Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability
Abstract
:1. Introduction
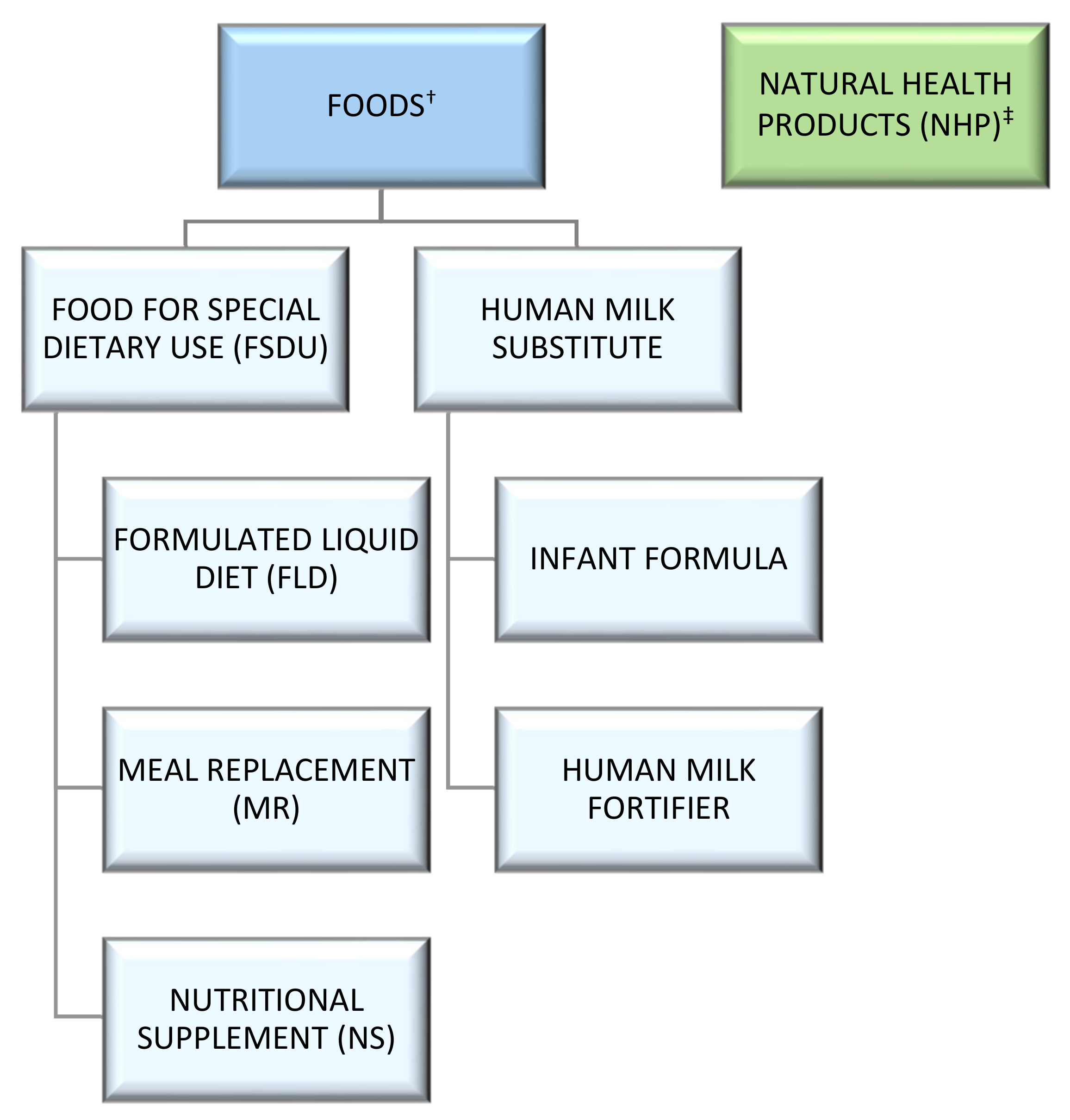
2. Specialized Product Use and Regulatory Categorization
2.1. United States
2.2. Canada
3. Role of Functional Packaging for Foods and Specialized Products
3.1. Preservation and Protection
3.2. Containment and Food Waste Reduction
3.3. Transportation and Traceability
3.4. Storage, Convenience, and Integrity
3.5. Marketing and Product Information
4. Packaging Considerations for the Manufacturing Process and Applicable Regulations
4.1. Manufacturing for Specialized Products
4.2. Packaging Materials for Specialized Products
4.3. Packaging and Food Contact Substance Regulations
5. Sustainability and Waste Reduction Strategies and Challenges
5.1. Reducing Packaging Materials
5.2. Reusing Packaging Materials
5.3. Recycling Packaging Materials
5.4. Biobased Packaging and Compostability
6. Recycling and Sustainability Policy Framework
6.1. United States
6.2. Canada
7. Innovation and Policy Development for Sustainability
7.1. Implications for Emerging Innovation
7.2. Implications for Policy Development for Sustainability
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFR | Code of Federal Regulations |
| EPR | Extended Producer Responsibility |
| FAP | Food Additive Petition |
| FCN | Food Contact Notification |
| FCS | Food Contact Substance |
| FD&C Act | Food Drug and Cosmetic Act |
| FDA | US Food and Drug Administration |
| FDR | Health Canada’s Food and Drug Regulations |
| FLD | Formulated Liquid Diet |
| FSDU | Food for Special Dietary Use |
| GMP | Good Manufacturing Practice |
| GRAS | Generally Recognized as Safe |
| HDPE | High Density Polyethylene |
| HPFB | Health Products and Food Branch |
| LDPE | Low Density Polyethylene |
| LONO | Letter of No Objection |
| MR | Meal Replacement |
| NHP | Natural Health Product |
| NHPR | Natural Health Product Regulations |
| NS | Nutritional Supplement |
| PCR | Post consumer recycled |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| USC | United States Code |
| USDA | United States Department of Agriculture |
References
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in packaging material for food products: Historical background, current scenario, and future prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Use of Recycled Plastics in Food Packaging (Chemistry Considerations). Available online: https://www.fda.gov/media/150792/download (accessed on 14 July 2022).
- Government of Canada. Food Safety for Vulnerable Populations. Available online: https://www.canada.ca/en/health-canada/services/food-safety-vulnerable-populations.html (accessed on 7 June 2022).
