Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products
Abstract
1. Introduction
1.1. Introduction to e-EDCs
| E-EDCs | MRL (s) or TDI (s) | Products | Provenances | Refs. |
|---|---|---|---|---|
| E2 | Not Detected | Animal foods | The CAC and the Chinese National Food Safety Standard | [12,13] |
| E2 | Not more than 50 ng·kg−1/day | Food intake | The EU and the CAC | [14,15] |
| DES and E2 | Not Detected | Animal feedstuffs and drinking water | The EU and Ministry of Agriculture of China | [17,18] |
| BPA | 50 µg·kg−1/day | Food intake | The FDA | [20] |
| BPA | 0.05 mg·kg−1 | Foods | The EU | [21] |
| Zeranol | Not Detected | Beef and other cattle food products | The EU and China | [24] |
1.2. Toxic Effects of e-EDCs
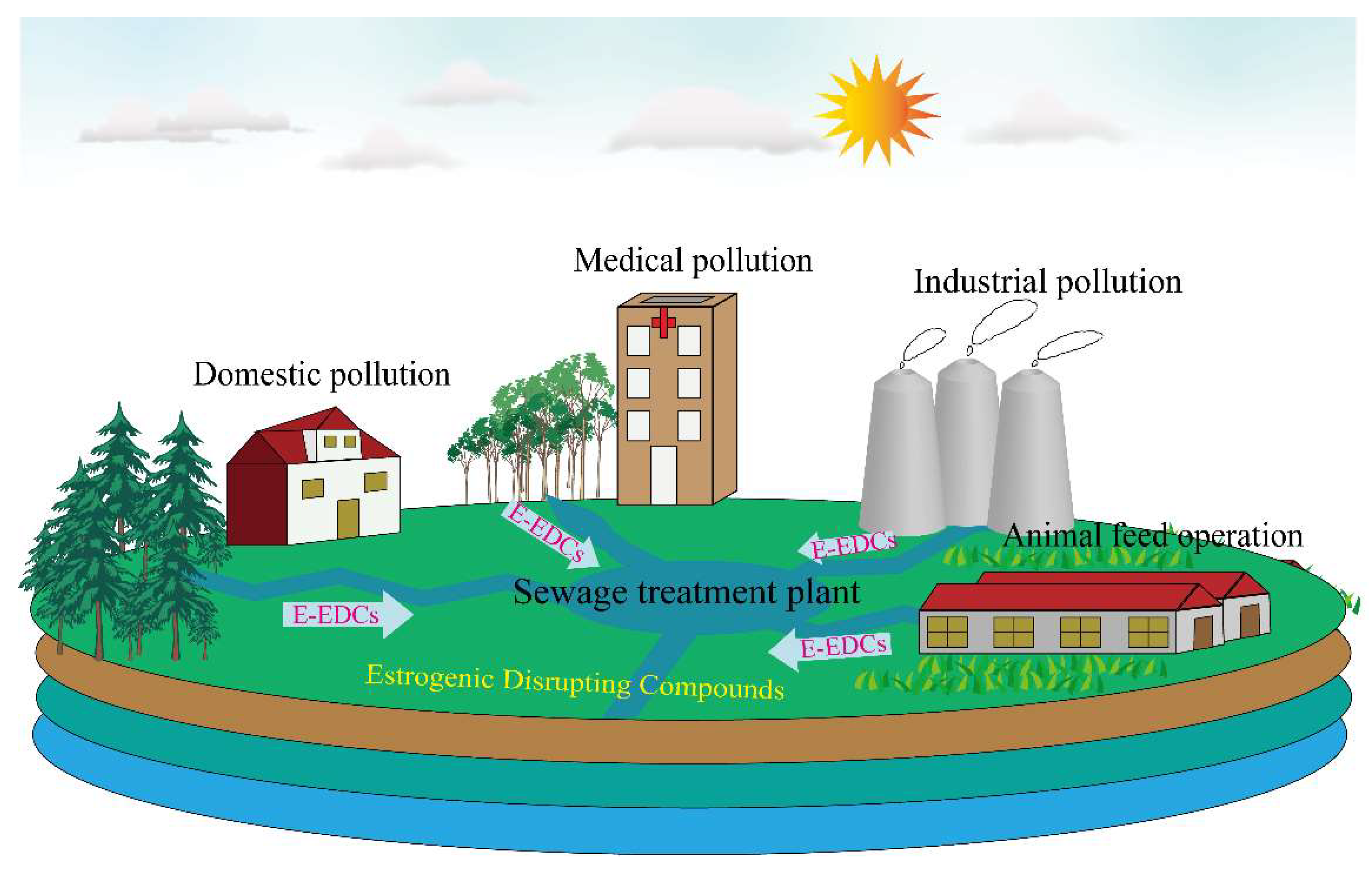
1.3. Exposure Routes of e-EDCs
2. Sample Preparation of e-EDCs Detection Methods
2.1. SPE
2.2. SPME
2.3. MSPE
2.4. LPME
2.4.1. DLLME
2.4.2. HF-LPME
2.5. The Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS)
3. Detection Methods
3.1. LC-MS
3.2. GC-MS
3.3. Biosensors for Estrogens
3.3.1. Electrochemical Biosensors
3.3.2. Optical Biosensors
3.3.3. Photoelectrochemical Biosensors
3.4. ELISA
3.5. Surface-Enhanced Raman Spectroscopy (SERS)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nesan, D.; Kurrasch, D.M. Gestational exposure to common endocrine disrupting chemicals and their impact on neurodevelopment and behavior. Annu. Rev. Physiol. 2020, 82, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Beykal, B.; Szafran, A.T.; Onel, M.; Stossi, F.; Mancini, M.G.; Lloyd, D.; Wright, F.A.; Zhou, L.; Mancini, M.A.; et al. Classification of estrogenic compounds by coupling high content analysis and machine learning algorithms. PLoS Comput. Biol. 2020, 16, e1008191. [Google Scholar] [CrossRef] [PubMed]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Lanková, D.; Urbancová, K.; Krtková, V.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á.; Pulkrabová, J.; Hajšlová, J. Multiclass analytical method for the determination of natural/synthetic steroid hormones, phytoestrogens, and mycoestrogens in milk and yogurt. Anal. Bioanal. Chem. 2017, 409, 4467–4477. [Google Scholar] [CrossRef]
- Hartmann, S.; Lacorn, M.; Steinhart, H. Natural occurrence of steroid hormones in food. Food Chem. 1998, 62, 7–20. [Google Scholar] [CrossRef]
- Pape-Zambito, D.A.; Magliaro, A.L.; Kensinger, R.S. 17β-estradiol and estrone concentrations in plasma and milk during bovine pregnancy. J. Dairy Sci. 2008, 91, 127–135. [Google Scholar] [CrossRef]
- Malekinejad, H.; Scherpenisse, P.; Bergwerff, A.A. Naturally occurring estrogens in processed milk and in raw milk (from gestated cows). J. Agric. Food Chem. 2006, 54, 9785–9791. [Google Scholar] [CrossRef]
- Du, B.; Fan, G.; Yu, W.; Yang, S.; Zhou, J.; Luo, J. Occurrence and risk assessment of steroid estrogens in environmental water samples: A five-year worldwide perspective. Environ. Pollut. 2020, 267, 115405. [Google Scholar] [CrossRef]
- Cook, M.M.; Symonds, E.M.; Gerber, B.; Hoare, A.; Van Vleet, E.S.; Breitbart, M. Removal of six estrogenic endocrine-disrupting compounds (EDCs) from municipal wastewater using aluminum electrocoagulation. Water 2016, 8, 128. [Google Scholar] [CrossRef]
- Nazari, E.; Suja, F. Effects of 17β-estradiol (E2) on aqueous organisms and its treatment problem: A review. Rev. Environ. Health 2016, 31, 465–491. [Google Scholar] [CrossRef] [PubMed]
- CX/MRL 2-2018; Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. Codex Committee on Residues of Veterinary Drugs in Foods (CCRVDF): Chicago, IL, USA, 2018.
