. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, R.C.; Seth, A.K.; Gupta, A. Reversal of fluorosis in children. Acta Paediatr. Jpn. 1996, 38, 513–519. [Google Scholar] [CrossRef]
- Khairnar, M.R.; Dodamani, A.S.; Jadhav, H.C.; Naik, R.G.; Deshmukh, M.A. Mitigation of fluorosis-A review. J. Clin. Diagn. Res. 2015, 9, ZE05–ZE09. [Google Scholar] [CrossRef]
- Vida, F.; Marsland, R. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Elsevier Inc.: London, UK, 2017; pp. 539–550. [Google Scholar] [CrossRef]
- Mondal, N.K. Diagnosis of fluorosis and recovery through easy to practice interventions. Fluoride 2018, 51, 230–242. [Google Scholar]
- Castro-Lara, D.; Basurto-Peña, F.; Bye-Boettler, R.A. Etnobotánica de quelites en Tuxtla, comunidad Totonaca de la Sierra Norte de Puebla, México. In En: Especies Vegetales Poco Valoradas, Una Alternativa Para la Seguridad Alimentaria; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2011. [Google Scholar]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, nutritional and antioxidant characterization of three vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as potential sources of phytochemicals and bioactive compounds. J. Food Meas. Charact 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R. Nutritional importance and biological activity of bioactive compounds from quelites consumed in Mexico. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Saenz, Y.O.; López-Palestina, C.U.; Gutiérrez-Tlahque, J.; Monroy-Torres, R.; Pinedo-Espinoza, J.M.; Hernández-Fuentes, A.D. Nutritional and functional evaluation of three powder mixtures based on mexican quelites: Alternative ingredients to formulate food supplements. Food Sci. Technol. 2020, 40, 1029–1037. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Chenopodium album-An overview. IJPSM 2015, 5, 10–17. [Google Scholar]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Moneim, A.E. Protective effects of Portulaca oleracea against rotenone mediated depletion of glutathione in the striatum of rats as an animal model of Parkinson’s disease. Pest. Biochem. Physiol. 2012, 103, 108–114. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana (NOM-127-SSA1-1994). Salud Ambiental. Agua para uso y Consumo Humano. Límites Permisibles de Calidad y Tratamientos a que debe Someterse el agua para su Potabilización. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=2063863&fecha=31/12/1969#gsc.tab=0 (accessed on 5 February 2022).
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, Illinois, USA, 1988. [Google Scholar]
- Shamah-Levy, T.; Villalpando-Hernández, S.; Rivera-Dommarco, J. Manual de Procedimientos Para Proyectos de Nutrición; Instituto Nacional de Salud Pública: Cuernavaca, México, 2006. [Google Scholar]
- Grossman, C.M. The effect of amino acids on serum and urine creatine. J. Clin. Investig. 1945, 24, 380–383. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Occupational Safety and Health (NIOSH); US department of health and human services. Fluoride in urine. Manual of Analytical Methods, 3rd ed.; NIOSH: Washington, DC, USA, 1984; pp. 8308-1–8308-3. [Google Scholar]
