Anti-Obesity Effect of Theabrownin from Dark Tea in C57BL/6J Mice Fed a High-Fat Diet by Metabolic Profiles through Gut Microbiota Using Untargeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
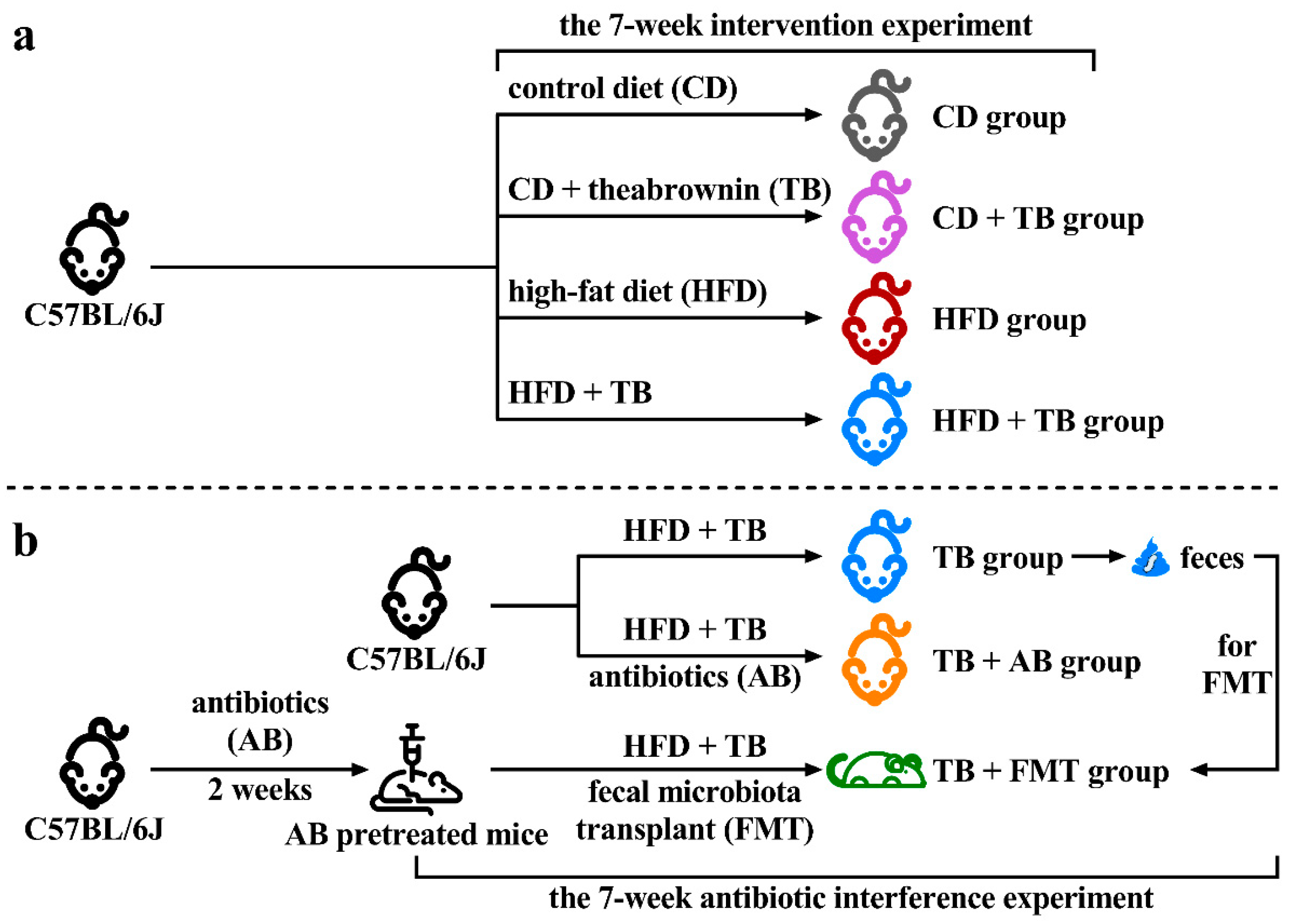
2.2. Animal Experimental Design
2.3. Fecal Microbiota Transplant
2.4. Blood Biochemical Tests and Nuclear Magnetic Resonance
2.5. Liver Histopathology
2.6. Untargeted Metabolomics
2.7. Statistical Analysis
3. Results
3.1. Effects of Theabrownin (TB) on Body Weight and Appetite
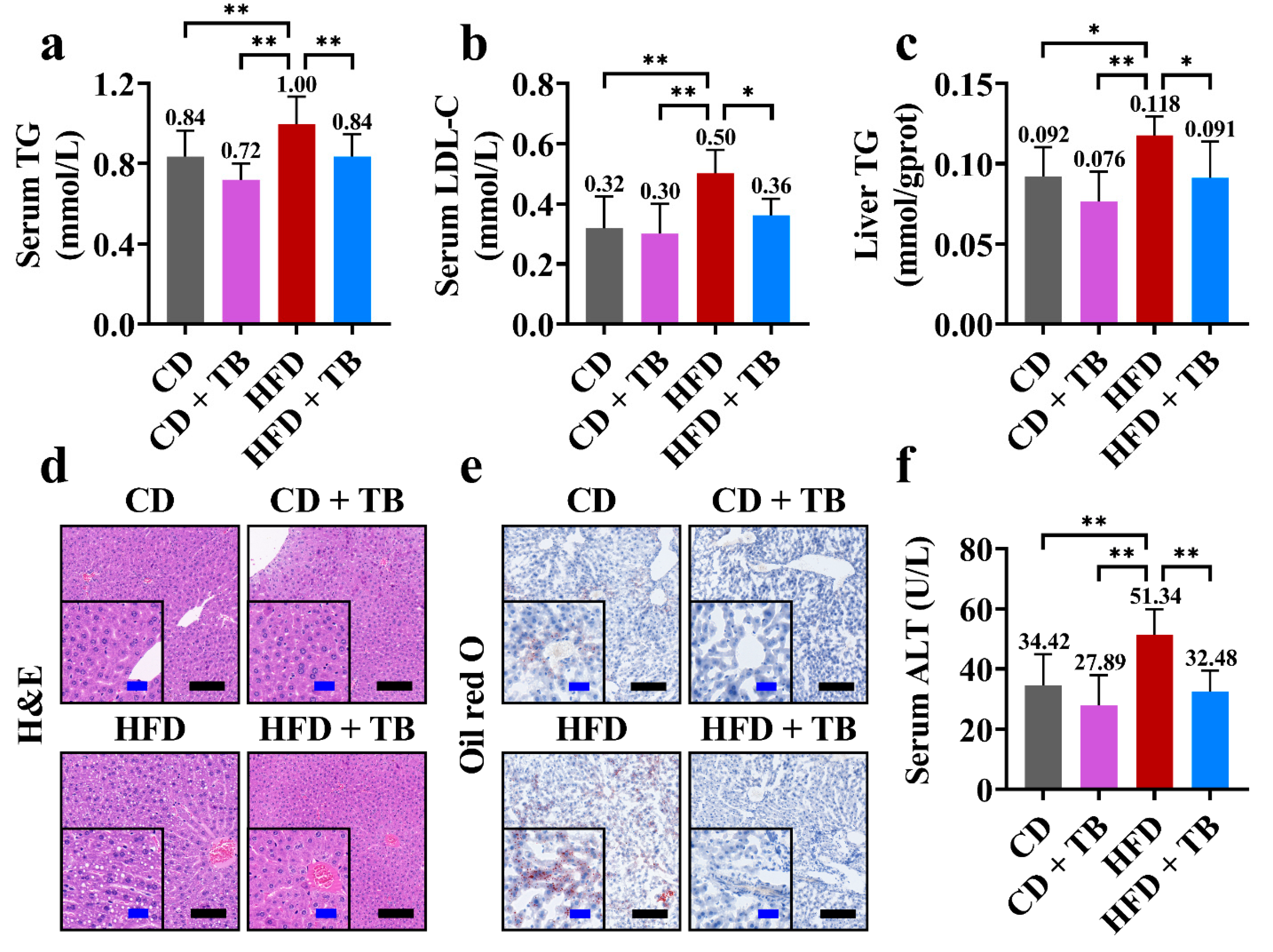
3.2. Effects of TB on Hepatic Indexes
3.3. TB, Gut Microbiota, and Body Weight
3.4. The OPLS-DA among the TB, TB + Antibiotics, and TB + Fecal Microbiota Transplant Groups
3.5. The Identification of Differential Metabolites in the Untargeted Metabolomics
3.6. The Evaluation of Metabolic Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western diet causes obesity-induced nonalcoholic fatty liver disease development by differentially compromising the autophagic response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef]
- Raptou, E. The role of snack choices, body weight stereotypes and smoking behavior in assessing risk factors for adolescent overweight and obesity. Foods 2021, 10, 557. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Wang, Y.B.; Shivappa, N.; Hebert, J.R.; Page, A.J.; Gill, T.K.; Melaku, Y.A. Association between dietary inflammatory index, dietary patterns, plant-based dietary index and the risk of obesity. Nutrients 2021, 13, 1536. [Google Scholar] [CrossRef]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y.; Tang, G.Y.; Corke, H.; Gan, R.Y.; Li, H.B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Liu, J.Y.; He, D.; Xing, Y.F.; Zeng, W.; Ren, K.; Zhang, C.; Lu, Y.; Yang, S.; Ou, S.J.; Wang, Y.; et al. Effects of bioactive components of Pu-erh tea on gut microbiomes and health: A review. Food Chem. 2021, 353, 129439. [Google Scholar] [CrossRef]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Li, H.B.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of tea against alcoholic fatty liver disease by modulating gut microbiota in chronic alcohol-exposed mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Li, B.Y.; Meng, J.M.; Gan, R.Y.; Xu, X.Y.; Gu, Y.Y.; Wang, X.H.; Li, H.B. Effects of several tea extracts on nonalcoholic fatty liver disease in mice fed with a high-fat diet. Food Sci. Nutr. 2021, 9, 2954–2967. [Google Scholar] [CrossRef]