- Government of Canada. Food Safety Information for Children Ages 5 and Under. Available online: https://www.canada.ca/en/health-canada/services/food-safety-vulnerable-populations/food-safety-information-children-ages-5-under.html (accessed on 14 July 2022).
- Government of Canada. Food Safety for People with a Weakened Immune System. Available online: https://www.canada.ca/en/health-canada/services/food-safety-vulnerable-populations/food-safety-people-with-weakened-immune-system.html (accessed on 14 July 2022).
- Devine, A.; Lawlis, T. Nutrition and Vulnerable Groups. Nutrients 2019, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Food Safety for Adults Ages 60 and Over. Available online: https://www.canada.ca/en/health-canada/services/food-safety-vulnerable-populations/food-safety-adults-ages-60-over.html (accessed on 14 July 2022).
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, bmj.k2173. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Rouster, A. Infant Nutrition Requirements and Options; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560758/ (accessed on 6 June 2022).
- Medical Nutrition International Industry. Oral Nutritional Supplements to Tackle Malnutrition: A Summary of the Evidence Base. Available online: http://medicalnutritionindustry.com/files/user_upload/documents/publications/Dossier2012FINAL2012-09-04.pdf (accessed on 10 August 2022).
- Office of Law Revision Counsel of the U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 360ee(b)(3). Gov. Print. Off. 2020; p. 372. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- US Food and Drug Administration. Frequently Asked Questions About Medical Foods; Second Edition. Available online: https://www.fda.gov/media/97726/download (accessed on 7 June 2022).
- US Food and Drug Administration. Compliance Program Guidance Manual. Available online: https://www.fda.gov/media/71685/download (accessed on 15 August 2022).
- U.S Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 105.3-105.66. Fed. Regist. 2021; pp. 210–212. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- Office of Law Revision Counsel of the, U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 321(z). Gov. Print. Off. 2020; p. 38. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- U.S. Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 107.1-107.280. Fed. Regist. 2021; pp. 239–249. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- American Society for Parenteral and Enteral Nutrition. Definitions of Terms, Style, and Conventions Used in ASPEN Board of Directors—Approved Documents. Available online: https://www.nutritioncare.org/uploadedFiles/Documents/Guidelines_and_Clinical_Resources/ASPEN%20Definition%20of%20Terms,%20Style,%20and%20Conventions%20Used%20in%20ASPEN%20Board%20of%20Directors%E2%80%93Approved%20Documents.pdf (accessed on 5 August 2022).