- Wyat, J.C.; López-Quiroga, V.; Olivas-Acosta, R.T.; Méndez, R.O. Excretion of arsenic (As) in urine of children, 7–11 Years, exposed to elevated levels of As in the city water supply in Hermosillo, Sonora, México. Environ. Res. 1998, 78, 19–24. [Google Scholar] [CrossRef]
- González-Horta, C.; Ballinas-Casarrubias, L.; Sánchez-Ramírez, B.; Ishida, M.C.; Barrera-Hernández, A.; Gutiérrez-Torres, D.; Zacarías, O.L.; Saunders, R.J.; Drobná, Z.; Mendez, M.A.; et al. A concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2015, 12, 4587–4601. [Google Scholar] [CrossRef] [Green Version]
- Ailani, V.; Gupta, R.C.; Gupta, S.K.; Gupta, K. Oxidative stress in cases of chronic fluoride intoxication. Indian J. Clin. Biochem. 2009, 24, 426–429. [Google Scholar] [CrossRef] [Green Version]
- Abuhaloob, L.; Abed, Y. Dietary behaviors and dental fluorosis among Gaza strip children. EMHJ 2013, 19, 657–663. [Google Scholar] [CrossRef]
- Rabelo, M.A.; Milton, G. Fluoride metabolism. Monogr. Oral. Sci. Basel Karger 2011, 22, 20–36. [Google Scholar] [CrossRef]
- Peres, C.; Leite, A.L.; Rabelo, M.A. Fluorine: Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: London, UK, 2015; pp. 54–72. [Google Scholar]
- Florian, L. Fluoride bioavailability-Nutritional and clinical aspects. Nutr. Res. 1997, 17, 907–929. [Google Scholar]
- Zheng, Y.; Wu, J.; Ng, J.C.; Wang, G.; Lian, W. The absorption and excretion of fluoride and arsenic in humans. Toxicol. Lett. 2002, 133, 77–82. [Google Scholar] [CrossRef]
- Chouhan, S.; Flora, S.J.S. Arsenic and fluoride: Two major ground water pollutants. Indian J. Exp. Biol. 2010, 48, 666–678. [Google Scholar] [PubMed]
- Mann, J.; Mahmoud, W.; Ernest, M.; Sgan-cohen, H.; Shoshan, N.; Gedalia, I. Fluorosis and dental caries in 6-8 year-old children in a 5 ppm area. Community Dent. Oral. Epidemiol. 1990, 18, 77–79. [Google Scholar] [CrossRef]
- Aminabadi, N.; Gangi, A.T.; Balayi, E.; Sadighi, M. Prevalence of fluorosis in 5-12 year-old children in the North-Western Villages of Makoo in 2004. JODDD 2007, 1, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Retta, N.; Abuye, C.; Susan, J. Dietary fluoride intake and associated skeletal and dental fluorosis in school age children in rural ethiopian rift valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A review of use effects on health. Mater. Socio Med 2016, 28, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Mier, E.A. Fluoride: Its metabolism, toxicity, and role in dental health. J. Evidence-Based Integr. Med. 2012, 17, 28–32. [Google Scholar] [CrossRef]
- Aguilar-Díaz, F.C.; De la Fuente-Hernandez, J.; Cintra-Viveiro, C.A. Dental fluorosis, fluoride in urine, and nutritional status in adolescent students living in the rural areas of Guanajuato, Mexico. J. Int. Soc. Prev. Community Dent. 2016, 6, 517–522. [Google Scholar] [CrossRef] [Green Version]