- Peng, C.X.; Liu, J.; Liu, H.R.; Zhou, H.J.; Gong, J.S. Influence of different fermentation raw materials on pyrolyzates of Pu-erh tea theabrownin by Curie-point pyrolysis-gas chromatography-mass spectroscopy. Int. J. Biol. Macromol. 2013, 54, 197–203. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Zhang, D. Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes. Food Funct. 2015, 6, 2008–2016. [Google Scholar] [CrossRef]
- Ma, W.; Shi, Y.; Yang, G.; Shi, J.; Ji, J.; Zhang, Y.; Wang, J.; Peng, Q.; Lin, Z.; Lv, H. Hypolipidaemic and antioxidant effects of various Chinese dark tea extracts obtained from the same raw material and their main chemical components. Food Chem. 2022, 375, 131877. [Google Scholar] [CrossRef]
- Peng, C.X.; Wang, Q.P.; Liu, H.R.; Gao, B.; Sheng, J.; Gong, J.S. Effects of Zijuan pu-erh tea theabrownin on metabolites in hyperlipidemic rat feces by Py-GC/MS. J. Anal. Appl. Pyrolysis 2013, 104, 226–233. [Google Scholar] [CrossRef]
- Long, P.; Wen, M.; Granato, D.; Zhou, J.; Wu, Y.; Hou, Y.; Zhang, L. Untargeted and targeted metabolomics reveal the chemical characteristic of Pu-erh tea (Camellia assamica) during pile-fermentation. Food Chem. 2020, 311, 125895. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.X.; Gao, B.; Gong, J.S. Serum metabolomics analysis of rat after intragastric infusion of Pu-erh theabrownin. J. Sci. Food Agric. 2016, 96, 3708–3716. [Google Scholar] [CrossRef]
- Yue, S.; Zhao, D.; Peng, C.; Tan, C.; Wang, Q.; Gong, J. Effects of theabrownin on serum metabolites and gut microbiome in rats with a high-sugar diet. Food Funct. 2019, 10, 7063–7080. [Google Scholar] [CrossRef]
- Yue, S.; Shan, B.; Peng, C.; Tan, C.; Wang, Q.; Gong, J. Theabrownin-targeted regulation of intestinal microorganisms to improve glucose and lipid metabolism in Goto-Kakizaki rats. Food Funct. 2022, 13, 1921–1940. [Google Scholar] [CrossRef]