- U.S. Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 107.50. Fed. Regist. 2021; pp. 243–245. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.01.001-B.29.032. Minist. Justice. 2022; pp. 11–719. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Health Canada. Natural Health Products Regulations, SOR/2003-196. Minist. Justice. 2022; pp. 1–75. Available online: https://laws-lois.justice.gc.ca/PDF/SOR-2003-196.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drugs Act, R.S.C., 1985, c. F-27. Minist. Justice. 2021; pp. 1–66. Available online: https://laws-lois.justice.gc.ca/PDF/F-27.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.24.001. Minist. Justice. 2022; pp. 593–595. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.01.001. Minist. Justice. 2022; pp. 11–24. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.25.001. Minist. Justice. 2022; pp. 615–616. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Health Canada. Classification of Products at the Food-Natural Health Product Interface: Products in Food Formats. Available online: https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/classification-products-at-food-natural-health-product-interface-eng.pdf (accessed on 19 July 2022).
- Government of Canada. Packaging Materials. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/packaging-materials.html (accessed on 14 June 2022).
- Centers for Disease Control and Prevention. Estimates of Foodborne Illness in the United States. Available online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 14 June 2022).
- Government of Canada. Yearly Food-Borne Illness Estimates for Canada. Available online: https://www.canada.ca/en/public-health/services/food-borne-illness-canada/yearly-food-borne-illness-estimates-canada.html (accessed on 14 June 2022).
- UN Environment Programme. UNEP Food Waste Index Report 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 14 June 2022).
- Marsh, K.; Bugusu, B. Food packaging–roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Ameripen. Ameripen Report: Quantifying the Value of Packaging as a Strategy to Prevent Food Waste in America. Available online: https://www.ameripen.org/page/foodwastereport (accessed on 14 June 2022).
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-related food losses and waste: An overview of drivers and issues. Sustainability 2019, 11, 264. [Google Scholar] [CrossRef]
- Mahalik, N.P. Advances in packaging methods, processes and systems. Challenges 2014, 5, 374–389. [Google Scholar] [CrossRef]
- Office of Law Revision Counsel of the, U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 2201–2252. Gov. Print. Off. 2020; pp. 941–957. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- US Food and Drug Administration. New Era of Smarter Food Safety Blueprint. Available online: https://www.fda.gov/food/new-era-smarter-food-safety/new-era-smarter-food-safety-blueprint (accessed on 14 June 2022).
- US Food and Drug Administration. FSMA Proposed Rule for Food Traceability. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-proposed-rule-food-traceability (accessed on 14 June 2022).
- Health Canada. Safe Food for Canadians Act, S.C. 2012, c. 24. Minist. Justice. 2019; pp. 1–48. Available online: https://laws-lois.justice.gc.ca/PDF/2012_24.pdf (accessed on 29 September 2022).
- Government of Canada. Regulatory Requirements: Traceability. Available online: https://inspection.canada.ca/food-safety-for-industry/traceability/traceability/eng/1522294721005/1522294781171 (accessed on 14 June 2022).
- Oliveira, V.; Prell, M.; Smallwood, D.; Frazão, E. Infant Formula Prices and Availability—Final Report to Congress. Available online: https://www.ers.usda.gov/webdocs/publications/43025/35759_efan02001.pdf?v=0 (accessed on 11 August 2022).
- National Retail Foundation. 2020 Organized Retail Crime Survey. Available online: https://nrf.com/research/2020-organized-retail-crime-survey (accessed on 14 June 2022).
- US Food and Drug Administration. Questions & Answers for Consumers Concerning Infant Formula. Available online: https://www.fda.gov/food/people-risk-foodborne-illness/questions-answers-consumers-concerning-infant-formula#2 (accessed on 7 June 2022).
- Fenko, A.; Schifferstein, H.N.; Hekkert, P. Shifts in Sensory Dominance between Various Stages of User-Product Interactions. Appl. Ergon. 2010, 41, 34–40. [Google Scholar] [CrossRef]
- Bell, A.F.; Walton, K.L.; Tapsell, L.C. Easy to open? Exploring the ‘openability’ of hospital food and beverage packaging by older adults. Appetite 2016, 98, 125–132. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 107.10-107.30. Fed. Regist. 2021; pp. 240–243. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.25.045-B.25.060. Minist. Justice. 2022; pp. 624–633. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Gilmore, L.A.; Altazan, A.D.; Flanagan, E.W.; Beyer, A.G.; Olson, K.N.; O’Connell, A.A.; Nguyen, T.H.; Beyl, R.A.; Redman, L.M. Modifications to infant formula instructions improve the accuracy of formula dispensing. Nutrients 2020, 12, 1150. [Google Scholar] [CrossRef]
- Emenhiser, C. Formulation and Manufacturing of Infant and Toddler Foods. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2003/october/features/developing-foods_formulation-and-manufacturing-of-infant-and-toddler-foods (accessed on 11 August 2022).