- GB 31650-2019; Maximum Residue Limits for Veterinary Drugs in Foods. China National Food Safety Standards: Beijing, China, 2019.
- CAC/MRL 2-2015; Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. Codex Alimentarius Commission (CAC): Geneva, Switzerland, 2015.
- European Commission. Directive 2003/74/EC of the European Parliament and of the Council of 22 September 2003 Amending Council Directive 96/22/EC Concerning the Prohibition on the Use in Stock Farming of Certain Substances Having a Hormonal or Thyrostatic Action and of Beta-Agonists; European Commission: Brussels, Belgium, 2003.
- Schreiber, E.; Garcia, T.; González, N.; Esplugas, R.; Sharma, R.P.; Torrente, M.; Kumar, V.; Bovee, T.; Katsanou, E.S.; Machera, K.; et al. Maternal exposure to mixtures of dienestrol, linuron and flutamide. Part i: Feminization effects on male rat offspring. Food Chem. Toxicol. 2020, 139, 111256. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 96/23/EC of 29 April 1996 on Measures to Monitor Certain Substances and Residues Thereof in Live Animals and Animal Products and Repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC; European Commission: Brussels, Belgium, 1996.
- Announcement No. 176; Catalog of Drug Varieties Prohibited for Use in Animal Feed and Animal Drinking Water. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2002.
- Mercogliano, R.; Santonicola, S. Investigation on bisphenol A levels in human milk and dairy supply chain: A review. Food Chem. Toxicol. 2018, 114, 98–107. [Google Scholar] [CrossRef] [PubMed]
- The, U.S. Food and Drug Administration (FDA). Draft Assessment of Bisphenol A for Use in Food Contact Applications; FDA: Silver Spring, MD, USA, 2008.
- The European Union (EU). Commission Regulation (EU) 2018/213 of 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food and Amending Regulation (EU) No 10/2011 as Regards the Use of That Substance in Plastic FOOD contact Materials; EU: Brussels, Belgium, 2018. [Google Scholar]
- Njåstad, K.M.; Adler, S.A.; Hansen-Møller, J.; Thuen, E.; Gustavsson, A.M.; Steinshamn, H. Gastrointestinal metabolism of phytoestrogens in lactating dairy cows fed silages with different botanical composition. J. Dairy Sci. 2014, 97, 7735–7750. [Google Scholar] [CrossRef] [PubMed]
- Becker Algeri, T.A.; Castagnaro, D.; Bortoli, K.; Souza, C.; Drunkler, D.A.; Badiale Furlong, E. Mycotoxins in bovine milk and dairy products: A review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 96/22/EC of 29 April 1996 Concerning the Prohibition on the Use in Stockfarming of Certain Substances Having a Hormonal or Thyrostatic Action and of Beta-Agonists, and Repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC; European Commission: Brussels, Belgium, 1996.