- Kuang, J.; Zheng, X.; Huang, F.; Wang, S.; Li, M.; Zhao, M.; Sang, C.; Ge, K.; Li, Y.; Li, J.; et al. Anti-adipogenic effect of theabrownin is mediated by bile acid alternative synthesis via gut microbiota remodeling. Metabolites 2020, 10, 475. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, A.; Du, H.; Liu, Y.; Qi, B.; Yang, X. Theabrownin from Fu Brick tea exhibits the thermogenic function of adipocytes in high-fat-diet-induced obesity. J. Agric. Food Chem. 2021, 69, 11900–11911. [Google Scholar] [CrossRef]
- Yue, S.; Peng, C.; Zhao, D.; Xia, X.; Tan, C.; Wang, Q.; Gong, J. Theabrownin isolated from Pu-erh tea regulates Bacteroidetes to improve metabolic syndrome of rats induced by high-fat, high-sugar and high-salt diet. J. Sci. Food Agric. 2022, 102, 4250–4265. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Gong, S.; Lan, T.; Zeng, L.; Luo, H.; Yang, X.; Li, N.; Chen, X.; Liu, Z.; Li, R.; Win, S.; et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 2018, 69, 51–59. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Yu, Q.; Wang, J.; Deng, Y.; Hua, J.; Zhou, Q.; Yuan, H.; Jiang, Y. Chemical profile of a novel ripened Pu-erh tea and its metabolic conversion during pile fermentation. Food Chem. 2022, 378, 132126. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Jin, W.; Yan, B.; Chen, X.; He, Y.; Yang, W.; Du, W.; Zhang, Q.; Guo, Y.; et al. Theabrownin inhibits cell cycle progression and tumor growth of lung carcinoma through c-myc-related mechanism. Front. Pharmacol. 2017, 8, 75. [Google Scholar] [CrossRef]
- Liu, T.; Xiang, Z.; Chen, F.; Yin, D.; Huang, Y.; Xu, J.; Hu, L.; Xu, H.; Wang, X.; Sheng, J. Theabrownin suppresses in vitro osteoclastogenesis and prevents bone loss in ovariectomized rats. Biomed. Pharmacother. 2018, 106, 1339–1347. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Kim, B.; Tsujimoto, T.; So, R.; Tanaka, K. Changes in lower extremity muscle mass and muscle strength after weight loss in obese men: A prospective study. Obes. Res. Clin. Pract. 2015, 9, 365–373. [Google Scholar] [CrossRef]