- US Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 113.3-113.100. Fed. Regist. 2021; pp. 343–377. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- US Food and Drug Administration. Aseptic Processing and Packaging for the Food Industry. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/aseptic-processing-and-packaging-food-industry (accessed on 11 August 2022).
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-143-986-241-4. [Google Scholar]
- Blakistone, B.; Chen, Y.; Song, Y.S.; Bourque, R.A. The Wiley Encyclopedia of Packaging Technology, 3rd ed.; Yam, K.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Chapter 8; pp. 567–580. ISBN 978-047-054-139-5. [Google Scholar]
- Weddig, L.M.; Balestrini, C.G.; Shafer, B.D. Canned Foods: Principles of Thermal Process Control, Acidification and Container Closure Evaluation, 7th ed.; GMA Science and Education Foundation: Washington, DC, USA, 2007; ISBN 978-093-777-458-8. [Google Scholar]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Ye, Y.; Engholm-Keller, K.; Fang, Y.; Nielsen, C.F.; Jordà, A.; Lund, M.N.; Chatterton, D.E.W. UHT treatment and storage of liquid infant formula affects protein digestion and release of bioactive peptides. Food Funct. 2022, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Sand, C.K. Testing the Integrity of Package Seals. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2019/july/columns/packaging-seals-tests-standards (accessed on 11 August 2022).
- US Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 106.50. Fed. Regist. 2021; pp. 222–223. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol2/pdf/CFR-2021-title21-vol2.pdf (accessed on 29 September 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.27.001-B.27.005. Minist. Justice. 2022; pp. 641–643. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Kirwan, M.J.; Plant, S.; Strawbridge, J.W. Plastics in food packaging. In Food and Beverage Packaging Technology, 2nd ed.; Coles, R., Kirwan, M., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 157–212. ISBN 978-144-439-218-0. [Google Scholar]
- Durusoy, R.; Karababa, A.O. Plastic food packaging and health. TAF Prev. Med. Bull. 2011, 10, 87–96. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Office of Law Revision Counsel, U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 321(s). Gov. Print. Off. 2020; p. 37. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- US Food and Drug Administration. Code of Federal Regulations: Title 21—Food and Drugs, 21 CFR 170.3-170.285. Fed. Regist. 2021; pp. 5–28. Available online: https://www.govinfo.gov/content/pkg/CFR-2021-title21-vol3/pdf/CFR-2021-title21-vol3.pdf (accessed on 29 September 2022).
- Office of Law Revision Counsel, U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 348(h)(6). Gov. Print. Off. 2020; p. 119. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- Office of Law Revision Counsel, U.S. House of Representatives. United States Code: Title 21—Food and Drugs, 21 USC 348(h). Gov. Print. Off. 2020; pp. 118–119. Available online: https://www.govinfo.gov/content/pkg/USCODE-2020-title21/pdf/USCODE-2020-title21.pdf (accessed on 29 September 2022).
- US Food and Drug Administration. Preparation of Food Contact Substance Notifications (Administrative): Guidance for Industry. Available online: https://www.fda.gov/media/153218/download (accessed on 11 August 2022).
- US Food and Drug Administration. Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 11 August 2022).
- Health Canada. Food and Drug Regulations: Part B—Foods, FDR B.23.001-B.23.008. Minist. Justice. 2022; pp. 591–593. Available online: https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf (accessed on 29 September 2022).
- Government of Canada. Lists of Acceptable Polymers for Use in Food Packaging Applications. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/lists-acceptable-polymers-use-food-packaging-applications.html (accessed on 11 August 2022).
- Government of Canada. Health Products and Food Branch Packaging Materials Assessment Process. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/health-products-food-branch-packaging-materials-assessment-process-food-nutrition.html (accessed on 11 August 2022).
- Government of Canada. Guidelines for Determining the Acceptability and Use of Recycled Plastics in Food Packaging Applications. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/guidelines-determining-acceptability-use-recycled-plastics-food-packaging-applications-1996.html (accessed on 11 August 2022).
- US Department of Agriculture. USDA Takes Significant Steps to Build More Sustainable, Resilient and Inclusive Food Systems. Available online: https://www.usda.gov/media/press-releases/2021/09/23/usda-takes-significant-steps-build-more-sustainable-resilient-and (accessed on 21 June 2022).