- Fucic, A.; Gamulin, M.; Ferencic, Z.; Katic, J.; von Krauss, M.K.; Bartonova, A.; Merlo, D.F. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ. Health 2012, 11 (Suppl. S1), S8. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hwang, Y.; Chen, P.; Sung, F.; Su, T. Association of serum levels of 4-tertiary-octylphenol with cardiovascular risk factors and carotid intima-media thickness in adolescents and young adults. Environ. Health 2019, 246, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Maciuszek, M.; Pijanowski, L.; Pekala-Safinska, A.; Palichleb, P.; Błachut, M.; Verburg-van Kemenade, B.M.L.; Chadzińska, M. 17α-ethinylestradiol and 4-tert-octylphenol concurrently disrupt the immune response of common carp. Fish Shellfish Immunol. 2020, 107, 238–250. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- Yoon, K.; Kwack, S.J.; Kim, H.S.; Lee, B. Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of actions on putative human diseases. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 127–174. [Google Scholar] [CrossRef]
- Skinner, M.K. Environmental stress and epigenetic transgenerational inheritance. BMC Med. 2014, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 2016, 12, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Snoj, T.; Majdič, G. Mechanisms in endocrinology: Estrogens in consumer milk: Is there a risk to human reproductive health? Eur. J. Endocrinol. 2018, 179, R275–R286. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Wang, T.; Sun, F.F.; Wang, L.H.; Ji, R. Effects of veterinary antibiotics on the fate and persistence of 17β-estradiol in swine manure. J. Hazard. Mater. 2019, 375, 198–205. [Google Scholar] [CrossRef]
- Gudda, F.O.; Ateia, M.; Waigi, M.G.; Wang, J.; Gao, Y.Z. Ecological and human health risks of manure-borne steroid estrogens: A 20-year global synthesis study. J. Environ. Manag. 2022, 301, 113708. [Google Scholar] [CrossRef]
- Dang, Z.C.; Kienzler, A. Changes in fish sex ratio as a basis for regulating endocrine disruptors. Environ. Int. 2019, 130, 104928. [Google Scholar] [CrossRef]
- Hu, Q.; Guan, X.Q.; Song, L.L.; Wang, H.N.; Xiong, Y.; Liu, J.L.; Yin, H.; Cao, Y.F.; Hou, J.; Yang, L.; et al. Inhibition of pancreatic lipase by environmental xenoestrogens. Ecotoxicol. Environ. Saf. 2020, 192, 110305. [Google Scholar] [CrossRef]
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J.J. Analytical methodologies for the determination of endocrine disrupting compounds in biological and environmental samples. BioMed Res. Int. 2013, 2013, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.B.; Huang, Z.B.; Sun, D.W.; Fu, H.H. Recent advances in the detection of 17β-estradiol in food matrices: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.H.; Wu, J.X.; Yuan, L.; Wang, Y.Q.; Du, X.D.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.H.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.F.; Praveena, S.M. Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: A mini review. Environ. Monit. Assess. 2017, 189, 178. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Capriotti, A.L.; Foglia, P.; Piovesana, S.; Samperi, R.; Ventura, S.; Laganà, A. Natural estrogens in dairy products determination of free and conjugated forms by ultra high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 3599–3606. [Google Scholar] [CrossRef]
- Tso, J.; Aga, D.S. A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk. J. Chromatogr. A 2010, 1217, 4784–4795. [Google Scholar] [CrossRef]
- Mesa, R.; Kabir, A.; Samanidou, V.; Furton, K.G. Simultaneous determination of selected estrogenic endocrine disrupting chemicals and bisphenol A residues in whole milk using fabric phase sorptive extraction coupled to HPLC-UV detection and LC-MS/MS. J. Sep. Sci. 2019, 42, 598–608. [Google Scholar] [CrossRef]
- Höjer, A.; Adler, S.; Purup, S.; Hansen-Møller, J.; Martinsson, K.; Steinshamn, H.; Gustavsson, A.M. Effects of feeding dairy cows different legume-grass silages on milk phytoestrogen concentration. J. Dairy Sci. 2012, 95, 4526–4540. [Google Scholar] [CrossRef]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- van Asselt, E.D.; van der Fels-Klerx, H.J.; Marvin, H.; van Bokhorst-van, D.V.H.; Groot, M.N. Overview of food safety hazards in the european dairy supply chain. Compr. Rev. Food Sci. Food Saf. 2017, 16, 59–75. [Google Scholar] [CrossRef]
- Tang, J.W.; Wang, J.X.; Yuan, L.J.; Xiao, Y.; Wang, X.; Yang, Z. Trace analysis of estrogens in milk samples by molecularly imprinted solid phase extraction with genistein as a dummy template molecule and high-performance liquid chromatography-tandem mass spectrometry. Steroids 2019, 145, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, L.; Queiroz, J.F.; Magalhães, L.M.; Silva, A.M.T.; Segundo, M.A. Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices—A review. Microchem. J. 2016, 126, 243–262. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Bu, Y.; Luo, C. Hollow fiber membrane-coated functionalized polymeric ionic liquid capsules for direct analysis of estrogens in milk samples. Anal. Bioanal. Chem. 2016, 408, 1679–1685. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. Part ii. Trends Analyt. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Poole, C.F. Principles and practice of solid-phase extraction. Compr. Anal. Chem. 2002, 37, 341–387. [Google Scholar]