- Seebacher, F.; Tallis, J.; McShea, K.; James, R.S. Obesity-induced decreases in muscle performance are not reversed by weight loss. Int. J. Obes. 2017, 41, 1271–1278. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Richter, L.R.; Wan, Q.; Wen, D.; Zhang, Y.; Yu, J.; Kang, J.K.; Zhu, C.; McKinnon, E.L.; Gu, Z.; Qiang, L.; et al. Targeted delivery of notch inhibitor attenuates obesity-induced glucose intolerance and liver fibrosis. ACS Nano 2020, 14, 6878–6886. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, C.N.; Li, B.Y.; Tang, G.Y.; Shang, A.; Gan, R.Y.; Feng, Y.B.; Li, H.B. Effects and mechanisms of tea on obesity. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Chen, H.H.; Tseng, Y.J.; Wang, S.Y.; Tsai, Y.S.; Chang, C.S.; Kuo, T.C.; Yao, W.J.; Shieh, C.C.; Wu, C.H.; Kuo, P.H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. 2015, 39, 1241–1248. [Google Scholar] [CrossRef]
- Libert, D.M.; Nowacki, A.S.; Natowicz, M.R. Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: Amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ 2018, 6, e5410. [Google Scholar] [CrossRef]
- Silveira, A.M.; Lima, P.L.; Alves, M.R.A.; Soares, R.D.L.; Kanufre, V.C.; Rodrigues, V.M.; Starling, A.L.P.; Norton, R.C.; Aguiar, M.J.B. Overweight/obesity in adolescents with phenylketonuria: Protective and predisposing factors. J. Pediatr. 2022, 98, 104–110. [Google Scholar] [CrossRef]
- Men, L.; Pi, Z.; Zhou, Y.; Wei, M.; Liu, Y.; Song, F.; Liu, Z. Urine metabolomics of high-fat diet induced obesity using UHPLC-Q-TOF-MS. J. Pharm. Biomed. Anal. 2017, 132, 258–266. [Google Scholar] [CrossRef]
- Bai, L.; Gao, M.; Cheng, X.; Kang, G.; Cao, X.; Huang, H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Fact. 2020, 19, 94. [Google Scholar] [CrossRef]
- Bellissimo, M.P.; Cai, Q.; Ziegler, T.R.; Liu, K.H.; Tran, P.H.; Vos, M.B.; Martin, G.S.; Jones, D.P.; Yu, T.; Alvarez, J.A. Plasma high-resolution metabolomics differentiates adults with normal weight obesity from lean individuals. Obesity 2019, 27, 1729–1737. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Cardellini, M.; Hoyles, L.; Latorre, J.; Davato, F.; Moreno-Navarrete, J.M.; Arnoriaga-Rodriguez, M.; Serino, M.; Abbott, J.; Barton, R.H.; et al. Iron status influences non-alcoholic fatty liver disease in obesity through the gut microbiome. Microbiome 2021, 9, 104. [Google Scholar] [CrossRef]