- Government of Canada. Canada Joins International Sustainable Agriculture Production and Food Systems Coalition. Available online: https://www.canada.ca/en/agriculture-agri-food/news/2022/01/canada-joins-international-sustainable-agriculture-production-and-food-systems-coalition.html (accessed on 28 June 2022).
- Licciardello, F. Packaging, Blessing in Disguise. Review on Its Diverse Contribution to Food Sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Coussy, H.; Guillard, V.; Guillaume, C.; Gontard, N. Role of packaging in the smorgasbord of action for sustainable food consumption. Agro-Food-Ind. Hi Tech. 2013, 24, 15–19. [Google Scholar]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef]
- Heller, M. Food Product Environmental Footprint Literature Summary: Packaging and Wasted Food. Available online: https://www.oregon.gov/deq/FilterDocs/PEF-Packaging-FullReport.pdf (accessed on 12 August 2022).
- Food and Agriculture Organization of the United Nations. Reduce, Reuse, Recycle: A Mantra for Food Packaging. Available online: https://www.fao.org/fao-stories/article/en/c/1441299/ (accessed on 28 June 2022).
- Berg, P.; Feber, D.; Granskog, A.; Nordigården, D.; Ponkshe, S. The Drive Toward Sustainability in Packaging—Beyond the Quick Wins. Available online: https://www.mckinsey.com/industries/paper-forest-products-and-packaging/our-insights/the-drive-toward-sustainability-in-packaging-beyond-the-quick-wins (accessed on 28 June 2022).
- Coelho, P.M.; Corona, B.; ten Klooster, R.; Worrell, E. Sustainability of reusable packaging–Current situation and trends. Resour. Conserv. Recycl. 2020, 6, 100037. [Google Scholar] [CrossRef]
- Jetten, J.; de Kruijf, N.; Castle, L. Quality and safety aspects of reusable plastic food packaging materials: A European study to underpin future legislation. Food Addit. Contam. 1999, 16, 25–36. [Google Scholar] [CrossRef]
- Tisler, S.; Christensen, J.H. Non-target screening for the identification of migrating compounds from reusable plastic bottles into drinking water. J. Hazard Mater. 2022, 429, 128331. [Google Scholar] [CrossRef]
- Sand, C.K. Orchestrating More Sustainable Reusable Food Packaging. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2020/december/columns/packaging-orchestrating-more-sustainable-reusable-food-packaging (accessed on 12 August 2022).
- US Food and Drug Administration. Packaging & Food Contact Substances (FCS). Available online: https://www.fda.gov/food/food-ingredients-packaging/packaging-food-contact-substances-fcs (accessed on 30 June 2022).
- US Food and Drug Administration. Guidance for Industry: Preparation of Food Contact Notifications for Food Contact Substances in Contact with Infant Formula and/or Human Milk. Available online: https://www.fda.gov/media/124714/download (accessed on 15 August 2022).
- Baughan, J.S. Future trends in global food packaging regulation. In Global Legislation for Food Contact Materials; Baughan, J.S., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 65–74. ISBN 978-1-78242-014-9. [Google Scholar]
- US Environmental Protection Agency. Advancing Sustainable Materials Management: 2018 Fact SHEET. Available online: https://www.epa.gov/sites/default/files/2020-11/documents/2018_ff_fact_sheet.pdf (accessed on 28 June 2022).
- Government of Canada. Solid Waste Diversion and Disposal. Available online: https://www.canada.ca/en/environment-climate-change/services/environmental-indicators/solid-waste-diversion-disposal.html (accessed on 28 June 2022).
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Leissner, S.; Ryan-Fogarty, Y. Challenges and opportunities for reduction of single use plastics in healthcare: A case study of single use infant formula bottles in two Irish maternity hospitals. Resour. Conserv. Recycl. 2019, 151, 104462. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of poly (ethylene terephthalate)-A review focusing on chemical methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Recycling of post-consumer packaging materials into new food packaging applications—Critical review of the European approach and future perspectives. Sustainability 2022, 14, 824. [Google Scholar] [CrossRef]
- AMERIPEN. US Company Recycled Plastic Content Goals Analysis—Supply & Demand. Available online: https://www.ameripen.org/resource/resmgr/docs/AMERIPEN-recycled-content-su.pdf (accessed on 13 August 2022).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S.; Van Geem, K.M.; Roosen, M.; Delbeke, E.I.P.; De Meester, S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manag. 2020, 104, 148–182. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.-S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and redesign challenges in multilayer flexible food packaging—A review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J.; Andersson, A.-M.; Backhaus, T.; Boucher, J.M.; Carney Almroth, B.; Castillo Castillo, A.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.-B.; et al. Impacts of food contact chemicals on human health: A consensus statement. Environ. Health 2020, 19, 25. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Recycled Plastics in Food Packaging. Available online: https://www.fda.gov/food/packaging-food-contact-substances-fcs/recycled-plastics-food-packaging (accessed on 13 August 2022).