- Zhang, J.H.; Zang, L.G.; Wang, T.; Wang, X.W.; Jia, M.; Zhang, D.; Zhang, H.Y. A solid-phase extraction method for estrogenic disrupting compounds based on the estrogen response element. Food Chem. 2020, 333, 127529. [Google Scholar] [CrossRef]
- Liu, K.; Kang, K.; Li, N.; An, J.; Lian, K.Q.; Kang, W.J. Simultaneous determination of five hormones in milk by automated online solid-phase extraction coupled to high-performance liquid chromatography. J. AOAC Int. 2020, 103, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef]
- Mo, Z.; Pang, Y.; Yu, L.; Shen, X. Membrane-protected covalent organic framework fiber for direct immersion solid-phase microextraction of 17β-estradiol in milk. Food Chem. 2021, 359, 129816. [Google Scholar] [CrossRef]
- Lan, H.; Gan, N.; Pan, D.; Hu, F.; Li, T.; Long, N.; Qiao, L. An automated solid-phase microextraction method based on magnetic molecularly imprinted polymer as fiber coating for detection of trace estrogens in milk powder. J. Chromatogr. A 2014, 1331, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Alampanos, V.; Samanidou, V.; Papadoyannis, I. Trends in sample preparation for the HPLC determination of penicillins in biofluids. J. Appl. Bioanal 2019, 5, 9–17. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, Z. Determination of tetracyclines in bovine milk using laccaic acid-loaded magnetite nanocomposite for magnetic solid-phase extraction. J. Chromatogr. Sci. 2017, 55, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Zachariadis, G.A. Recent advances in the extraction of polycyclic aromatic hydrocarbons from environmental samples. Molecules 2020, 25, 2182. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Espinosa, J.; Aguilar-Arteaga, K.; Ibarra, I.S.; Miranda, J.M. Determination of tetracyclines in milk samples by magnetic solid phase extraction flow injection analysis. Mikrochim. Acta 2010, 171, 407–413. [Google Scholar] [CrossRef]
- Li, N.; Zhao, T.W.; Du, L.; Zhang, Z.Y.; Nian, Q.X.; Wang, M.M. Fast and simple determination of estrogens in milk powders by magnetic solid-phase extraction using carbon nitride composites prior to HPLC. Anal. Bioanal. Chem. 2021, 413, 215–223. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Chen, H.; Dang, X.; Ai, Y.; Liu, X.; Chen, H. Mesoporous yolk–shell structure molecularly imprinted magnetic polymers for the extraction and detection of 17β-estradiol. Anal. Methods 2020, 12, 507–513. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Ren, S.; Li, S.; Wang, Y.; Han, D.; Qin, K.; Peng, Y.; Han, T.; Gao, Z.; et al. Fabrication of magnetic Al-based Fe3O4@MIL-53 metal organic framework for capture of multi-pollutants residue in milk followed by HPLC-UV. Molecules 2022, 27, 2088. [Google Scholar] [CrossRef]
- Huang, X.C.; Ma, J.K.; Wei, S.L. Preparation and application of a novel magnetic molecularly imprinted polymer for simultaneous and rapid determination of three trace endocrine disrupting chemicals in lake water and milk samples. Anal. Bioanal. Chem. 2020, 412, 1835–1846. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H. Recent development and applications of dispersive liquid–liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vela-Soria, F.; Jiménez-Díaz, I.; Díaz, C.; Pérez, J.; Iribarne-Durán, L.M.; Serrano-López, L.; Arrebola, J.P.; Fernández, M.F.; Olea, N. Determination of endocrine-disrupting chemicals in human milk by dispersive liquid–liquid microextraction. Bioanalysis 2016, 8, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, X.; Liu, Z.; Xue, S.; Zhang, L. Novel functionalized magnetic ionic liquid green separation technology coupled with high performance liquid chromatography: A rapid approach for determination of estrogens in milk and cosmetics. Talanta 2020, 209, 120542. [Google Scholar] [CrossRef]
- Villegas-Álvarezab, M.C.; Callejón-Leblicab, B.; Rodríguez-Moroab, G.; Gómez-Arizaab, J.L.; Arias-Borregoab, A.; García-Barrera, T. Optimization of hollow-fiber liquid phase microextraction for polychlorinated biphenyls in human breast milk. J. Chromatogr. A 2020, 1626, 461381. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Pakade, V.E.; Ncube, S.; Tutu, H.; Chimuka, L. Application of hollow fibre-liquid phase microextraction technique for isolation and pre-concentration of pharmaceuticals in water. Membranes 2020, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Analysis of oestrogenic compounds in dairy products by hollow-fibre liquid-phase microextraction coupled to liquid chromatography. Food Chem. 2014, 149, 319–325. [Google Scholar] [CrossRef]
- Wang, P.J.; Xiao, Y.; Liu, W.J.; Wang, J.; Yang, Y.L. Vortex-assisted hollow fibre liquid-phase microextraction technique combined with high performance liquid chromatography-diode array detection for the determination of oestrogens in milk samples. Food Chem. 2015, 172, 385–390. [Google Scholar] [CrossRef]
- Xu, X.; Liang, F.H.; Shi, J.Y.; Zhao, X.; Liu, Z.; Wu, L.J.; Song, Y.; Zhang, H.Q.; Wang, Z.M. Determination of hormones in milk by hollow fiber-based stirring extraction bar liquid–liquid microextraction gas chromatography mass spectrometry. Anal. Chim. Acta 2013, 790, 39–46. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-based liquid chromatography-tandem mass spectrometry method for the simultaneous determination of nine zearalenone-like mycotoxins in pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef]