- González-Dávila, O. Dental fluorosis in children from Aguascalientes, Mexico: A persistent public health problem. Water 2021, 13, 1125. [Google Scholar] [CrossRef]
- Andezhath, K.S.; Gupta, R.; Mondal, N.K. Anemia in adolescent girls: An intervention of diet editing and counselling. Natl. Med. J. India 2016, 29, 200–204. [Google Scholar]
- Indian Natural Resource Economics and Management Foundation (INREM Foundation). Nutritional Interventions for Fluorosis Mitigation under INREM Foundation’s Programme in Jhabua. INREM Foundation. 2020. Available online: http://inremfoundation.org/fluorosis/pdf/nutrition.pdf (accessed on 5 February 2022).
- Mittal, M.; Flora, S.J.S. Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem.-Biol. Interact. 2006, 162, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Flora, S.J.S. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem. Toxicol. 2007, 30, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Torres, R.; Hernández-Luna, M.A.; Ramírez-Gómez, X.S.; López-Briones, S. Role of the Microbiome as the First Metal Detoxification Mechanism. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; Franco-Robles, E., Ramírez-Emiliano, J., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
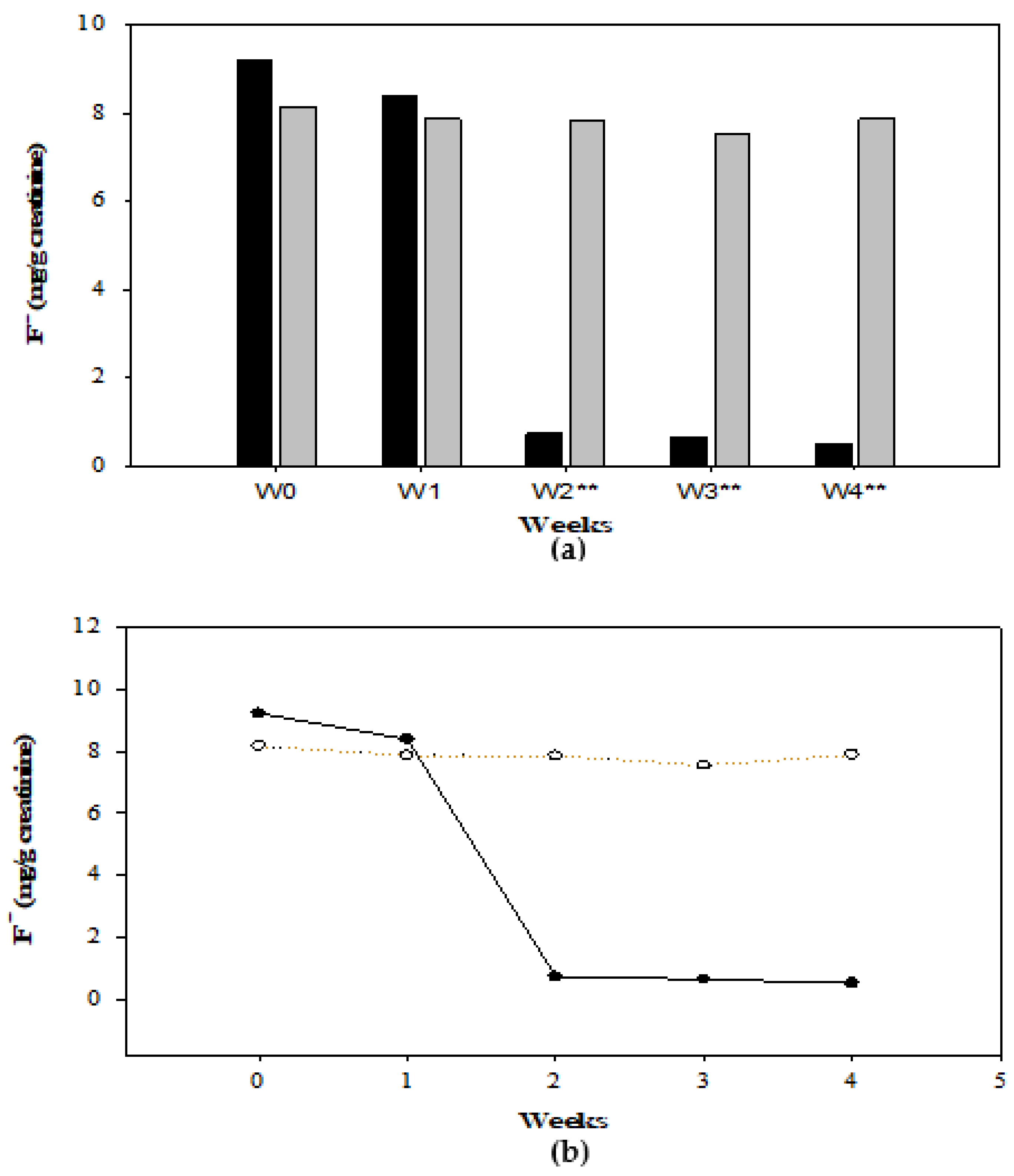
| G1 Total Sample n = 15 | Boys n = 9 | Girls n = 6 | G2 Total Sample n = 12 | Boys n = 4 | Girls n = 8 | |||
|---|---|---|---|---|---|---|---|---|
| Weeks | F− (mg/g Creatinine) | |||||||
| p | p | |||||||
| 0 | 9.23 | 9.42 | 9.01 | NS | 8.17 | 8.61 | 7.55 | NS |
| 1 | 8.39 | 8.21 | 8.48 | NS | 7.87 | 8.29 | 6.64 | NS |
| 2 | 0.73 | 0.79 | 0.53 | <0.05 | 7.86 | 8.08 | 7.16 | NS |
| 3 | 0.64 | 0.69 | 0.46 | <0.05 | 7.55 | 7.73 | 7.46 | NS |
| 4 | 0.52 | 0.68 | 0.43 | <0.05 | 7.89 | 8.16 | 7.09 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Saenz, Y.O.; Monroy-Torres, R.; Rocha-Amador, D.O.; López-Palestina, C.U. Fluoride Concentration in Urine after Supplementation with Quelites in a Population of Adolescents. Foods 2022, 11, 3071. https://doi.org/10.3390/foods11193071
Santiago-Saenz YO, Monroy-Torres R, Rocha-Amador DO, López-Palestina CU. Fluoride Concentration in Urine after Supplementation with Quelites in a Population of Adolescents. Foods. 2022; 11(19):3071. https://doi.org/10.3390/foods11193071
Chicago/Turabian StyleSantiago-Saenz, Yair Olovaldo, Rebeca Monroy-Torres, Diana Olivia Rocha-Amador, and César Uriel López-Palestina. 2022. "Fluoride Concentration in Urine after Supplementation with Quelites in a Population of Adolescents" Foods 11, no. 19: 3071. https://doi.org/10.3390/foods11193071
APA StyleSantiago-Saenz, Y. O., Monroy-Torres, R., Rocha-Amador, D. O., & López-Palestina, C. U. (2022). Fluoride Concentration in Urine after Supplementation with Quelites in a Population of Adolescents. Foods, 11(19), 3071. https://doi.org/10.3390/foods11193071