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A literature review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, H.; Adams, C.D.; Ma, Y. Assessment of metal contaminations leaching out from recycling plastic bottles upon treatments. Environ. Sci. Pollut. Res. Int. 2010, 17, 1323–1330. [Google Scholar] [CrossRef]
- Whitt, M.; Vorst, K.; Brown, W.; Baker, S.; Gorman, L. Survey of heavy metal contamination in recycled polyethylene terephthalate used for food packaging. J. Plast. Film Sheeting 2012, 29, 163–173. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. Quality-determination of recycled plastic packaging waste by identification of contaminants by GC–MS after microwave assisted extraction (MAE). Polym. Degrad. Stab. 2000, 71, 123–134. [Google Scholar] [CrossRef]
- Watson, A. Companies Putting Public Health at Risk by Replacing One Harmful Chemical with Similar, Potentially Toxic, Alternatives. Available online: https://chemtrust.org/toxicsoup/ (accessed on 13 August 2022).
- Environment and Climate Change Canada. Assessing the State of Food Grade Recycled Resin in Canada & the United States. Available online: https://www.plasticsmarkets.org/jsfcontent/ECCC_Food_Grade_Report_Oct_2021_jsf_1.pdf (accessed on 28 June 2022).
- Tullo, A. Plastic Has a Problem; is Chemical Recycling the Solution? Available online: https://cen.acs.org/environment/recycling/Plastic-problem-chemical-recycling-solution/97/i39 (accessed on 28 June 2022).
- Cecon, V.S.; da Silva, P.H.F.; Curtzwiler, G.W.; Vorst, K.L. The challenges in recycling post-consumer polyolefins for food contact applications: A review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Song, T.; Qian, S.; Lan, T.; Wu, Y.; Liu, J.; Zhang, H. Recent advances in bio-based smart active packaging materials. Foods 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Cornall, J. Nestlé Launches Bio-Based Lids and Scoops for Infant Formula. Available online: https://www.dairyreporter.com/Article/2021/03/16/Nestle-launches-bio-based-lids-and-scoops-for-infant-formula (accessed on 13 August 2022).
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Byrnes, H.; Frohlich, T.C. Canada Produces the Most Waste in the World. The US Ranks Third. Available online: https://www.usatoday.com/story/money/2019/07/12/canada-united-states-worlds-biggest-producers-of-waste/39534923/ (accessed on 28 June 2022).
- The World Bank. Trends in Solid Waste Management. Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 28 June 2022).
- Schultz, J.; Hildreth, K. State and Federal Efforts to Revitalize Recycling. Available online: https://www.ncsl.org/research/environment-and-natural-resources/state-and-federal-efforts-to-revitalize-recycling.aspx (accessed on 28 June 2022).
- Quinn, M.; Rosengren, C. Tracking the Future of US Recycling Policy in Congress. Available online: https://www.wastedive.com/news/tracking-the-future-of-us-recycling-policy-in-congress/570778/ (accessed on 28 June 2022).
- US Government Accountability Office. Building on Existing Federal Efforts Could Help Address Cross-Cutting Challenges. Available online: https://www.gao.gov/products/gao-21-87 (accessed on 28 June 2022).
- US Environmental Protection Agency. Resource Conservation and Recovery Act (RCRA) Overview. Available online: https://www.epa.gov/rcra/resource-conservation-and-recovery-act-rcra-overview (accessed on 28 June 2022).
- US Environmental Protection Agency. US National Recycling Goal. Available online: https://www.epa.gov/recyclingstrategy/us-national-recycling-goal (accessed on 28 June 2022).
- US Environmental Protection Agency. National Recycling Strategy: Part One of a Series on Building a Circular Economy for All. Available online: https://www.epa.gov/system/files/documents/2021-11/final-national-recycling-strategy.pdf (accessed on 15 August 2022).
- Canadian Council of Ministers of the Environment. Canada-Wide Action Plan for Extended Producer Responsibility. Available online: https://ccme.ca/en/res/cap-epr_e.pdf (accessed on 15 August 2022).