- Xiong, L.; Yan, P.; Chu, M.; Gao, Y.; Li, W.; Yang, X. A rapid and simple HPLC-FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk. Food Chem. 2018, 244, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.T.; Li, Z.M.; Deng, L.G.; Zhao, S.C.; Wang, M.L. Analysis of 13 kinds of steroid hormones in raw milk using modified QuEChERS method combined with UPLC-QTOF-MS. J. Integr. Agric. 2016, 15, 2163–2174. [Google Scholar] [CrossRef]
- Yu, Y.; Kuang, M.; Zheng, B.; Wang, M.; Liu, Z.; Xu, H.; Wang, J. Detection of multiple endocrine-disrupting chemicals in milk: Improved and safe high performance liquid chromatography tandem mass spectrometry method. J. Sep. Sci. 2022, 45, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Arain, M.B.; Soylak, M. Nanomaterials-based solid phase extraction and solid phase microextraction for heavy metals food toxicity. Food Chem. Toxicol. 2020, 145, 111704. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Perez, J.F.; Arroyo-Manzanares, N.; Garcia-Campana, A.M.; Gamiz-Gracia, L. Solid phase extraction as sample treatment for the determination of ochratoxin A in foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3405–3420. [Google Scholar] [CrossRef]
- Wathudura, P.D.; Kavinda, T.; Gunatilake, S.R. Determination of steroidal estrogens in food matrices: Current status and future perspectives. Curr. Opin. Food Sci. 2019, 28, 104–113. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental applications of solid-phase microextraction. Trends Analyt. Chem. 2019, 112, 1–12. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2017, 90, 302–360. [Google Scholar] [CrossRef]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef]
- Huang, S.M.; Chen, G.S.; Ye, N.R.; Kou, X.X.; Zhu, F.; Shen, J.; Ouyang, G.F. Solid-phase microextraction: An appealing alternative for the determination of endogenous substances—A review. Anal. Chim. Acta 2019, 1077, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic solid-phase extraction of organic compounds based on graphene oxide nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Sorouraddin, S.M.; Mogaddam, M.R.A. Liquid phase microextraction of pesticides: A review on current methods. Mikrochim. Acta 2014, 181, 829–851. [Google Scholar] [CrossRef]
- Yamini, Y.; Rezazadeh, M.; Seidi, S. Liquid-phase microextraction—The different principles and configurations. Trends Analyt. Chem. 2019, 112, 264–272. [Google Scholar] [CrossRef]
- Hansen, F.; Øiestad, E.L.; Pedersen-Bjergaard, S. Bioanalysis of pharmaceuticals using liquid-phase microextraction combined with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113446. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Trujillo-Rodríguez, M.J.; Pino, V.; Anderson, J.L. Non-conventional solvents in liquid phase microextraction and aqueous biphasic systems. J. Chromatogr. A 2017, 1500, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Arain, M.B.; Yamini, Y.; Shah, N.; Kazi, T.G.; Pedersen-Bjergaard, S.; Tajik, M. Hollow fiber-based liquid phase microextraction followed by analytical instrumental techniques for quantitative analysis of heavy metal ions and pharmaceuticals. J. Pharm. Anal. 2020, 10, 109–122. [Google Scholar] [CrossRef]
- Mogaddam, M.R.A.; Farajzadeh, M.A.; Mohebbi, A.; Nemati, M. Hollow fiber–liquid phase microextraction method based on a new deep eutectic solvent for extraction and derivatization of some phenolic compounds in beverage samples packed in plastics. Talanta 2020, 216, 120986. [Google Scholar] [CrossRef]
- Esrafili, A.; Baharfar, M.; Tajik, M.; Yamini, Y.; Ghambarian, M. Two-phase hollow fiber liquid-phase microextraction. Trends Analyt. Chem. 2018, 108, 314–322. [Google Scholar] [CrossRef]
- Hong, R.X.; Wu, P.P.; Lin, J.; Huang, L.Y.; Yang, L.J.; Wu, Y.J.; Yao, H. Three-phase hollow fiber liquid-phase microextraction combined with HPLC for determination of three trace acidic plant growth regulators in anoectochilus roxburghii (Wall.) Lindl. J. Sep. Sci. 2020, 43, 2773–2783. [Google Scholar] [CrossRef]
- McCarthy, O.J.; Singh, H. Physico-chemical properties of milk. In Advanced Dairy Chemistry Volume 3: Lactose, Water, Salts and Minor Constituents; Springer Science & Business Media: New York, NY, USA, 2009; pp. 691–758. [Google Scholar]
- Adamusova, H.; Bosakova, Z.; Coufal, P.; Pacakova, V. Analysis of estrogens and estrogen mimics in edible matrices—A review. J. Sep. Sci. 2014, 37, 885–905. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, K.; Watanabe, T. Excretion of bisphenol A into rat milk. Toxicol. Mech. Methods 2010, 20, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Preindl, K.; Braun, D.; Aichinger, G.; Sieri, S.; Fang, M.L.; Marko, D.; Warth, B. A generic liquid chromatography−tandem mass spectrometry exposome method for the determination of xenoestrogens in biological matrices. Anal. Chem. 2019, 91, 11334–11342. [Google Scholar] [CrossRef] [PubMed]