- Norton, M.; Murphy, K.G. Targeting gastrointestinal nutrient sensing mechanisms to treat obesity. Curr. Opin. Pharmacol. 2017, 37, 16–23. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Angelakis, E.; Lagier, J.C. Samples and techniques highlighting the links between obesity and microbiota. Microb. Pathog. 2017, 106, 119–126. [Google Scholar] [CrossRef]




| Groups | Metabolic Pathways | Total | Hits | p-Value | Impact |
|---|---|---|---|---|---|
| TB vs. TB + AB | Alanine, aspartate, and glutamate metabolism | 28 | 3 | 0.024287 | 0.071023 |
| Caffeine metabolism | 22 | 3 | 0.012576 | 0.14706 | |
| Central carbon metabolism in cancer | 37 | 4 | 0.009072 | 0.15094 | |
| Citrate cycle (TCA cycle) | 20 | 4 | 0.00088982 | 0.13333 | |
| Glucagon signaling pathway | 26 | 4 | 0.0024774 | 0.20455 | |
| HIF-1 signaling pathway | 15 | 2 | 0.043847 | 0.26316 | |
| Histidine metabolism | 47 | 4 | 0.020681 | 0.144 | |
| Phenylalanine metabolism | 60 | 6 | 0.0020825 | 0.10714 | |
| Protein digestion and absorption | 47 | 4 | 0.020681 | 0.085106 | |
| Pyrimidine metabolism | 65 | 5 | 0.014892 | 0.13541 | |
| Pyruvate metabolism | 31 | 3 | 0.031759 | 0.14286 | |
| Tyrosine metabolism | 78 | 7 | 0.0016704 | 0.072 | |
| TB + AB vs. TB + FMT | ABC transporters | 138 | 10 | 0.042268 | 0.072464 |
| Arginine biosynthesis | 23 | 5 | 0.0016344 | 0.18239 | |
| beta-Alanine metabolism | 32 | 5 | 0.0073705 | 0.17241 | |
| Caffeine metabolism | 22 | 5 | 0.0013201 | 0.20588 | |
| Central carbon metabolism in cancer | 37 | 6 | 0.0027667 | 0.15094 | |
| Histidine metabolism | 47 | 5 | 0.035216 | 0.112 | |
| Pentose phosphate pathway | 35 | 4 | 0.046187 | 0.059701 | |
| Phenylalanine metabolism | 60 | 7 | 0.0082768 | 0.17857 | |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 34 | 4 | 0.042145 | 0.13158 | |
| Protein digestion and absorption | 47 | 6 | 0.0092762 | 0.12766 | |
| Tryptophan metabolism | 83 | 12 | 0.0000713 | 0.13897 |
| Pathways | Total | Hits | Compounds |
|---|---|---|---|