- Ontario. Ontario Enhancing Blue Box Program. Available online: https://news.ontario.ca/en/release/1000259/ontario-enhancing-blue-box-program (accessed on 29 June 2022).
- Environment and Climate Change Canada. Consultation Paper: A Proposed Federal Plastics Registry for Producers of Plastic Products. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/cepa/consultationplasticproductreg/Federal%20Plastic%20Registry%20Consultation%20Paper%20EN.pdf (accessed on 15 August 2022).
- Environment and Climate Change Canada. Federal Government, Provinces, and Territories Push Forward on a Canada-Wide Zero-Plastic-Waste Strategy. Available online: https://www.canada.ca/en/environment-climate-change/news/2018/11/federal-government-provinces-and-territories-push-forward-on-a-canada-wide-zero-plastic-waste-strategy.html (accessed on 29 June 2022).
- Environment and Climate Change Canada. Government of Canada Delivers on Commitment to Ban Harmful Single-Use Plastics. Available online: https://www.canada.ca/en/environment-climate-change/news/2022/06/government-of-canada-delivers-on-commitment-to-ban-harmful-single-use-plastics.html (accessed on 29 June 2022).
- Environment and Climate Change Canada. Technical Issues Paper: Recycled Content for Certain Plastic Manufactured Items Regulations. Available online: https://www.canada.ca/en/environment-climate-change/services/canadian-environmental-protection-act-registry/technical-issues-paper-recycled-content-plastic-manufactured-regulations.html (accessed on 15 July 2022).
- Environment and Climate Change Canada. Consultation Paper: Towards Canada-Wide Rules to Strengthen Recycling and Composting of Plastics through Accurate Labelling. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/cepa/consultationplasticproductreg/Labelling%20consultation%20paper_final%20EN.pdf (accessed on 15 August 2022).
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front. Sustain. Food Syst. 2021, 5, 125. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Corte-Tarazón, J.A.; Rochín-Wong, S.; Fernández-Quiroz, J.D.; Garzón-García, A.M.; Santos-Sauceda, I.; Plascencia-Martínez, D.F.; Chan-Chan, L.H.; Vásquez-López, C.; Barreras-Urbina, C.G.; et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. J. Food Eng. 2022, 334, 111153. [Google Scholar] [CrossRef]
- Lee, H.; An, D.; Lee, D.S. Use-friendly active packaging of powdered infant formula in single-serve portion augmented with anti-oxidative function. KOPAST 2019, 25, 95–99. [Google Scholar]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Newhart, B. Making Baby Formula Transparent with Danone’s Track & Connect. Available online: https://www.dairyreporter.com/Article/2020/02/21/Making-baby-formula-transparent-with-Danone-s-Track-Connect (accessed on 30 June 2022).
- Packaging South Asia. FrieslandCampina Uses Intelligent Packaging to Tackle Infant Formula Counterfeits in China. Available online: https://packagingsouthasia.com/technology/track-and-trace/frieslandcampina-uses-intelligent-packaging-to-tackle-infant-formula-counterfeits-in-china/ (accessed on 30 June 2022).
- State of New Jersey 219th Legislature. Senate Committee Substitute for Senate, No. 2515. Available online: https://pub.njleg.gov/bills/2020/S3000/2515_R5.PDF (accessed on 15 August 2022).
- Recycling Leadership Council. Blueprint for America’s Recycling System. Available online: https://consumerbrandsassociation.org/wp-content/uploads/2021/02/RLC_Blueprint.pdf (accessed on 30 June 2022).
- Canada Plastics Pact. Roadmap to 2025: A Shared Action Plan to Build a Circular Economy for Plastics Packaging. Available online: https://roadmap.plasticspact.ca/#resources (accessed on 30 June 2022).