- Farke, C.; Rattenberger, E.; Roiger, S.U.; Meyer, H.H.D. Bovine colostrum: Determination of naturally occurring steroid hormones by liquid chromatography−tandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 2011, 59, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Regal, P.; Cepeda, A.; Fente, C. Development of an LC-MS/MS method to quantify sex hormones in bovine milk and influence of pregnancy in their levels. Food Addit. Contam. Part A 2012, 29, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Cai, J.Z.; Kraehenbuehl, K.; Hartmann, C.; Shao, B.; Mottier, P. Determination of steroid hormones in bovine milk by LC-MS/MS and their levels in swiss holstein cow milk. Food Addit. Contam. Part A 2016, 33, 804–816. [Google Scholar] [CrossRef]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Cheng, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC-MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef]
- Palacios Colón, L.; Rascón, A.J.; Hejji, L.; Azzouz, A.; Ballesteros, E. Validation and use of an accurate, sensitive method for sample preparation and gas chromatography–mass spectrometry determination of different endocrine-disrupting chemicals in dairy products. Foods 2021, 10, 1040. [Google Scholar] [CrossRef]
- Cao, X.L.; Popovic, S.; Arbuckle, T.E.; Fraser, W.D. Determination of free and total bisphenol A in human milk samples from canadian women using a sensitive and selective GC-MS method. Food Addit. Contam. Part A 2015, 32, 120–125. [Google Scholar] [CrossRef]
- Courant, F.; Antignac, J.P.; Maume, D.; Monteau, F.; Andre, F.; Le Bizec, B. Determination of naturally occurring oestrogens and androgens in retail samples of milk and eggs. Food Addit. Contam. Part A 2007, 24, 1358–1366. [Google Scholar] [CrossRef][Green Version]
- D’Orazio, G.; Hernández-Borges, J.; Herrera-Herrera, A.V.; Fanali, S.; Rodríguez-Delgado, M.Á. Determination of estrogenic compounds in milk and yogurt samples by hollow-fibre liquid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 7447–7459. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.P.; Zhao, G.Z.; Wei, M.; Wang, Y.J.; Zhao, B. Design of nanogold electrochemical immunosensor for detection of four phenolic estrogens. Chem. Phys. Lett. 2019, 732, 136657. [Google Scholar] [CrossRef]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, D. Highly-sensitive and simple fluorescent aptasensor for 17β-estradiol detection coupled with HCR-HRP structure. Talanta 2022, 240, 123094. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Pu, H.; Sun, D. A fluorescence aptasensor based on carbon quantum dots and magnetic Fe3O4 nanoparticles for highly sensitive detection of 17β-estradiol. Food Chem. 2022, 373, 131591. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Li, H. Diamond-based electrochemical aptasensor realizing a femtomolar detection limit of bisphenol A. Biosens. Bioelectron. 2017, 92, 21–25. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Karthika, P.; Shanmuganathan, S.; Viswanathan, S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensor for the determination of endocrine disruptor bisphenol-A in bovine milk. Food Chem. 2021, 363, 130287. [Google Scholar] [CrossRef]
- Tschmelak, J.; Käppel, N.; Gauglitz, G. TIRF-based biosensor for sensitive detection of progesterone in milk based on ultra-sensitive progesterone detection in water. Anal. Bioanal. Chem. 2005, 382, 1895–1903. [Google Scholar] [CrossRef]
- Daems, D.; Lu, J.; Delport, F.; Mariën, N.; Orbie, L.; Aernouts, B.; Adriaens, I.; Huybrechts, T.; Saeys, W.; Spasic, D.; et al. Competitive inhibition assay for the detection of progesterone in dairy milk using a fiber optic SPR biosensor. Anal. Chim. Acta 2017, 950, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Partridge, A.; Wu, Y. Improving nanoparticle-enhanced surface plasmon resonance detection of small molecules by reducing steric hindrance via molecular linkers. Talanta 2019, 198, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Colazo, M.G.; Serpe, M.J. Poly(N-isopropylacrylamide) microgel-based etalons for the label-free quantitation of estradiol-17β in aqueous solutions and milk samples. Anal. Bioanal. Chem. 2018, 410, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, Q.; Li, Y.; Li, S.; Han, T.; Wang, J.; Peng, Y.; Bai, J.; Ning, B.; Gao, Z. Upconversion fluorescent aptasensor for bisphenol A and 17β-estradiol based on a nanohybrid composed of black phosphorus and gold, and making use of signal amplification via DNA tetrahedrons. Mikrochim. Acta 2019, 186, 151. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, Z.; Dou, L.; Zhao, B.; He, Y.; Wang, J.; Sun, J.; Li, T.; Zhang, D. An innovative immunochromatography assay for highly sensitive detection of 17β-estradiol based on an indirect probe strategy. Sens. Actuators B Chem. 2019, 289, 48–55. [Google Scholar] [CrossRef]
- Lu, L.; Wang, M.; Zhang, D.; Zhang, H. Establishment of an immunofiltration strip for the detection of 17β-estradiol based on the photothermal effect of black phosphorescence. Analyst 2019, 144, 6647–6652. [Google Scholar] [CrossRef]
- Pan, J.; Liu, Z.; Chen, J. An amplifying DNA circuit coupled with Mg2+-dependent DNAzyme for bisphenol A detection in milk samples. Food Chem. 2021, 346, 128975. [Google Scholar] [CrossRef]