| Phenylalanine metabolism | 60 | 10 | 2-Hydroxyphenylacetate (0.75, 3.09) #, 2-Phenylacetamide * (37.54, 0.19), Hippuric acid (16.80, 0.39), L-Phenylalanine (1.79, 4.00), Phenylacetic acid (0.82, 1.05), Phenylacetylglycine * (9.73, 0.19), Phenyllactate (1.15, 1.29), Pyruvic acid (2.15, 0.68), Succinic acid (1.90, 1.57), trans-Cinnamate (1.16, 4.05) |
| Histidine metabolism | 47 | 7 | 4-Oxoglutaramate (0.26, 0.21), Anserine (0.75, 0.35); Histamine (1.65, 0.47), Imidazol-5-yl-pyruvate (1.57, 4.00), L-Aspartic acid (1.66, 0.32), N-Formyl-L-glutamic acid * (8.98, 0.04), Urocanic acid (0.84, 2.11) |
| Protein digestion and absorption | 47 | 9 | Butyric acid (3.74, 9653.20), Histamine (1.65, 0.47), Indole (1.01, 1.20), L-Arginine (1.18, 0.49), L-Aspartic acid (1.66, 0.32), L-Phenylalanine (1.79, 4.00), Isobutyric acid (3.03, 1.53), p-Cresol (8.16, 1.10); Phenol * (38.05, 0.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-Y.; Huang, S.-Y.; Xiong, R.-G.; Wu, S.-X.; Zhou, D.-D.; Saimaiti, A.; Luo, M.; Zhu, H.-L.; Li, H.-B. Anti-Obesity Effect of Theabrownin from Dark Tea in C57BL/6J Mice Fed a High-Fat Diet by Metabolic Profiles through Gut Microbiota Using Untargeted Metabolomics. Foods 2022, 11, 3000. https://doi.org/10.3390/foods11193000
Li H-Y, Huang S-Y, Xiong R-G, Wu S-X, Zhou D-D, Saimaiti A, Luo M, Zhu H-L, Li H-B. Anti-Obesity Effect of Theabrownin from Dark Tea in C57BL/6J Mice Fed a High-Fat Diet by Metabolic Profiles through Gut Microbiota Using Untargeted Metabolomics. Foods. 2022; 11(19):3000. https://doi.org/10.3390/foods11193000
Chicago/Turabian StyleLi, Hang-Yu, Si-Yu Huang, Ruo-Gu Xiong, Si-Xia Wu, Dan-Dan Zhou, Adila Saimaiti, Min Luo, Hui-Lian Zhu, and Hua-Bin Li. 2022. "Anti-Obesity Effect of Theabrownin from Dark Tea in C57BL/6J Mice Fed a High-Fat Diet by Metabolic Profiles through Gut Microbiota Using Untargeted Metabolomics" Foods 11, no. 19: 3000. https://doi.org/10.3390/foods11193000
APA StyleLi, H.-Y., Huang, S.-Y., Xiong, R.-G., Wu, S.-X., Zhou, D.-D., Saimaiti, A., Luo, M., Zhu, H.-L., & Li, H.-B. (2022). Anti-Obesity Effect of Theabrownin from Dark Tea in C57BL/6J Mice Fed a High-Fat Diet by Metabolic Profiles through Gut Microbiota Using Untargeted Metabolomics. Foods, 11(19), 3000. https://doi.org/10.3390/foods11193000