| Regulatory Category | Statute/Regulation | Definition |
|---|---|---|
| Food for Special Dietary Use (FSDU) | 21 CFR 105.3 | “The term special dietary uses, as applied to food for man, means particular (as distinguished from general) uses of food, as follows:
|
| Medical Food | 21 USC 360ee(b)(3) | “A food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation” [11] |
| Infant Formula | 21 USC 321(z) | “A food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk” [15] |
| Exempt Infant Formula | 21 CFR 107.3 | “An infant formula intended for commercial or charitable distribution that is represented and labeled for use by infants who have inborn errors of metabolism or low birth weight, or who otherwise have unusual medical or dietary problems” [16] |
| Regulatory Category | Regulation | Definition |
|---|---|---|
| Food for Special Dietary Use (FSDU) | FDR B.24.001 | “Food that has been specially processed or formulated to meet the particular requirements of a person
|
| Formulated Liquid Diet (FLD) | FDR B.24.001 | “A food that
|
| Meal Replacement (MR) | FDR B.24.200 | “A formulated food that, by itself, can replace one or more daily meals” [23] |
| Nutritional Supplement(NS) | FDR B.24.201 | “A food sold or represented as a supplement to a diet that may be inadequate in energy and essential nutrients” [23] |
| Human Milk Substitute (Infant Formula) | FDR B.25.001 | “Any food that is labelled or advertised
|
| Human Milk Fortifier (HMF) | FDR B.25.001 | “A food that
|
| Natural Health Product (NHP) | Natural Health Product Regulations (NHPR) | “A substance … that is manufactured, sold or represented for use in
|
| Manufacturing Process | Description | Advantages/Disadvantages | Product Examples | Common Packaging Materials * |
|---|---|---|---|---|
| Retort | Non-sterile product filled into hermetically sealed, non-sterile packaging that is bulk loaded into retort bin; bin subjected to high heat/pressure for extended time to commercially sterilize product/packaging | Advantage
| Low-acid foods (pH > 4.6, water activity > 0.85) including liquid forms of infant formulas, oral nutrition supplements |
|
| Hot-fill | Heated, commercially sterile product filled into non-sterile packaging that is sealed, held at high temperature for short time (to commercially sterilize inner surface of package), and then immediately cooled | Advantages
| High-acid foods (pH < 4.6) including oral electrolyte solutions |
|
| Aseptic | Product and packaging commercially sterilized, product filled into packaging in commercially sterile environment | Advantages
| Low- and high-acid foods including liquid forms of infant formulas, oral nutrition supplements, oral electrolyte solutions |
|
| Powdered | Product prepared, evaporated/dried, then product filled into package | Advantage
| Infant formulas, oral nutrition supplements, oral electrolyte solutions |
|
| Packaging Requirement | Potential Quality/Safety Benefits from Effective Packaging Materials | Potential Quality/Safety Risks from Ineffective Packaging Materials |
|---|---|---|
| Material Performance: Withstand requisite processing and heat treatment conditions | Maintains packaging integrity throughout manufacturing process | Packaging degrades or fails during manufacturing resulting in product loss *,†,‡ |
| Packaging Performance: Withstand rigors of supply chain and distribution process | Maintains packaging integrity during transportation, warehousing, retail sale, and consumer storage/use | Packaging is damaged during distribution, resulting in unsaleable product †,‡ |
| Shelf Life: Maintain commercial sterility | Keeps product shelf-stable and safe to consume for an extended period without refrigeration | Biological protection not maintained, increasing product susceptibility to spoilage and risk for foodborne illness †,‡,§ |
| Barrier Requirements: Maintain nutrition value | Packaging barrier (light, oxygen, moisture) properties ensure product nutrient levels meet label claims throughout product shelf life | Packaging lacks adequate barrier properties, allowing nutrients to degrade and resulting in product not able to adequately meet consumer nutrition needs and label claims †,§ |
| Food Safety: Use is safe for food contact | Proper material selection limits migration of unintended, unsafe contaminants from the packaging materials to the product during processing and throughout product shelf life | Unintended contaminants, compatibilizers, and/or byproducts that are restricted from food contact use migrate to product, jeopardizing consumer health and ability to meet food safety regulations *,† |
| Potential Contaminants of Concern | Possible Health Issues |
|---|---|
| Endocrine disruptors—potential implications for reproductive systems, metabolic disorders including diabetes and obesity [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascall, M.A.; DeAngelo, K.; Richards, J.; Arensberg, M.B. Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods 2022, 11, 3043. https://doi.org/10.3390/foods11193043
Pascall MA, DeAngelo K, Richards J, Arensberg MB. Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods. 2022; 11(19):3043. https://doi.org/10.3390/foods11193043
Chicago/Turabian StylePascall, Melvin A., Kris DeAngelo, Julie Richards, and Mary Beth Arensberg. 2022. "Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability" Foods 11, no. 19: 3043. https://doi.org/10.3390/foods11193043
APA StylePascall, M. A., DeAngelo, K., Richards, J., & Arensberg, M. B. (2022). Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods, 11(19), 3043. https://doi.org/10.3390/foods11193043