- Du, X.; Dai, L.; Jiang, D.; Li, H.; Hao, N.; You, T.; Mao, H.; Wang, K. Gold nanrods plasmon-enhanced photoelectrochemical aptasensing based on hematite/N-doped graphene films for ultrasensitive analysis of 17β-estradiol. Biosens. Bioelectron. 2017, 91, 706–713. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Li, J.; Li, H.B.; Fang, H.L.; Fan, D.H.; Wang, W. A label-free photoelectrochemical aptasensor for bisphenol A based on surface plasmon resonance of gold nanoparticle-sensitized ZnO nanopencils. Biosens. Bioelectron. 2016, 86, 315–320. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, J.Y.; Liu, S.Z.; Zhang, W.Y.; Zhang, J.; He, J.; Li, P.D.; Li, X.H.; Jin, J.J.; Wang, Z.H. Production of antibodies and development of an enzyme-linked immunosorbent assay for 17β-estradiol in milk. Food Agric. Immunol. 2017, 28, 1519–1529. [Google Scholar] [CrossRef]
- Jia, M.; Chen, S.; Shi, T.T.; Li, C.Y.; Wang, Y.P.; Zhang, H.Y. Competitive plasmonic biomimetic enzyme-linked immunosorbent assay for sensitive detection of bisphenol A. Food Chem. 2021, 344, 128602. [Google Scholar] [CrossRef]
- Samiee, F.; Kharazi, A.; Elaridi, J.; Javad, M.T.; Leili, M. An assessment of the occurrence and nutritional factors associated with aflatoxin M1, ochratoxin A, and zearalenone in the breast milk of nursing mothers in Hamadan, Iran. Toxicon 2020, 187, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kuruto-Niwa, R.; Tateoka, Y.; Usuki, Y.; Nozawa, R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere 2007, 66, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Shen, Y.; Yang, M.; Li, X.; Han, X.; Jiang, X.; Zhao, B. SERS strategy based on the modified Au nanoparticles for highly sensitive detection of bisphenol A residues in milk. Talanta 2018, 179, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhang, Y.Y.; Kou, Q.W.; Zhang, Y.J.; Wang, Y.X.; Chen, L.; Sun, Y.T.; Zhang, H.L.; Jung, Y.M. Detection and identification of estrogen based on surface-enhanced resonance raman scattering (SERRS). Molecules 2018, 23, 1330. [Google Scholar] [CrossRef]
- Han, X.X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling reaction-based ultrasensitive detection of phenolic estrogens using surface-enhanced resonance Raman scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef]
- Broadbent, J.A.; Condina, M.R.; Colgrave, M.L. Quantitative mass spectrometry-based analysis of proteins related to cattle and their products–Focus on cows’ milk beta-casein proteoforms. Methods 2021, 186, 112–118. [Google Scholar] [CrossRef]
- Sargent, M.E. Guide to Achieving Reliable Quantitative LC-MS Measurements, 1st ed.; RSC Analytical Methods Committee: Middlesex, UK, 2013; p. 1. [Google Scholar]
- Chae, H.J.; Cho, S.E.; Park, H.D.; Chun, S.; Lee, Y.W.; Yun, Y.M.; Song, S.H.; Lee, S.G.; Lee, K.; Junghan, S.; et al. Use of liquid chromatography-tandem mass spectrometry for clinical testing in korean laboratories: A questionnaire survey. Ann. Lab. Med. 2019, 39, 447–453. [Google Scholar] [CrossRef]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.R.; Izadyar, M.; Bozorgmehr, M.R.; Verdian, A. Recent advances in computational methods for biosensor design. Biotechnol. Bioeng. 2021, 118, 555–578. [Google Scholar] [CrossRef]
- Farag, M.A.; Tanios, M.; AlKarimy, S.; Ibrahim, H.; Guirguis, H.A. Biosensing approaches to detect potential milk contaminants: A comprehensive review. Food Addit. Contam. Part A 2021, 38, 1169–1192. [Google Scholar] [CrossRef]
- Park, M. Surface display technology for biosensor applications: A review. Sensors 2020, 20, 2775. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Jalalvand, A.R.; Haseli, A.; Farzadfar, F.; Goicoechea, H.C. Fabrication of a novel biosensor for biosensing of bisphenol A and detection of its damage to DNA. Talanta 2019, 201, 350–357. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. Trends Analyt. Chem. 2020, 127, 115882. [Google Scholar] [CrossRef]
- Wu, T.; Yan, T.; Zhang, X.; Feng, Y.; Wei, D.; Sun, M.; Du, B.; Wei, Q. A competitive photoelectrochemical immunosensor for the detection of diethylstilbestrol based on an Au/UiO-66(NH2)/Cds matrix and a direct Z-scheme Melem/CdTe heterojunction as labels. Biosens. Bioelectron. 2018, 117, 575–582. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Dong, K.; Wu, Y.J.; Zhang, P.X. Study of raman technique applied to estrogen. Spectrosc. Spect. Anal. 2011, 31, 2127–2130. [Google Scholar]
- Ross, M.B.; Ashley, M.J.; Schmucker, A.L.; Singamaneni, S.; Naik, R.R.; Schatz, G.C.; Mirkin, C.A. Structure–function relationships for surface-enhanced raman spectroscopy-active plasmonic paper. J. Phys. Chem. C 2016, 120, 20789–20797. [Google Scholar] [CrossRef]
- Vedad, J.; Mojica, E.E.; Desamero, R.Z.B. Raman spectroscopic discrimination of estrogens. Vib. Spectrosc. 2018, 96, 93–100. [Google Scholar] [CrossRef]
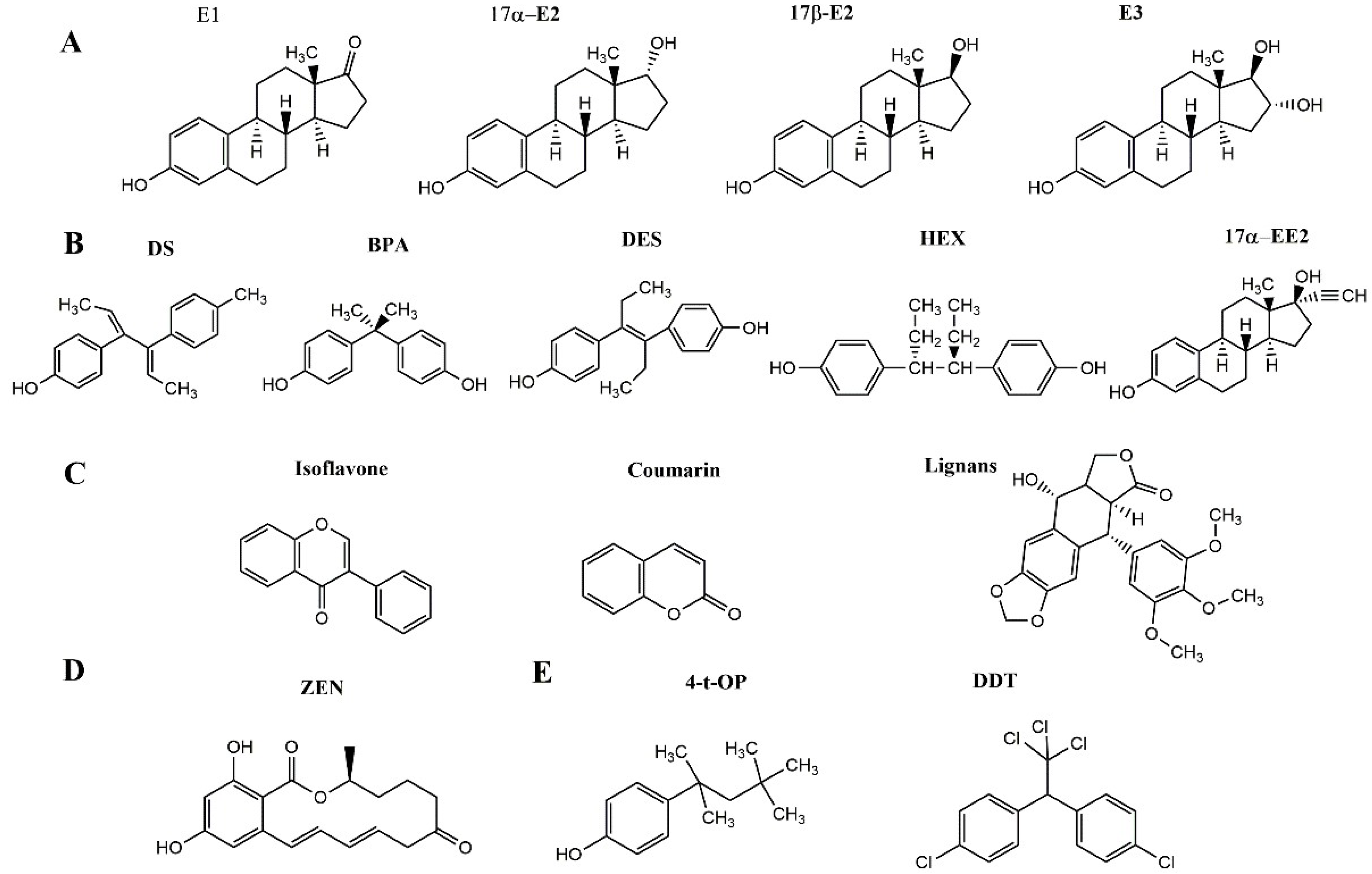
| Technology | Strengths | Limitations | Samples | E-EDCs | Recovery | Refs. |
|---|---|---|---|---|---|---|
| SPE | High recovery rate; Few operation steps; Short analysis time; Analyte stabilization. | Poor selectivity; Large solvent consumption. | Milk | BPA, E2, DES | 84.1 ± 8.2–113.6 ± 2.9% | [53,54,55,56] |
| Milk | E3, DES, E1 | 70.82–112.90% | ||||
| SPME | Short pretreatment time; Less organic solvent consumption; No need for special equipment; Low cost; High sensitivity. | Limited options for commercial stationary phases and fibrous coatings; Low thermal and chemical stability. | Milk | E2 | 77.27–108.26% | [57,58,59,60] |
| Milk powders | E1, E2, E3, DES | 80.8–96.6%; 81.5–93.3%; 77.3–95.1%; 79.4–92.2% | ||||
| MSPE | Fast; Clean; Short time; High recovery; Few steps; Little waste; No column blockage problem. | Difficult and complex synthesis of magnetic materials. | Milk powders | E2,17α-EE2, E1, HEX | 75.1–97.2%; | [61,62,63,64,65,66,67,68] |
| Milk powders | E2 | 88.3–102.4% | ||||
| Milk | BPA, E2, DES | 88.17–107.58% | ||||
| Milk samples | Nonylphenol, BPA, and HEX | 89.9–98.7% | ||||
| DLLME | Simple operation; Fast speed; Low consumption of solvents and reagents. | DLLME has higher requirements for extraction and dispersed solvents. | Human milk | BPA, BPF, BPS parabens, and benzophenones | Above 90.2% | [69,70,71,72] |
| Milk | E1, E2, CMA, MGA, HP, MPA | 98.5–109.3% | ||||
| HF-LPME | Simple operation; Low consumption of organic solvents; Low cost. | Within a specific pH range. | Different dairy products | E1, 17β-E2, 17α-E2, E3, 17α-EE2, DES, DS, HEX, 2-OHE2 | Above 82% | [73,74,75,76,77] |
| Whole milk and skim milk | E2, E1, DES | 98.5–109.3% | ||||
| Milk | E1, 17α-E2, 17β-E2, 17α-EE2, and E3 | 93.6–104.6% | ||||
| QuEChERS | Low cost; Simple operation; Sensitive detection; Short time consumption. | Highly dependent on the nature of the target analyte, substrate composition, experimental equipment, and temperature. | Milk | Nine bisphenols | 75.82–93.86% | [78,79,80,81,82] |
| Raw milk | 17β-E2, E3, E1, DES, progesterone | 74.2–99.7% | ||||
| Milk | E1, E2, E3, DES, BPA, and BPB | 77.7–107.5% |
| Technology | Strengths | Sample | E-EDCs | LOD (s) or LOQ (s) | Refs. |
|---|---|---|---|---|---|
| LC-MS | High selectivity and sensitivity. | Milk | BPA | ppm levels | [102] |
| LC-MS/MS | Analytical capability; High throughput. | Breast milk | Xenoestrogens | 0.03–4.6 µg·L−1 | [103] |
| Isolated colostrum and colostrum powder | E1, 17α-E2, 17β-E2; | E1 (5.51µg·L−1; 15 µg·kg−1), 17α-E2 (2.66 µg·L−1; 7.5 µg·kg−1) and 17β-E2 (2.28 µg·L−1; 3.3 µg·kg−1) | [104] | ||
| Milk | Progesterone, E1 | ng·dL−1 level | [105] | ||
| Milk | 17α-E2, 17β-E2, E1 | 31 ng·kg−1, 6 ng·kg−1, 159 ng·kg−1 | [106] | ||
| Milk | E1, 17β-E2, E3, 17α-EE2, and conjugated estrogen metabolites | ng·L−1 level | [45] | ||
| UHPLC-MS/MS | N.M. | Milk and yogurt | Various estrogenic compounds | 0.02–0.60 µg·L−1, 0.02–0.90 µg·kg−1 | [5] |
| Milk | E1, 17β-E2, 17α-E2, E3, 17α-EE2, DES, HEX, DS | 0.10–0.35 µg·L−1 | [50] | ||
| Milk | ZEN, and α-zearalenol | 0.003–0.015 µg·kg−1 | [107] | ||
| GC-MS | Good selectivity; high separation degree; High sensitivity; High repeatability; Relatively stable. | Dairy products | BPA; | 6–40 ng·kg−1; | [108] |
| Human milk | Free and total BPA | ng·g−1 level | [109] | ||
| Milk | E1, 17β-E2, 17α-E2 | 5 ng·kg−1 | [110] | ||
| Different kinds of dairy products | 17α-E2, 17β-E2, and 17α-EE2 | µg·L−1 level | [111] | ||
| Electrochemical biosensors | N.M. | Milk powder | EDS, DS, BPA, HEX | 0.25, 0.15, 0.20 and 0.25 ng·mL−1 | [112] |
| Milk | 17β-E2 | 0.7 pM | [113] | ||
| Milk | E2 | 0.2 pg·mL−1 | [114] | ||
| Milk | E2 | 3.48 × 10−12 M | [115] | ||
| Milk | BPA | 7.2 × 10−15 mol·L−1 | [116] | ||
| Liquid milk and milk powder | BPA | 5 µM | [117] | ||
| Bovine milk | BPA | 0.2 nmol·L−1 | [118] | ||
| Optical biosensors | N.M. | UHT milk, fresh milk and raw milk | Progesterone | 45.5–56.1 pg·mL−1 | [119] |
| Milk | Progesterone | 0.5 ng·mL−1 | [120] | ||
| Milk | Progesterone | 0.038 ng·mL−1 | [121] | ||
| Nonfat milk, 2% milk, and farm milk | E2 | 0.9 pg·mL−1, 8.4 pg·mL−1, and 4–9.1 pg·mL−1 | [122] | ||
| Milk | BPA and E2 | 7.8 pg·mL−1 and 92 pg·mL−1 | [123] | ||
| Milk | E2 | 0.2 ng·mL−1 | [124] | ||
| Milk | E2 | 0.104 ng·mL−1 | [125] | ||
| Milk | BPA | 50 fM | [126] | ||
| Photoelectrochemical biosensors | High sensitivity; Low cost; Easy miniaturization. | Milk powder | E2 | 3.3 × 10−16 M | [127] |
| Liquid milk | BPA | 0.5 nmol·L−1 | [128] | ||
| ELISA | Highly sensitive; Cost-effective; Simple to perform. | Milk | 17β-E2 | 0.093 µg·L−1 | [129] |
| Milk | BPA | 40 pg·mL−1 | [130] | ||
| Human milk | ZEN | 5 ng·L−1 | [131] | ||
| Human colostrum | BPA | ng·mL−1 | [132] | ||
| SERS | Highly sensitive. | Milk | BPA | 4.3 × 10−9 moL·L−1 | [133] |
| Infant formula | E1, E2 and BPA | 0.2 × 10−4 M | [134] | ||
| Infant formula | E2 | 0.1 µg·kg−1 | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Zhou, J.; Gao, M.; Zhang, H.; Wang, T. Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products. Foods 2022, 11, 3057. https://doi.org/10.3390/foods11193057
Chang J, Zhou J, Gao M, Zhang H, Wang T. Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products. Foods. 2022; 11(19):3057. https://doi.org/10.3390/foods11193057
Chicago/Turabian StyleChang, Jia, Jianhua Zhou, Mingyang Gao, Hongyan Zhang, and Tian Wang. 2022. "Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products" Foods 11, no. 19: 3057. https://doi.org/10.3390/foods11193057
APA StyleChang, J., Zhou, J., Gao, M., Zhang, H., & Wang, T. (2022). Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products. Foods, 11(19), 3057. https://doi.org/10.3390/foods11193057






